-
Multiple waves of coronavirus disease 2019 (COVID-19) outbreaks have affected numerous countries worldwide. The first case of SARS-CoV-2 reinfection was reported in Hong Kong in August 2020[1], leading to increased interest in the effectiveness of natural immunity acquired from primary infection. While data reports vary across countries, all findings indicate that prior SARS-CoV-2 infection provides substantial protection against reinfection[2]. However, natural immunity from infection with previous non-Omicron or early Omicron sub-lineages offers lower levels of protection against Omicron reinfection, with rates below 60%[3] and approximately 75%[4], respectively. Furthermore, this natural immunity wanes rapidly within 5–9 months post-infection[3,5]. The frequency of reinfection has increased during the Omicron wave with reinfection rates reaching up to 10.6%[6], compared to less than 1% prior to the Omicron epidemic[2].
The rapid spread of the Omicron variant across Chinese mainland, reaching its peak in December 2022, has likely led to a significant number of infections. Then a considerable number of individuals may have developed some level of immunity from prior infections. This acquired immunity could influence the course of future pandemic waves. Hence, it is crucial to evaluate the likelihood of reinfections within the population and pinpoint high-risk groups
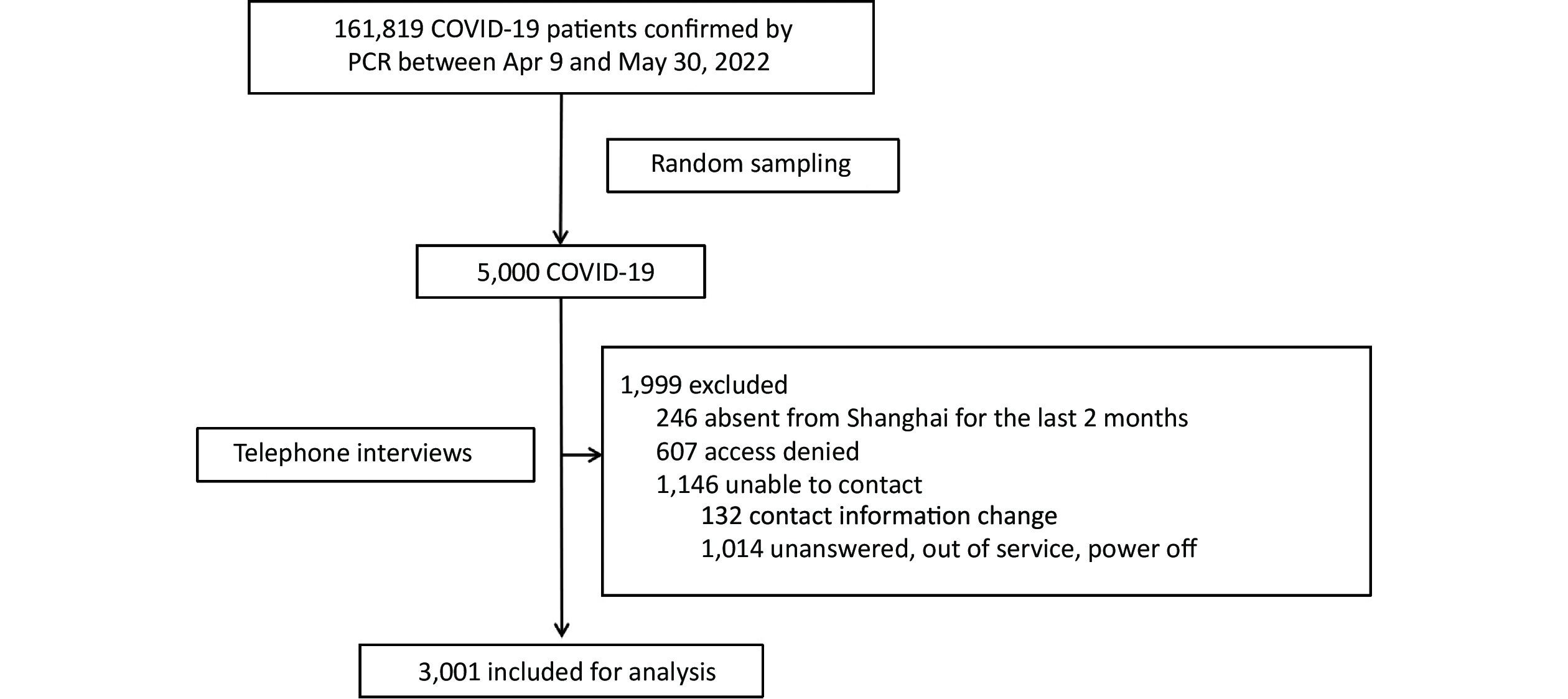
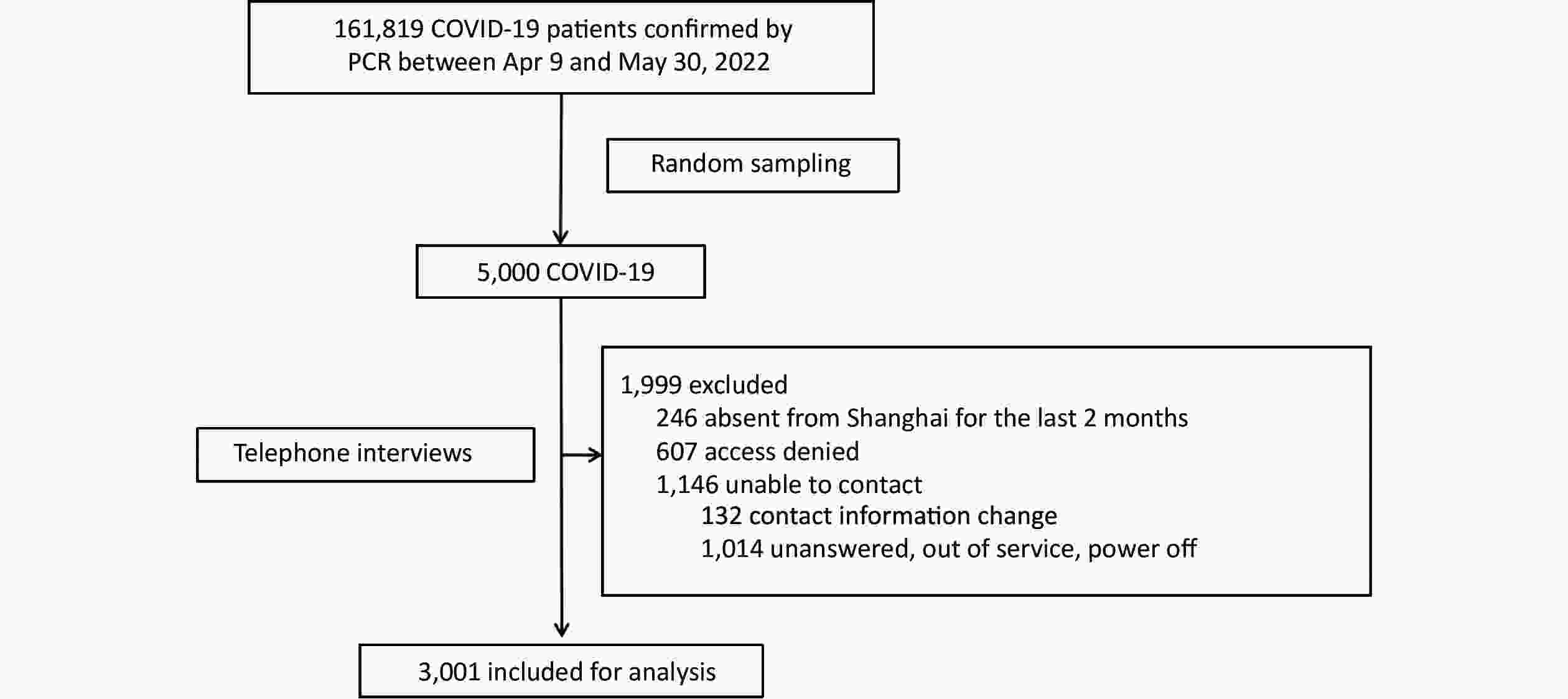
. This indicates that a majority of individuals have acquired immunity through previous infection, making reinfections the potential cause of future epidemic waves. Consequently, it is crucial to determine the likelihood of reinfection among the Chinese population and identify those at higher risk. Currently, only a small number of reinfection cases have been reported in mainland China . Furthermore, there is a lack of studies involving East Asian populations that examine the protective effect of natural immunity against SARS-CoV-2 reinfection and provide comprehensive data on associated risk factors[5]. Therefore, it is imperative to clarify the extent of immunity protection and identify the relevant risk factors for reinfection in the local region. To address the aforementioned concerns, a study was conducted involving a simple random sample of 5,000 individuals from a total of 161,819 PCR-confirmed patients with COVID-19 who were admitted to the mobile cabin hospital in the National Exhibition and Convention Center in Shanghai. This study took place between April 9 and May 30, 2022. Telephone interviews were conducted with the selected individuals to gather information about reinfection during the ongoing epidemic. Reinfection was defined as a positive result in either a reverse transcription-polymerase chain reaction (RT-PCR) test or a rapid antigen test (RAT) that occurred at least 90 days after the last positive test in the same individual.
During the telephone interviews, general information was collected, including the individual's area of residence during the previous two months, history of reinfection before January 10, 2023, vaccination status (including any catch-up vaccinations following infection), recurrence history (positive RT-PCR within one month after initial discharge), presence of comorbidities, and use of specific medications such as glucocorticoids, immunosuppressants, and anti-rejection drugs. The interviews were conducted between January 10 and January 30, 2023, with individuals infected with the Omicron variant. All patients included in the study were diagnosed according to the Diagnosis and Treatment Protocol for COVID-19 Patients (Tentative 9th Edition) issued by the National Health Commission of the People's Republic of China.
The inclusion criteria for the study were as follows: (a) individuals with asymptomatic or mild symptomatic COVID-19 confirmed by RT-PCR; (b) regardless of age and gender; (c) voluntary participation in the telephone interviews. The exclusion criteria were: (a) individuals who had been absent from Shanghai during the previous two months; (b) individuals with incorrect contact information or were unreachable; (c) individuals who refused to complete the telephone interview and follow-up. The study was approved by the Research Ethics Committees of the Shanghai Changzheng Hospital (no.2023SL001). Each participant in the telephone interview will be apprised of its primary purpose at the outset. Their cooperation in the interview shall be regarded as their informed consent to participate in the study. Throughout the interview process, participants reserve the right to refuse answering at any given moment and subsequently terminate the discussion.
Out of the initial sample, a total of 1,999 individuals were excluded from the study. This included 246 individuals who had been absent from Shanghai for at least two months, 607 individuals who refused to participate in the interviews, and 1,146 individuals who could not be reached. Ultimately, 3,001 patients agreed to cooperate and completed the interview, as illustrated in Figure 1.
Table 1 presents the demographic and clinical characteristics of the participants included in the study. Among the participants, there were 1,726 males (58.9%) and 1,232 females (41.1%). Additionally, 23 individuals (0.8%) identified as healthcare workers. The majority of patients fell within the age range of 18–65 years (88.9%). During their initial infection, a total of 2,141 individuals (71.3%) experienced symptoms, and 16 individuals (0.5%) had a history of recurrence.
Variables Total (N = 3,001) Reinfection (n = 260) Without reinfection (n = 2,741) P Age (y) 0.187 < 18 128 (4.3) 6 (2.3) 122 (4.5) 18– 2,667 (88.9) 239 (91.9) 2,428 (88.6) ≥ 65 206 (6.9) 15 (5.8) 191 (7.0) Sex 0.276 Male 1,769 (58.9) 145 (55.8) 1,624 (59.2) Female 1,232 (41.1) 115 (44.2) 1,117 (40.8) Vaccination 0.014** NO 257 (8.6) 24 (9.2) 233 (8.5) Partially vaccinated 96 (3.2) 17 (6.5) 79 (2.9) fully vaccinated 1,050 (35.0) 85 (32.7) 965 (35.2) Boosted 1,598 (53.2) 134 (51.5) 1,464 (53.4) Current smoker 704 (23.5) 59 (22.7) 645 (23.5) 0.760 Current drinker 559 (18.6) 40 (15.4) 519 (18.9) 0.160 Healthcare Workers 23 (0.8) 6 (2.3) 17 (0.6) 0.011** Symptomatic in primary infection 2,141 (71.3) 205 (78.8) 1,936 (70.6) 0.005** Recurrence history 88 (2.9) 10 (3.8) 78 (2.8) 0.361 Catch-up vaccination post-infection 105 (3.5) 6 (2.3) 99 (3.6) 0.274 Special drug use 16 (0.5) 3 (1.2) 13 (0.5) 0.156 With comorbidities 381 (12.7) 32 (12.3) 349 (12.7) 0.992 No. of comorbidities ≥ 2 91 (3.0) 11 (4.2) 80 (2.9) 0.238 Comorbidities Distribution Hypertension 274 (9.1) 23 (8.8) 251 (9.2) 0.868 Diabetes 82 (2.7) 10 (3.8) 72 (2.6) 0.249 Cardiovascular diseases 39 (1.3) 3 (1.2) 36 (1.3) 1.000 Endocrine diseases (except diabetes) 8 (0.3) 0 (0.0) 8 (0.3) 1.000 Cerebrovascular diseases 12 (0.4) 0 (0.0) 12 (0.4) 0.616 Chronic respiratory diseases
(Chronic bronchitis, COPD)19 (0.6) 1 (0.4) 18 (0.7) 1.000 Chronic respiratory diseases (asthma) 23 (0.8) 2 (0.8) 2 (0.8) 1.000 Cirrhosis 1 (0.0) 0 (0.0) 1 (0.0) 1.000 Chronic renal diseases 8 (0.3) 2 (0.8) 6 (0.2) 0.148 Cancer history 14 (0.5) 1 (0.4) 12 (0.4) 1.000 High-risk malignancies 8 (0.3) 2 (0.8) 6 (0.2) 0.148 Autoimmune diseases 9 (0.3) 2 (0.8) 7 (0.3) 0.180 Note. **P < 0.05. Table 1. Clinical characteristics of the study population with or without Omicron reinfection (n, %)
Antibodies generally develop quickly after SARS-CoV-2 infection, but their titers decline within 1–2 months[1]. However, the immune response to COVID-19 can vary depending on natural immunity within the human body. Factors such as immunosuppressive drugs and underlying pathological conditions can impact antibody release, potentially leading to prolonged viral shedding. This phenomenon of continuous low-level viral shedding has been frequently observed, making it challenging for physicians to differentiate between true reinfection and prolonged viral shedding in clinical settings. The current diagnostic criteria for reinfection are not always consistent, but it is generally recommended that reinfection be defined as a person becoming infected again after fully recovering from an initial infection, with a separation period of at least 90 days.
Although the reinfection rate of COVID-19 has been tentatively established, the evidence remains insufficient. Limited studies have shown that the overall rate of reinfection with the SARS-CoV-2 Omicron variant ranges from 0.3% to 10.6%[5,7,8], indicating its significant ability to evade immunity from previous infection. In our study, we reported a reinfection rate of 8.7% (260/3,001) during the second COVID-19 epidemic in the local region. However, it is important to note that several factors may have influenced previously reported reinfection rates. In some countries and regions, the detection of COVID-19 is hindered by a shortage of RT-PCR or RAT testing, and the reinfection rate is also closely related to the specific variant of SARS-CoV-2, which can dramatically increase with the emergence of new variant. In the first infection of our cohort, the main virus strains were Omicron BA2.2. However, during the second wave of the epidemic, BA5 and BF7 became the predominant strains according to data from the China CDC. Additionally, the sensitivity of diagnostic assays can vary. In our data, approximately half (131/260) of the reinfected patients were diagnosed using RAT rather than RT-PCR. Furthermore, the underreporting of asymptomatic cases may potentially result in a lower reported reinfection rate.
Previous studies have reported that natural immunity is associated with a 84%–95% lower risk of reinfection compared to non-immunity[8]. However, the duration of natural immunity remains unclear. Two studies demonstrated that immune memory lasted for at least 6 months in the majority of patients (87.8% and 95.0%, respectively)[9]. Another study found that the protective effect of natural immunity remained high (78%) even after 9 to 19 months of follow-up. The median time between primary infection and reinfection in our study was 8 months (IQR 7–8 m, range 3–9 m). One patient was reinfected 3 months after the initial infection, while three patients were reinfected 6 months after the initial infection. The remaining reinfections all occurred within 7–9 months after the primary infection. These findings align with previously reported results[3,5], suggesting that natural immunity may be long-lasting. However, it is worth noting that regional managements for epidemic control and prevention, as well as the environment in which an individual lives, also influence the length of the interval between infections.
Although individuals develop natural immunity after recovering from the initial infection, vaccination can further enhance protection, particularly for those with lower antibody levels following SARS-CoV-2 infection. This concept of “hybrid immunity” combines natural and vaccine-induced immunity. Vaccines play a crucial role in increasing population immunity, with efficacy ranging from 62% to 95% and satisfactory safety profiles. In our retrospective cohort study, we investigated whether individuals who had previously been infected still benefited from complete vaccination. The results indicated that complete vaccination significantly reduced the risk of reinfection (OR = 0.68, 95% CI: 0.47–0.98, P = 0.040), demonstrating that the vaccines remained effective against SARS-CoV-2 reinfection. Although a lower reinfection rate was observed in participants with catch-up vaccination, the difference was not statistically significant (OR = 0.52, 95% CI: 0.22–1.22, P = 0.133). In summary, the development and widespread use of effective vaccines against COVID-19 remain crucial in mitigating the pandemic crisis and reducing reinfection rates. Hybrid immunity, combining natural immunity and vaccination, may provide additional protection against reinfection[10].
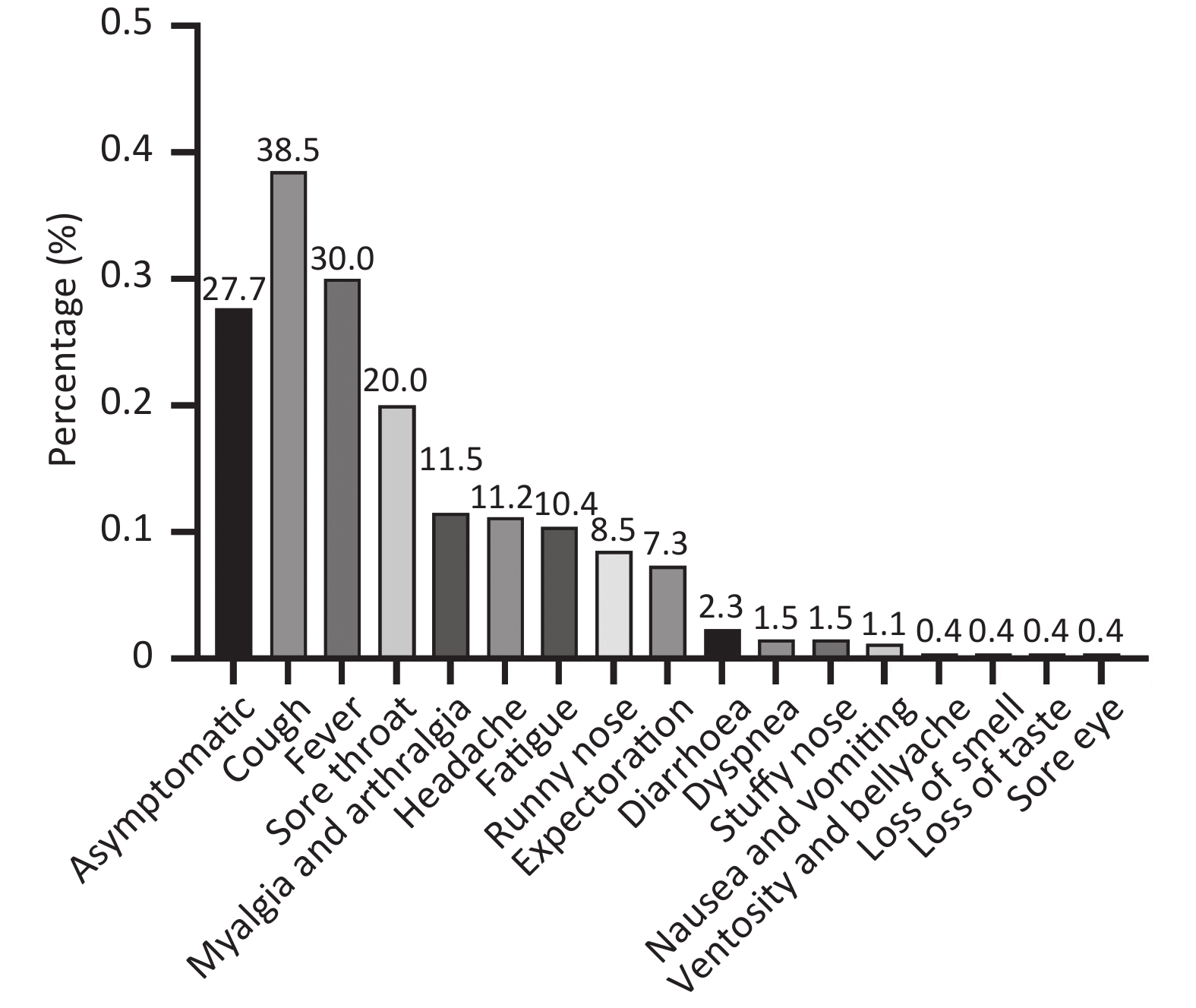
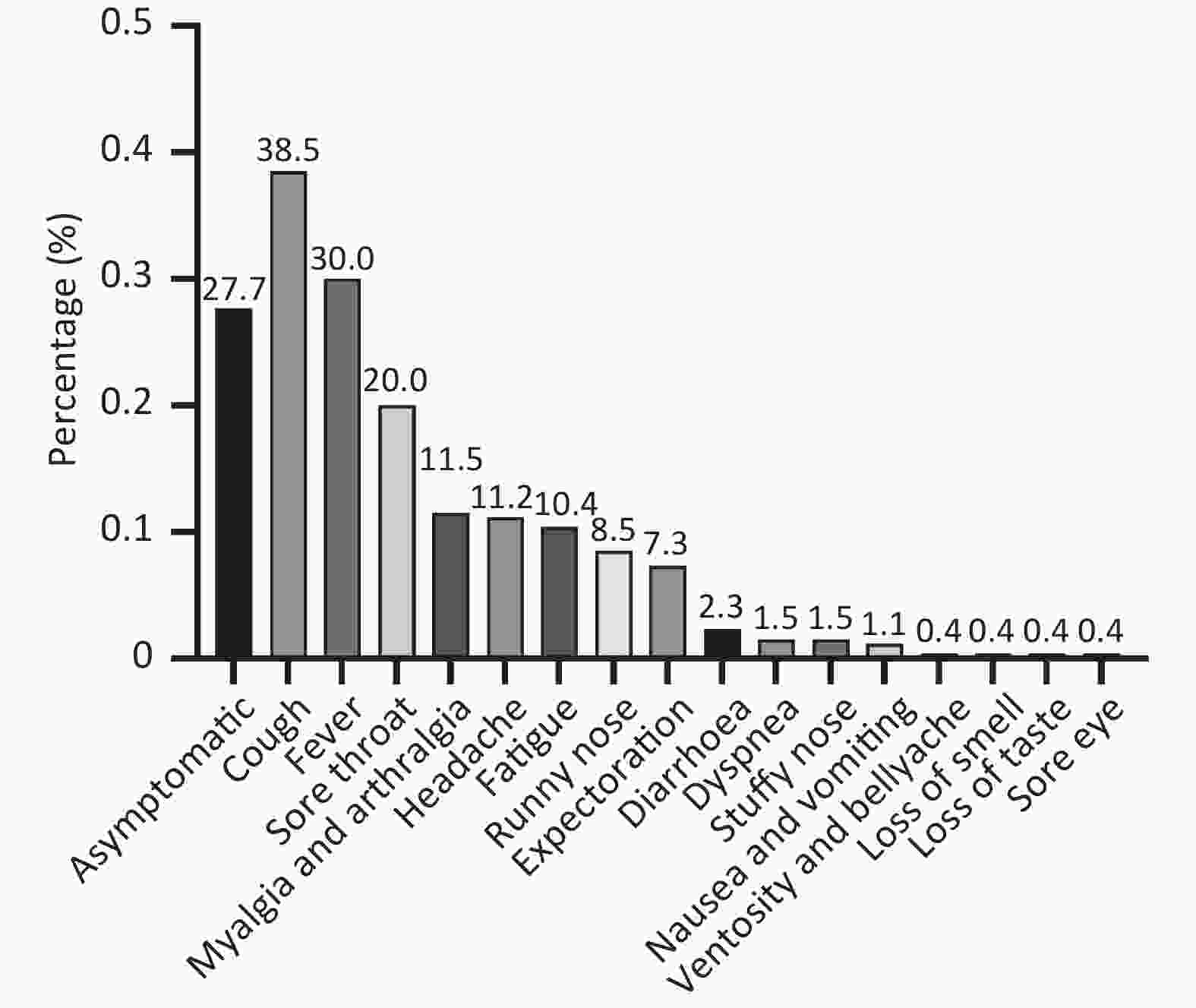
Numerous studies have focused on the clinical characteristics of COVID-19 patients, with several studies concluding that reinfection symptoms are generally less severe than those of the initial infection. In our cohort, the median duration of reinfection was 4 days (IQR 3–6 d), which was shorter than the median duration of 7 days (IQR 5–8 d) for the initial infection (P < 0.001). Among the 260 reinfected individuals, 188 (72.3%) exhibited symptoms, while 72 (27.7%) were asymptomatic. The most commonly reported symptoms after SARS-CoV-2 reinfection were cough (100/260, 38.5%) and fever (78/260, 30.0%), followed by sore throat (52/260, 20.0%) (Figure 2). None of the reinfected cases required hospitalization, but 43 (16.5%) experienced comparable severity of symptoms in both infections, while 52 (20.0%) had aggravated symptoms, and 165 (63.5%) had milder symptoms during reinfection. However, the severity of symptoms was milder in reinfection compared to the initial infection (P < 0.001).
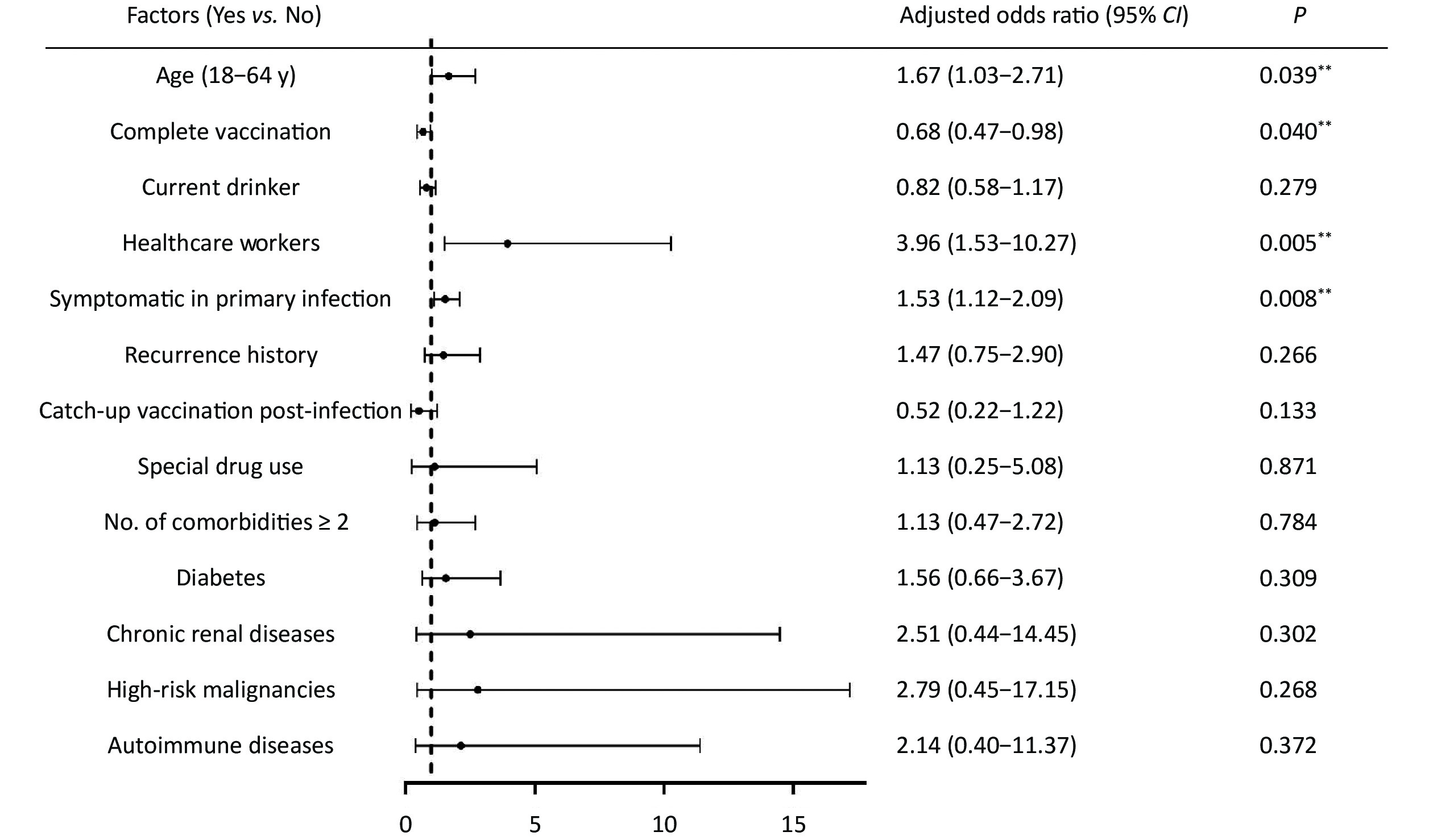
The identification of risk factors associated with SARS-CoV-2 reinfections is still not well established. Some studies have suggested that reinfection is associated with age, vaccination status, and occupational exposure, although the data are inconsistent due to variations in patient groups, mixed variant epidemics, diverse protective measures, and challenges in distinguishing persistent COVID-19 from reinfection[7]. In our study, we found that healthcare workers were more likely to experience reinfection (OR = 3.96, 95% CI: 1.53–10.27, P = 0.005), which may be attributed to higher occupational exposure. Additionally, individuals aged 18-65 years had a higher risk of reinfection compared to those over 65 years and younger than 18 years (OR = 1.67, 95% CI: 1.03–2.71, P = 0.039), possibly due to reduced social activity in the older population and better protection for minors. Furthermore, patients with symptomatic primary infection were at a higher risk of reinfection (OR = 1.53, 95% CI: 1.12–2.09, P = 0.008) (Figure 3). These findings provide important evidence for decision-making and the development of public health strategies to protect vulnerable populations at higher risk of reinfection during the resurgence of COVID-19 and the emergence of new variants. Emergency or reinforced vaccination against COVID-19 is essential for these populations to decrease the reinfection rate.
This study has several limitations. Firstly, as a retrospective study, patients may not accurately recall the conditions during the initial infection. Secondly, screening efforts using PCR or RAT tests decreased during the second wave of the epidemic, potentially leading to missed diagnoses of asymptomatic reinfection. As a result, the actual reinfection rate may be underestimated. Thirdly, while all patients were monitored for conversion by RT-PCR during the initial infection, the majority of patients were monitored using RAT. Adjustments to the conversion criteria may have biased the assessment of reinfection duration. Lastly, this study was conducted at a single center in a specific region, involving individuals previously admitted to a mobile cabin hospital. This may have omitted middle and severe cases of COVID-19 from the initial infection, as well as rural patients. Future multi-center studies covering a wider range of patient cohorts from different regions are urgently needed to further confirm our findings.
In conclusion, natural immunity appears to be durable, and the rate of reinfection with SARS-CoV-2 remains low in the current outbreak. For individuals with associated risk factors for reinfection, such as specific age groups, symptomatic primary infection, and healthcare workers, vaccination-induced immunity can serve as a necessary complement to prevent reinfection.
Acknowledgments We thank the participants for their contribution.
Declaration of Interests The authors declare that they have no conflict of interests. And this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions XIE Wei Fen and WANG Pei Qin designed the study and revised the manuscript. WANG Pei Qin, WANG Jian and SHI Zhi Wen contributed to literature search, data analysis, and manuscript drafting. WANG Xiao Hang, CHU Dong Mei, WANG Zhi Fei, ZHANG Mu Bai, LIU Wei and ZHOU Zhi Jie contributed to data collection. All authors had full access to all the data in the study, and they took responsibility for the decision to submit for publication.
Reinfection and Risk Factors of SARS-CoV-2 during an Omicron Wave 2022 in Shanghai
doi: 10.3967/bes2024.021
- Received Date: 2023-06-16
- Accepted Date: 2023-12-12
| Citation: | WANG Pei Qin, WANG Xiao Hang, WANG Jian, SHI Zhi Wen, CHU Dong Mei, WANG Zhi Fei, ZHANG Mu Bai, LIU Wei, ZHOU Zi Jie, XIE Wei Fen. Reinfection and Risk Factors of SARS-CoV-2 during an Omicron Wave 2022 in Shanghai[J]. Biomedical and Environmental Sciences, 2024, 37(2): 204-209. doi: 10.3967/bes2024.021 |








 Quick Links
Quick Links
 DownLoad:
DownLoad: