-
With the accelerated rate of urbanization in recent years, air pollution has become an environmental problem that requires urgent resolution, almost all of the world's population exposed to pollution on a daily basis. Among the various air pollutants, the excessive dispersion and suspension of particulate matter (PM) in the air, such as PM2.5 and PM10, is one of the main indicators of air pollution. Particulate matter 2.5 (PM2.5) is a term used to describe particles with an aerodynamic equivalent diameter of less than or equal to 2.5 μm in ambient air, and are often referred to as fine particles. PM2.5 damages the human respiratory and cardiovascular systems via inducing oxidative stress, immune-mediated inflammation, and mutagenic effects, all of which can adversely affect human health[1] (Graphical Abstract, available in www.besjournal.com).
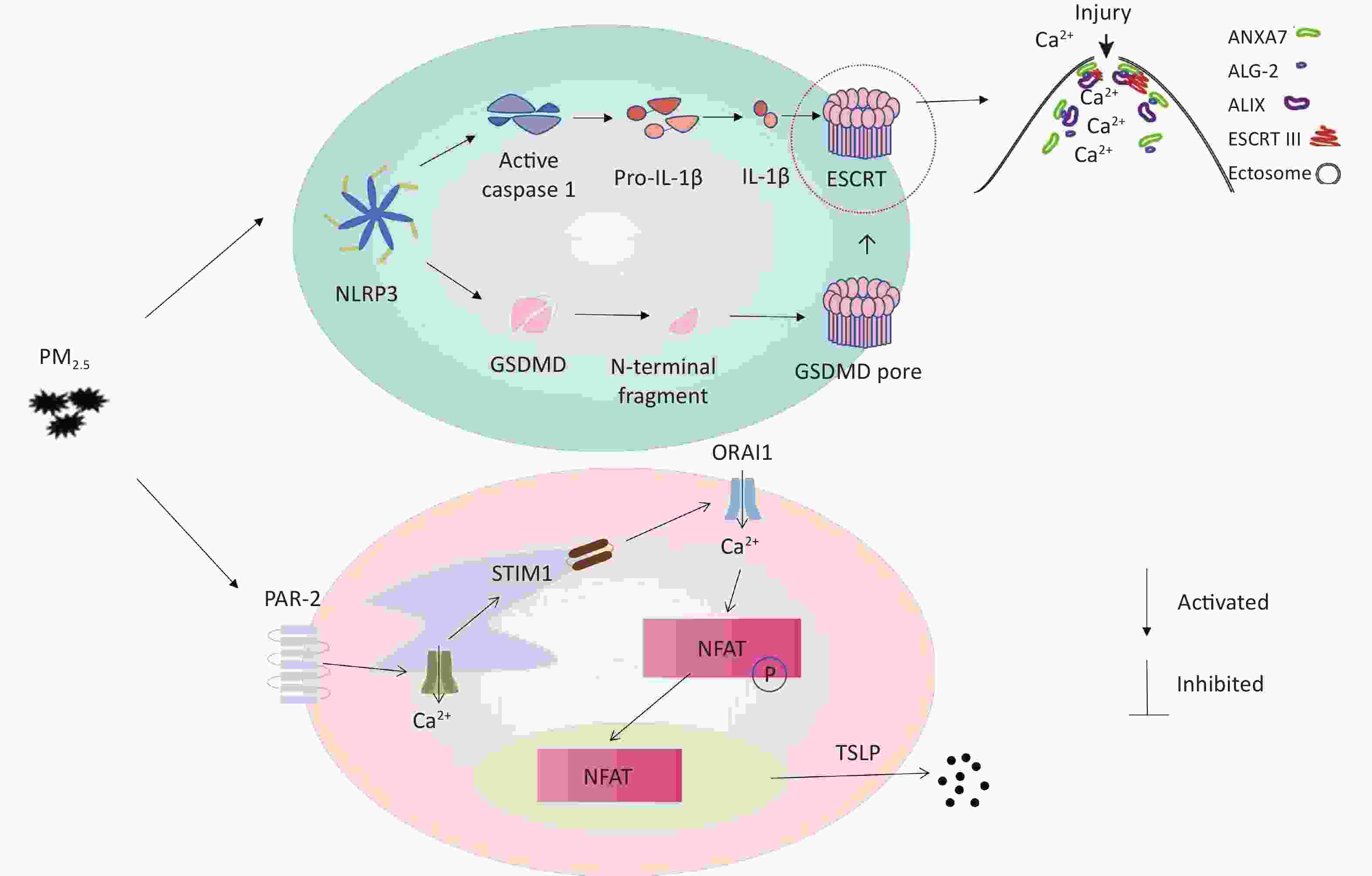
Pyroptosis is a type of inflammatory cell death that is intermediate between apoptosis and necrosis. The main features of pyroptosis are the formation of pores and vesicles in the cell membrane, cell swelling followed by rupture, and secretion of proinflammatory cytokines (IL-1β and IL-18) along with the release of cellular contents outside of the cell[2]. Previous literature showed that PM2.5 can activate nuclear factors κ B (NF- κ B) pathway and NALP3. Then NALP3 activates caspase-1 expression to cut off IL-1β Inflammatory factor precursors produce IL-1β[3]. Caspase-1 is the only caspase in the caspase family that can cleave IL-1β precursor protein to produce corresponding mature inflammatory factors. On the other hand, caspase-1 cleaves the Gasdermin D (GSDMD), the cell pyroptosis effector protein, from the self-repressed C-terminal structural domain to the N-terminal p30 structural domain. After cleavage, the N-terminal p30 structural domain of GSDMD binds to lipids on the cytoplasmic membrane and undergoes oligomerization to form larger oligomeric pores, leading to the release of cell contents and the occurrence of pyroptosis[4]. Meanwhile, the endosomal sorting complex required for transport (ESCRT), can repair cell membrane damage caused by GSDMD in pyroptosis. ESCRT is formed to allow cell pyrosis and GSDMD-dependent cytokine release while removing the site of membrane damage caused by GSDMD while membrane repair[5,6]. Protease-activated receptor 2 (PAR-2) is a G-protein-coupled receptor that plays a major role in regulating chronic pruritus. PAR-2 plays a key role in thymic stromal lymphopoietin (TSLP) production in keratinocytes[7]. At present, no research has shown the relationship between inflammation and cell scorch caused by PM2.5 and the expression of pruritus receptor in skin keratinocytes.
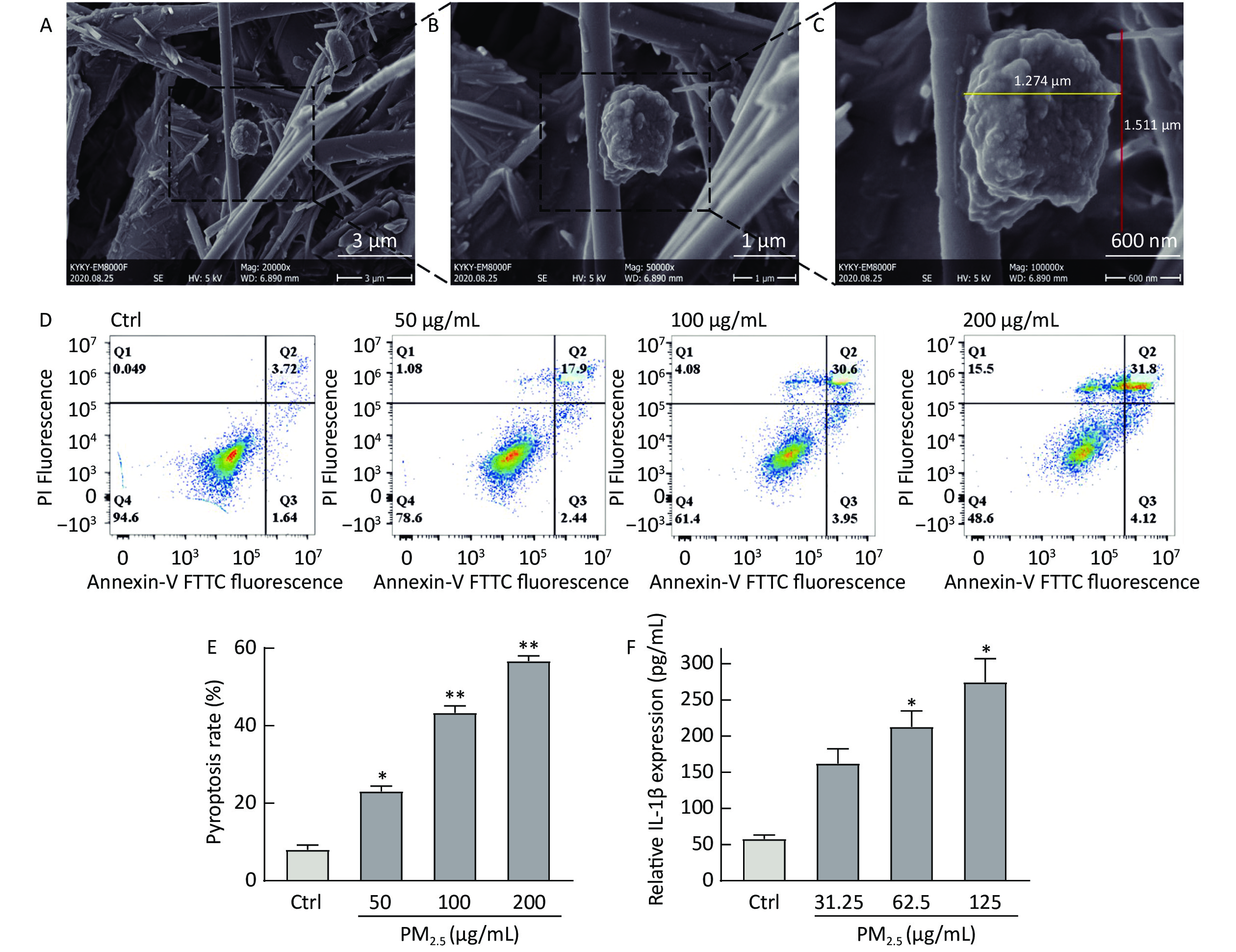
A smart medium-flow atmospheric particulate matter sampler TH-150H, with a flow rate of 100 m3/min, was used to collect PM2.5 particles on a glass fiber filter membrane from the air at the Fucheng Road, Haidian District, Beijing (coordinates: 39.93° N, 116.32° E). The basic morphology of the collected samples were used a scanning electron microscope (SEM). SEM analysis revealed that the diameter of pretreated PM2.5 was less than 2.5 μm (Figure 1C), indicating the feasibility of the pretreatment method.
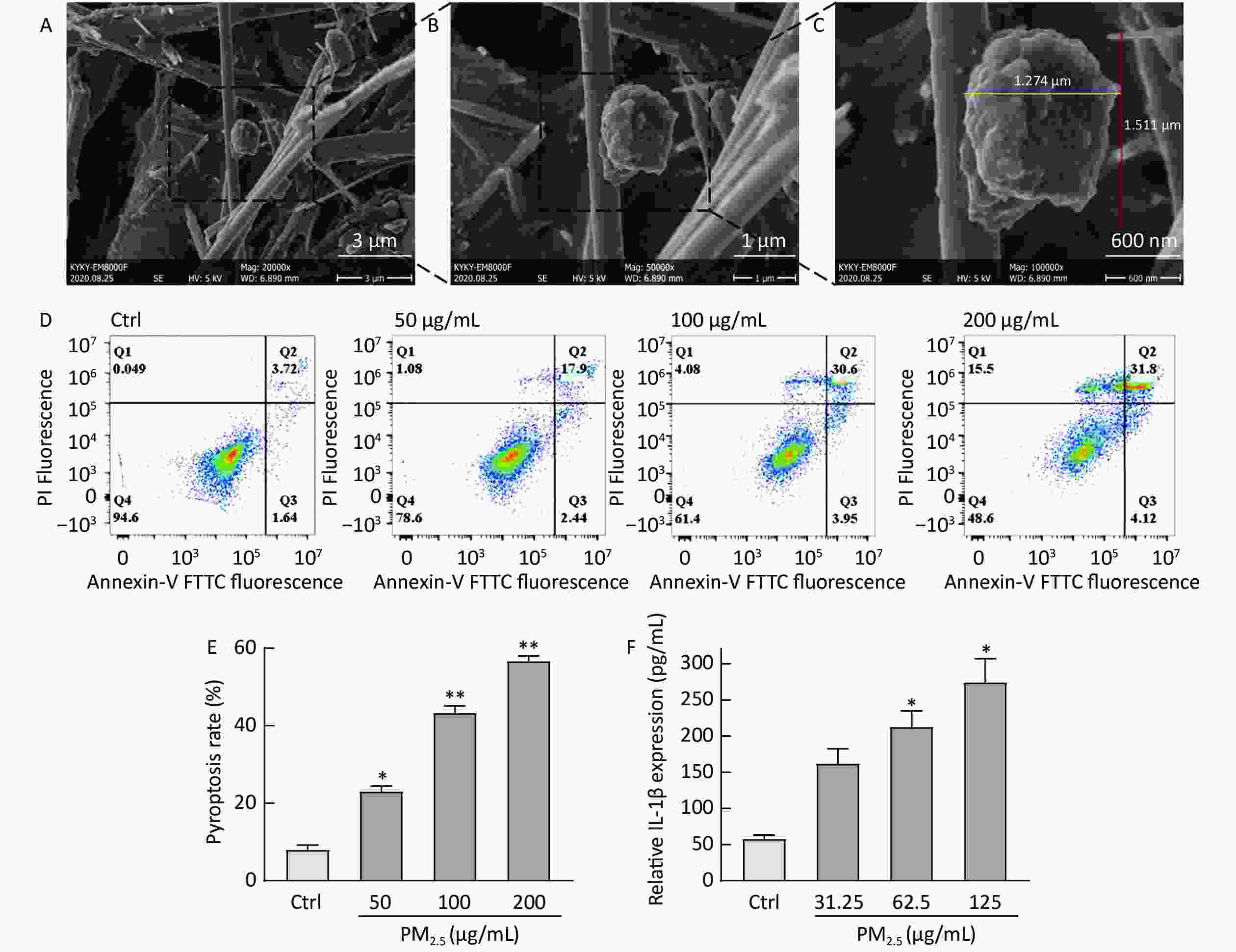
Figure 1. Figure 1. The basic morphology of collected PM2.5 and PM2.5 induces THP-1 cell damage. (A–C) The different magnifications of scanning electron microscopic image of PM2.5, 20,000x (A), 50,000x (B), 100,000x (C). (D) Flow cytometry of different concentrations of PM2.5 induced pyroptosis. (E) The effect of different concentrations of PM2.5 on the pyroptosis rate of THP-1. (F) The effect of different concentrations of PM2.5 on IL-1β released by THP-1. (**P < 0.01 vs. control; *P < 0.05 vs. control).
The pyroptosis rate assay was performed to determine whether PM2.5 enhances cell membrane damage and release of inflammatory factors. Briefly, Human monocytic-leukemia cells (THP-1) were seeded (2 × 106 cells/mL) in 24-well culture plates after 500 µL cell suspension was mixed with 500 µL PM2.5 samples for 24 h, using serum-free RPMI 1640 medium as a blank control. An annexin V–fluorescein isothiocyanate (FITC) kit was used to evaluate apoptosis. THP-1 cells were then seeded in 96-well plates, treated with various concentrations of PM2.5 for 12 h, and harvested for analyzing IL-1β levels. The pyroptosis rate (PI-positive area, Q1 and Q2) increased considerably when THP-1 cells were stimulated with PM2.5, and the increase in rate was concentration dependent (Figure 1D and E). Meanwhile, IL-1β release was concentration dependent (Figure 1F), which was consistent with the literature report of Zhang Y, up-regulating the expression of IL-1β when PM2.5-stimulated HaCaT cells[3]. IL-1β is a pro-inflammatory factor involved in various chronic and acute inflammatory responses and is mainly secreted by activated mononuclear macrophages. When PM2.5 invades hair follicles and irritates the skin, immune cells (such as monocytes) may recognize this foreign irritant through their membrane receptors, activate inflammasome assembly, induce pyrolysis and promote the release of IL-1β[5]. We successfully established a PM2.5-induced THP-1 cell damage model based on the amount of IL-1β released and pyrolysis rate.
PM2.5-induced cell membrane damage was evaluated based on the activity of caspase-1 and the expression of GSDMD. Briefly, THP-1 cells were seeded in 24-well plates (3 × 106 cells/mL) and were exposed to PM2.5 at 100 µg/mL for 24 h. The caspase-1 enzyme activity was evaluated using the caspase-Glo-1 inflammasome assay. We tested the expression of target genes and protein involved in inflammatory responses and pyroptosis (Supplementary Table S1, available in www.besjournal.com). Caspase-1 activation was assessed in 100 μg/mL PM2.5-induced THP-1 cell injury model at five time points (0, 2, 4, 6, and 8 h). The results (Figure 2A) showed that between 0 and 4 h, the activity of caspase-1 continuously increased over time. Between 2 and 4 h, the caspase-1 activity increased significantly, whereas between 4 and 8 h, the caspase-1 activity continuously decreased over time; caspase-1 activity was maximum at 4 h. The levels of GSDMD transcript did not differ between 100 μg/mL PM2.5-treated and blank control groups (Figure 2B). The same pattern was noted for the GSDMD protein level, which showed no significant changes in response to the treatments (Figure 2C, D). However, the N-GSDMD level significantly increased in response to PM2.5 treatment compared with the control (Figure 2E, F). PM2.5 induced caspase-1 expression as well as cleavage of GSDMD to N-GSDMD, which would promote cell membrane perforation. This facilitated the release of inflammatory cytokines into the cellular environment, and induced pyroptosis and skin inflammation.
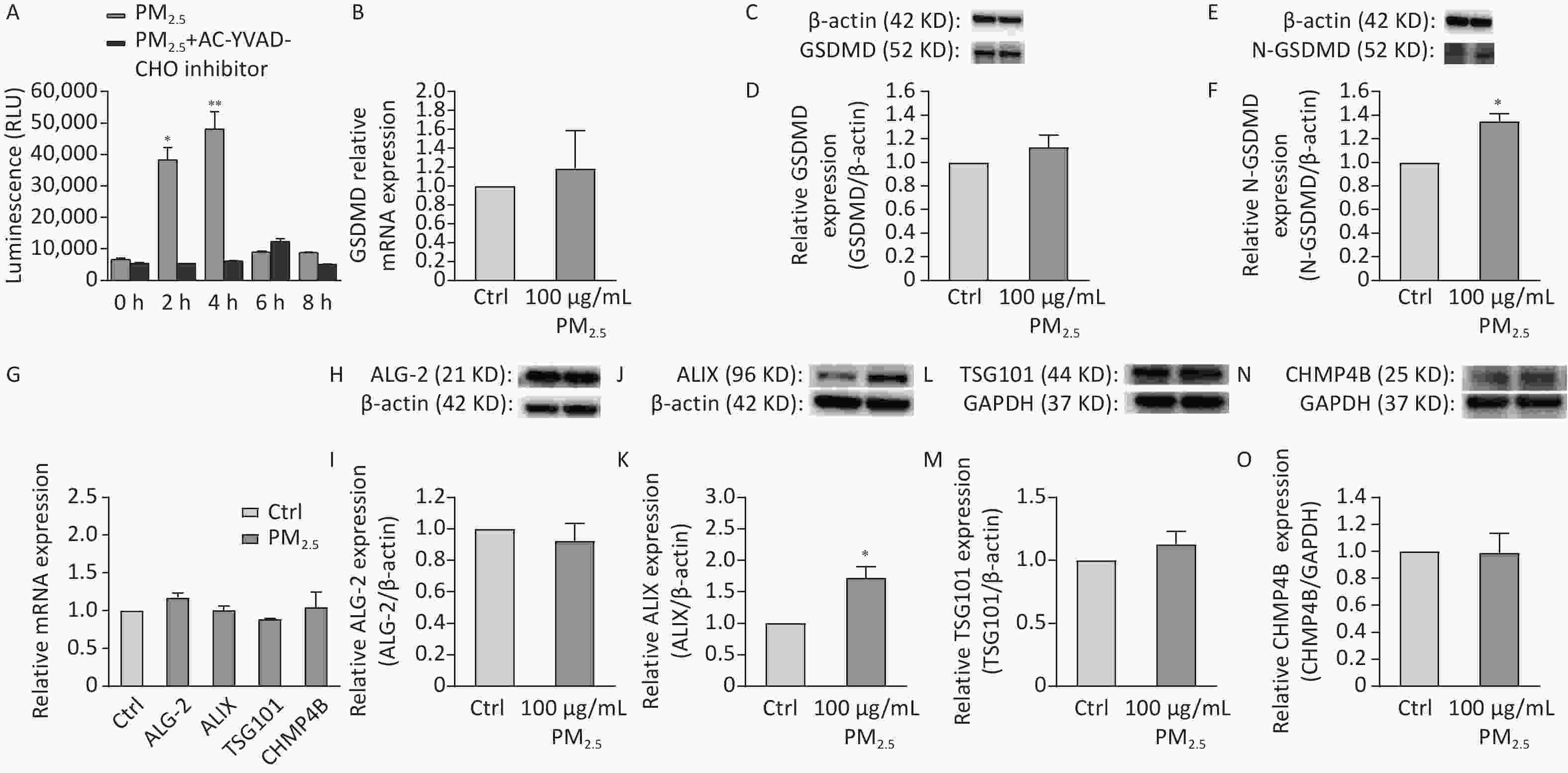
Figure 2. Figure 2. (A) The effect of PM2.5 on the activity of Caspase-1 enzyme in THP-1 cells stimulated at different times. (B) Results of 100 μg/mL PM2.5 on the transcription level of GSDMD. (C) Western blot raw image of GSDMD protein. (D) Results of 100 μg/mL PM2.5 on the expression level of GSDMD. (E) Western blot raw image of N-GSDMD protein. (F) Results of 100 μg/mL PM2.5 on the expression level of N-GSDMD. (G) Results of 100 μg/mL on ALG-2, ALIX, TSG101, CHMP4B transcription level. (H) Western blot raw image of ALG-2 protein. (I) Results of 100 μg/mL PM2.5 on the expression level of ALG-2. (J) Western blot raw image of ALIX protein. (K) Results of 100 μg/mL PM2.5 on the expression level of ALIX. (L) Western blot raw image of TSG101 protein. (M) Results of 100 μg/mL PM2.5 on the expression level of TSG101. (N) Western blot raw image of CHMP4B protein. (O) Results of 100 μg/mL PM2.5 on the expression level of CHMP4B. (**P < 0.01 vs. control; *P < 0.05 vs. control).
Gene Forward/reverse Primer Sequence (5' to 3') GSDMD Forward GGACAGGCAAAGATCGCAG Reverse CACTCAGCGAGTACACATTCATT ALG-2 Forward GGCAGACTGCATCTTAGTCAAC Reverse GGTCTATGTGAGACAGGGACTT Alix Forward ATCGCTGCTAAACATTACCAGTT Reverse AGGGTCCCAACAGTATCTGGA TSG101 Forward GAGAGCCAGCTCAAGAAAATGG Reverse TGAGGTTCATTAGTTCCCTGGA CHMP4B Forward TGCAGAGGAGATTTCAACAGC Reverse TGTTTCGGGTCCACTGATTTC TSLP Forward CTGTGGGTCTTTCTTTTCCGAA Reverse CAAGGGGAACCAGATGACAGA PAR-2 Forward TCCTCTGAAGACCTGACC Reverse TCTCCTTTCTCCCTA ATC CTC Table S1. Primer sequences of genes analyzed by quantitative real-time PCR
We explored the effect of PM2.5 on ESCRT-III mediated membrane repair related proteins. ESCRT is formed to allow cell pyrosis and GSDMD-dependent cytokine release while removing the site of membrane damage caused by GSDMD while membrane repair[6]. Real-time PCR revealed that PM2.5-treated cells had been conformable ALG-2, ALIX, TSG101, and CHMP4B mRNA levels with those in the blank control (Figure 2G). The protein expression levels of ALG-2, TSG101, and CHMP4B were also conformable between PM2.5-treated and blank control cells (Figure 2I, M, and O). However, ALIX expression was significantly upregulated in PM2.5-treated cells compared with that in the blank control (Figure 2K), which is consistent with the literature report of Rühl S. When cellular pyroptosis occurs in the cell, ESCRT-III complexes mediate the repair of the damaged cell membrane[6]. Although the mRNA of ALIX was not increased (Figure 2G), the protein levels was increased (Figure 2J and K), which is conformed to cellular regulatory mechanisms. These changes were consistent with the results of transcription analysis following these treatments. Our analysis revealed that PM2.5 only up-regulated the expression level of ALIX, the bridging protein of ESCRT-III protein, which is not associated with up- or down-regulation of other related proteins. A research reported that the ESCRT subunits were recruited to damaged lysosomes membranes[8], such as CHMP4B, ALIX, and TSG101, which is proved our results from the opposite perspective. Thus, PM2.5 induced cell scorch death in THP-1 cells leading to cell membrane damage and suppressed the transcription of ALG-2-ALIX-TSG101-CHMP4B. The research demonstrated that PM2.5-induced cell pyroptosis can inhibit the ESCRT mechanism to repair cell membrane damage caused by GSDMD.
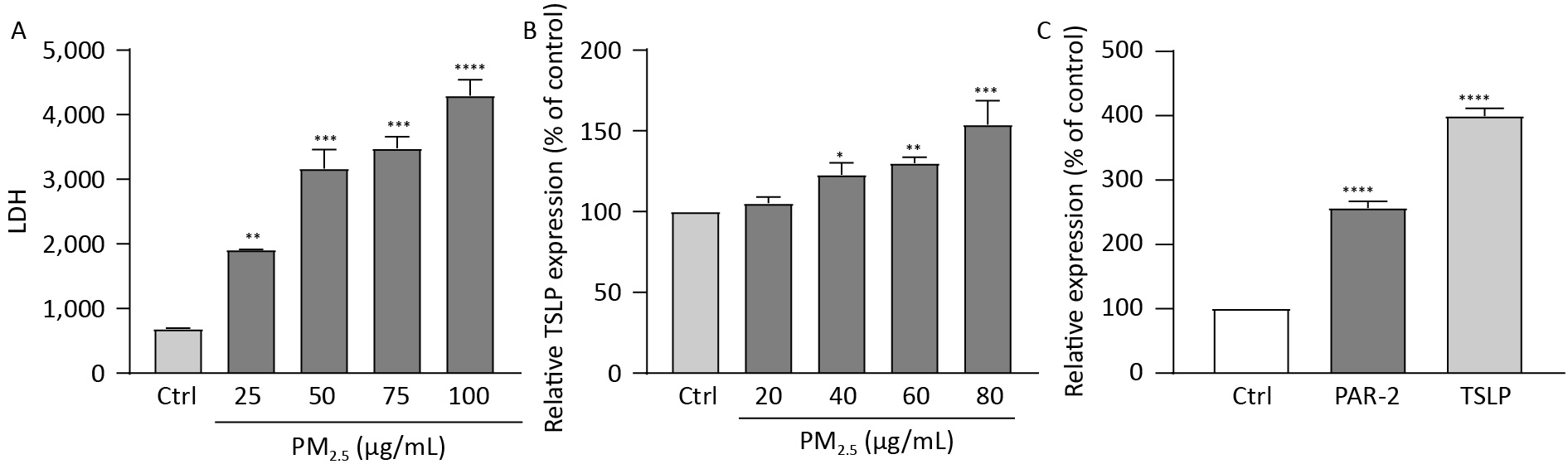
Human immortalized keratinocytes (HaCaT) cells were seeded in 96-well plates, treated with various concentrations of PM2.5 for 12 h, and were thereafter harvested for analyzing TSLP levels and the content of lactate dehydrogenase (LDH). When PM2.5 has stimulated HaCaT cells for 12 h, the content of LDH in HaCaT cells was significantly increased with the increase of PM2.5 concentration. (Figure 3A). Stimulation of HaCaT cells by different concentrations of PM2.5 for 12 h increased the relative content of TSLP, with a significant increase observed between 40–80 μg/mL concentration (Figure 3B). HaCaT cells were seeded in 6-well plates (5 × 105 cells/well) and were exposed to PM2.5 at 50 µg/mL for 12 h. Real-time quantitative PCR showed a significant increase in TSLP and PAR-2 receptor transcripts after stimulation by 50 μg/mL PM2.5 for 12 h compared to the blank control (Figure 3C). Previous studies reported that PAR-2 mediated Ca2+ influx in PM2.5-induced HaCaT cells, and pro-inflammatory cytokines IL-8, IL-6, and TNF-α were induced[9], indicating that the upregulation of PAR-2 could trigger an inflammatory response, accelerate cell migration and immune response, and cause the integrity of the skin barrier to be affected. This was consistent with the results of PM2.5 stimulating HaCaT cells to secrete TSLP.
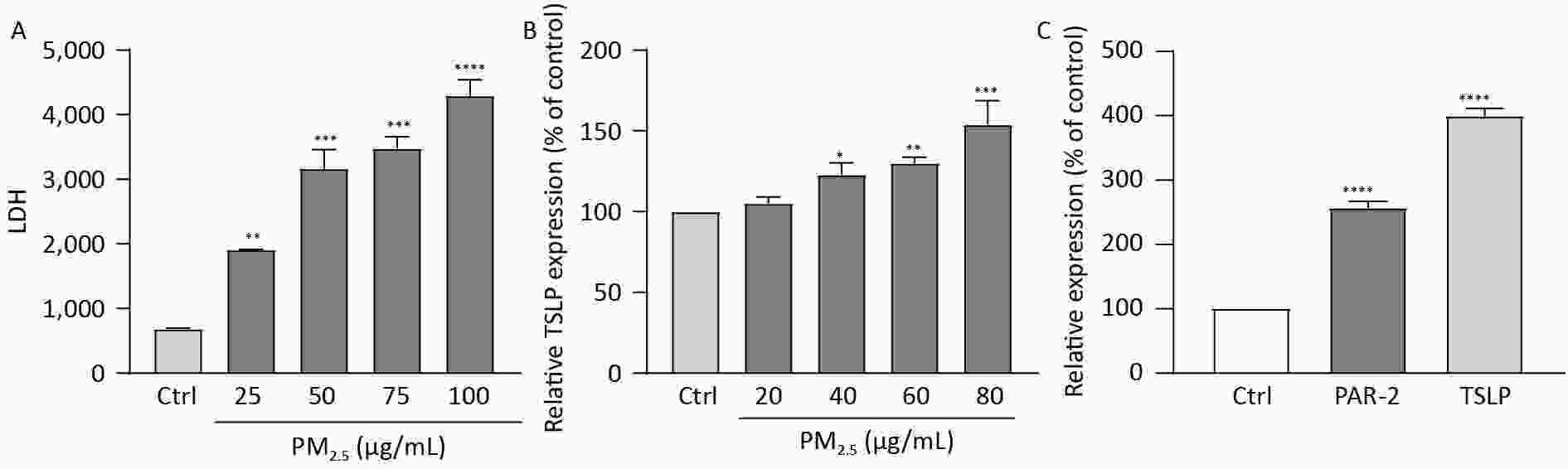
Figure 3. Figure 3. (A) The effect of different concentrations of PM2.5 on the secretion of LDH by HaCaT cells, LDH increased significantly with the increase of PM2.5 concentration; (B) The effect of different concentrations of PM2.5 on the secretion of TSLP by HaCaT cells, TSLP increased significantly with the increase of PM2.5 concentration; (C) Stimulation of HaCaT cells with 50 μg/mL PM2.5 resulted in an increase of TSLP and PAR-2 receptor transcript levels to increase. (****P < 0.0001 vs. control; ***P < 0.001 vs. control; **P < 0.01 vs. control; *P < 0.05 vs. control).
In summary, the skin, as an important barrier for the human body, will produce a series of reactions when exposed to harmful substances from the outside world. Our results showed that PM2.5 may increase the risk of specific dermatitis and other skin diseases. Mechanisms may be related to skin barrier damage, elevated inflammatory response, and activation of the innate immune system. In addition, on the treatment of PM-induced inflammation could consider antioxidants, vitamin D, and microbiota regulation strategies[10], especially plant derived antioxidant compounds.
Conflicts of Interest The authors declare no conflict of interest.
Authors’ Contributions HE Qiao conceived and designed the experiments, performed the experiments, analyzed the data and wrote the manuscript. XUE Wan Ting conceived, designed, and performed the experiments. LI Li, YI Fan, LING Xiao and GUO Miao Miao analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
HTML
 23272Supplementary Materials.pdf
23272Supplementary Materials.pdf
|

|








 Quick Links
Quick Links
 DownLoad:
DownLoad: