-
In critical care medicine, sepsis is a dangerous systemic condition that is highly prevalent and is associated with high morbidity and mortality rates[1]. The high mortality rate associated with sepsis is closely related to multi-organ dysfunction, with heart injury being particularly critical and considered the starting point of multi-organ injury[2]. However, the clinical treatment of sepsis-induced heart injury is challenging, because of an incomplete understanding of its molecular mechanism[3]. Therefore, it is important to understand the pathogenesis of sepsis and identify relevant therapeutic agents. Anthocyanins are the most potent water-soluble anti-inflammatory and oxidizing agents that play an important role in protecting against several diseases, particularly cardiovascular illnesses[4]. Malvidin, the most common anthocyanin in blueberries, is one of the most pervasive and widely distributed anthocyanins. Its antioxidant, anti-inflammatory, and immunomodulatory properties have been useful in treating various illnesses, including cardiovascular conditions. Thus, the purpose of this study is to demonstrate that malvidin treatment can alleviate sepsis-induced heart injury through in vivo and in vitro experiments and to investigate the specific mechanisms involved.
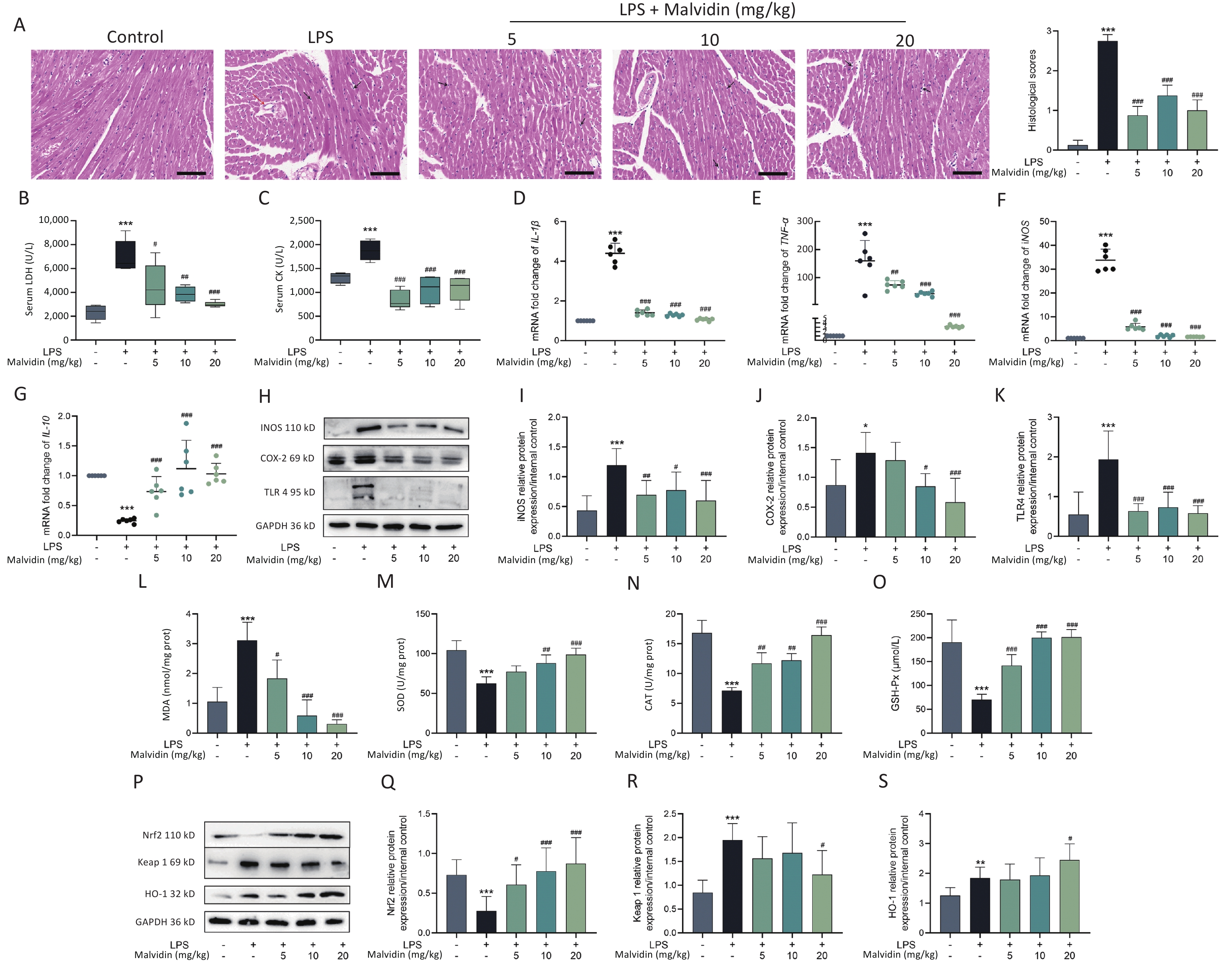
Lipopolysaccharide (LPS) has been widely used in animal studies to investigate the intracellular signaling pathways involved in sepsis. We investigated whether malvidin could prevent LPS-induced septic cardiac injury in mice by intraperitoneal injection of LPS followed by treatment with malvidin. In our study, the clinical manifestations of endotoxemia were more evident in LPS-treated mice than in healthy controls and malvidin-treated mice. Mice with LPS-induced sepsis exhibited reduced exercise tolerance, decreased food intake and water consumption, and increased lethargy, whereas malvidin-treated mice demonstrated improved mobility and feeding ability. This preliminarily indicates that malvidin has a certain improvement effect. Experimental septic myocardial injury is primarily characterized by morphological abnormalities in the myocardial structures and elevated levels of markers of myocardial injury, including lactate dehydrogenase (LDH) and creatine kinase (CK)[5]. Therefore, we examined structural anomalies of the myocardium using hematoxylin and eosin (HE) staining. As shown in Figure 1A, compared with the control group, the LPS group exhibited an increased myocardial cell gap, poor fiber alignment, dilated myocardial interstitial vessels, increased inflammatory cell infiltration, and unclear cell borders. Notably, although stimulated by LPS, the hearts in the malvidin-treated group exhibited varying degrees of improvement. The HE pathology scores showed similar results. To investigate the effect of malvidin on LPS-induced septic cardiac damage, we measured serum levels of LDH and CK. As shown in Figure 1B–C, LDH, and CK levels dramatically increased following LPS stimulation, indicating heart damage, and both biomarker levels decreased after malvidin treatment. These findings demonstrate that we successfully established a model of LPS-induced septic heart injury and that malvidin can temporarily reduce LPS-induced septic heart injury.
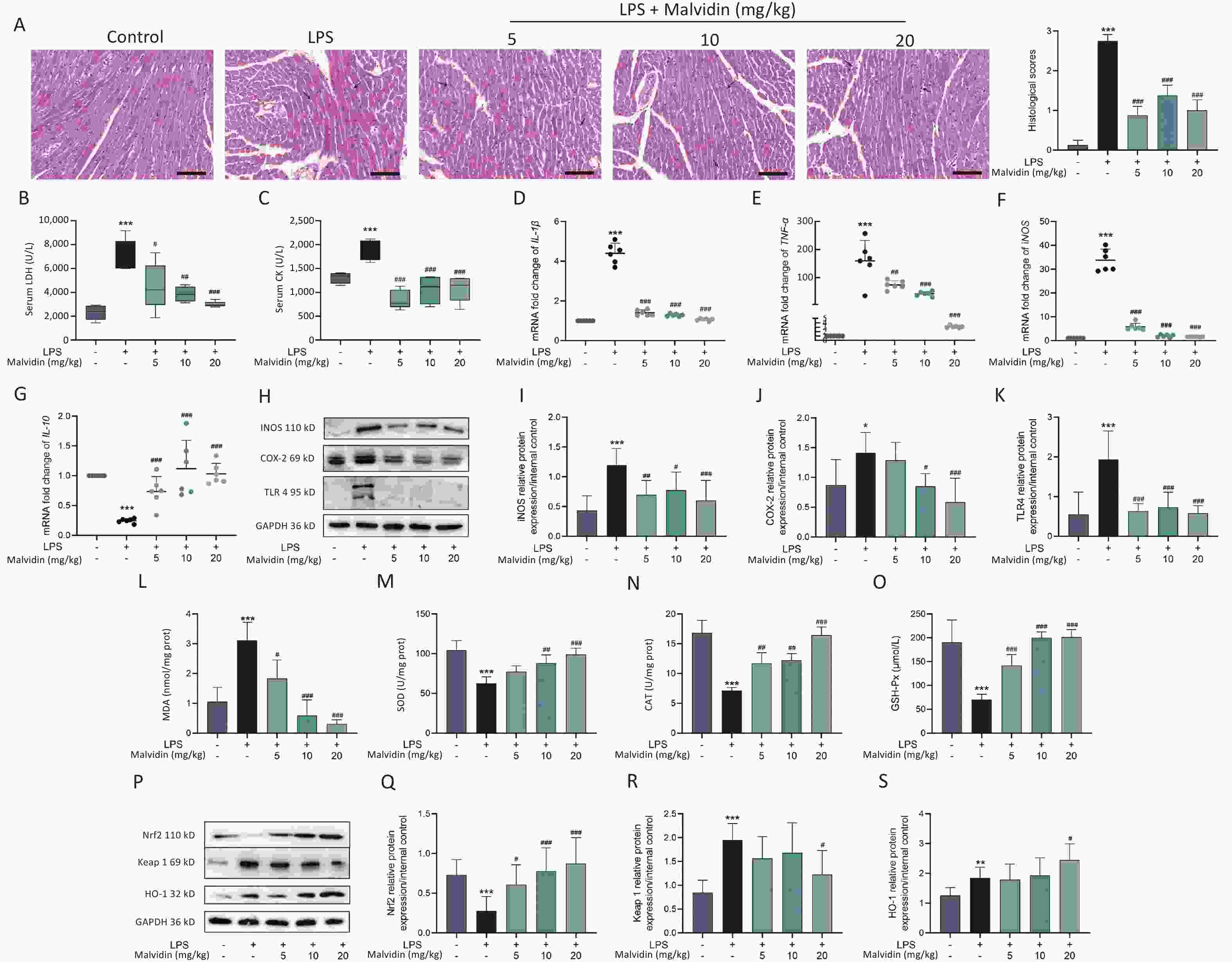
Figure 1. Malvidin significantly attenuates cardiac functional impairment in mice with sepsis and reduces inflammation and oxidative stress in the heart.
In this study, we separately examined the mRNA expression levels of inflammatory factors and found that LPS stimulation significantly elevated pro-inflammatory factor mRNA expression levels, whereas the anti-inflammatory factor interleukin (IL)-10 expression levels decreased (Figure 1D–G). We observed that the expression levels of toll-like receptor (TLR) 4, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) were substantially elevated in the hearts of each mice group (Figure 1H–K). However, the elevated expression of these genes was reduced after malvidin administration. These findings suggest that sepsis-induced heart injury is accompanied by a strong inflammatory response and that malvidin could effectively alleviate the inflammatory response in the septic heart by modulating TLR4-iNOS-COX-2.
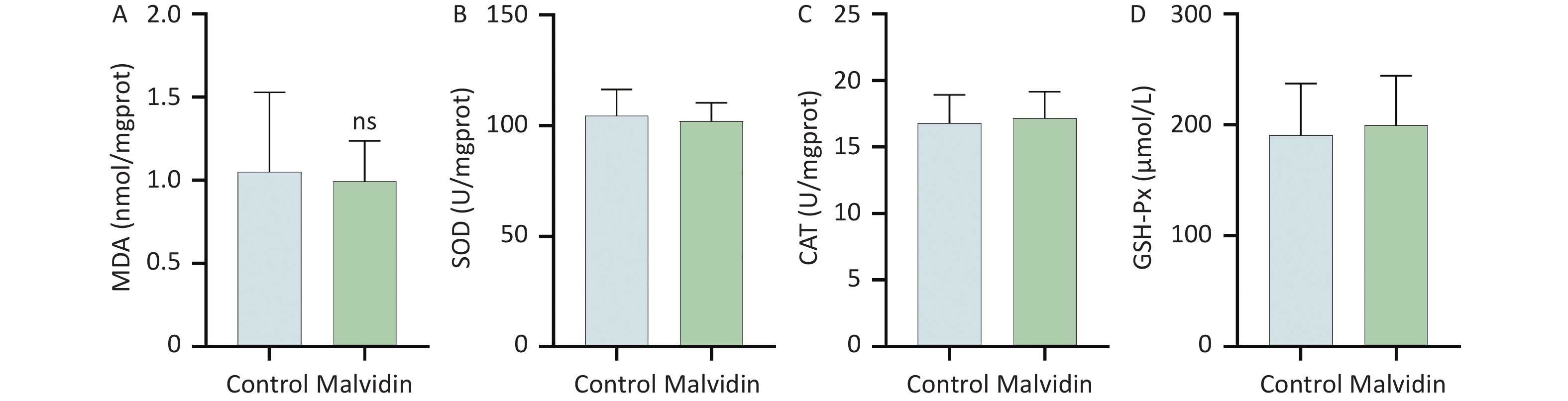
Myocardial injury in sepsis is primarily caused by oxidative stress and inflammatory responses[6]. The effects of malvidin on LPS-induced oxidative stress were evaluated using an assay kit. The degree of membrane lipid peroxidation was determined by assessing the level of malondialdehyde (MDA) in each group (Figure 1L). After LPS stimulation, the level of MDA increased substantially but was reversed after malvidin administration, indicating that malvidin reduces the extent of membrane lipid peroxidation. The activity levels of three important antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) were also assessed. The results revealed that the activities of these enzymes were reduced in response to LPS stimulation. However, this was alleviated by malvidin treatment and the activities of several antioxidant enzymes were restored (Figure 1M–O). In addition, we evaluated the effect of malvidin administration alone on oxidative stress and found no difference compared with the control group (Supplementary Figure S1, available in www.besjournal.com). Oxidative stress and inflammation are mediated by the kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2 (Keap 1/Nrf2) antioxidant response element (ARE) signaling cascade. As depicted in Figure 1P–S, the expression of Nrf2 and Keap 1 and their downstream target heme oxygenase-1 (HO-1) was examined. Malvidin was found to inhibit Nrf2 degradation by decreasing Keap 1 levels and increasing HO-1 expression. These results demonstrate that malvidin significantly inhibits LPS-induced oxidative stress in damaged hearts by activating the Nrf2-associated cytoprotective system.
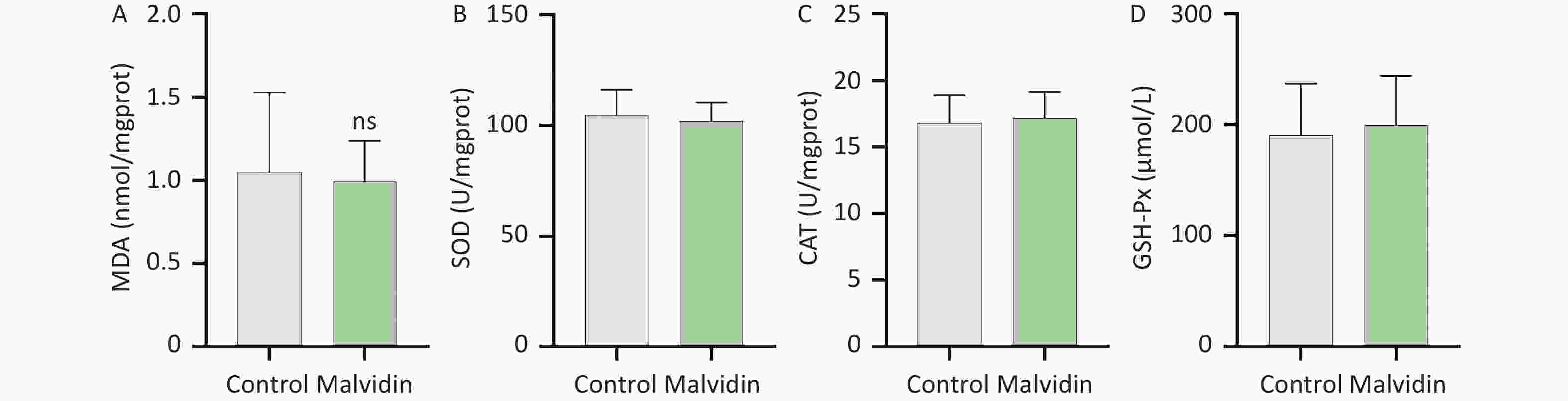
Figure S1. Effects of malvidin alone on oxidative stress-related enzymes. (A–D) Using the supernatant of heart tissue homogenates, the activities of malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) are assessed (n = 6 mice per group).
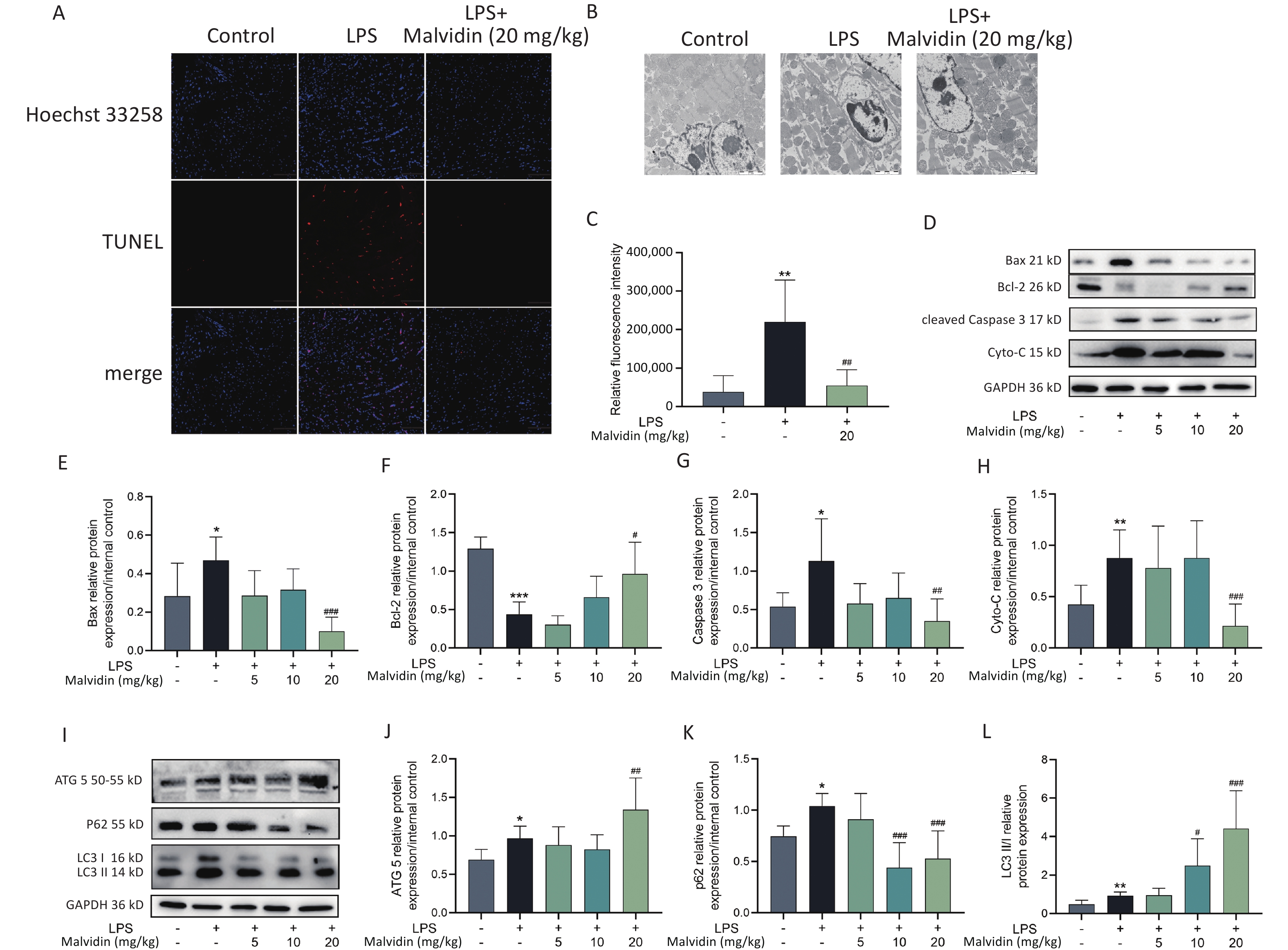
Cellular apoptosis is a terminal stage of sepsis cardiomyopathy is cellular apoptosis[7]. Mitochondria-dependent apoptotic signaling pathways are more active in various organs, including muscles and the heart. The balance between Bcl-2-associated X protein (Bax) and B-cell lymphoma 2 (Bcl-2) determines the cell destiny, and mitochondria have been shown to release cytochrome-C (Cyto-C), which is regulated by Bax and Bcl-2 homeostasis, thus playing a role in apoptosis. Therefore, targeting the Bax/Bcl-2/cyto-C mitochondrial apoptotic pathway is necessary for treating sepsis-induced heart injury. To investigate the effect of malvidin therapy on LPS-induced apoptosis, the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay and transmission electron microscopy (TEM) were employed to measure apoptosis (Figure 2A–C). LPS stimulation significantly increases apoptosis. A significant number of apoptotic cells were observed in the control group, but this was prevented by pretreatment with malvidin. Furthermore, examination of the expression levels of apoptosis-related proteins revealed that under LPS stimulation, Bax, cleaved Caspase 3, and cyto-C expression levels increased, whereas Bcl-2 expression levels decreased, indicating an abnormal increase in apoptotic cells in the heart and suggesting that mitochondrial apoptosis is a major pathway involved in sepsis-induced heart injury. However, this effect was alleviated by malvidin administration (Figure 2D–H).

Figure 2. Malvidin reduces LPS-induced apoptosis of septic heart cells and promotes autophagy. (A) Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) detection of apoptotic cells in cardiac tissue (n = 3 mice per group, Scale bar = 100 μm). (B) The morphology of cells in the cardiac tissue is investigated using transmission electron microscopy (TEM) (n = 3 mice per group, scale bar = 100 μm). (C) Quantitative analysis of the results of B. (D–H) To assess the expression of the apoptosis-related proteins Bcl-2-associated X protein (Bax), B-cell lymphoma 2 (Bcl-2), cytochrome-C (Cyto-C), and Caspase 3, protein extracts from heart tissues are used (n = 3 mice per group). Subsequently, a grayscale value analysis is performed. (I–L) The expression of autophagy-related proteins autophagy-related gene 5 (ATG5), (ATG5), sequestosome 1 (SQSTM1) (P62), and microtubule-associated protein 1A/1B-light chain 3 (LC3) in heart tissue extract proteins is investigated by western blot analysis and greyscale value analysis (n = 3 mice per group). LPS, Lipopolysaccharide.
Previous studies have implicated autophagy in sepsis[8]. Common autophagy markers include autophagy-related gene 5 (ATG5), sequestosome 1 (SQSTM1) (P62), and microtubule-associated protein 1A/1B-light chain 3 (LC3). To further investigate the role of autophagy, we examined these markers in subsequent experiments. Our findings demonstrated that malvidin administration counteracted LPS-induced damage by enhancing autophagy in the heart. Specifically, LPS treatment increased autophagy in the heart, which was further potentiated by malvidin administration (Figure 2I–L).
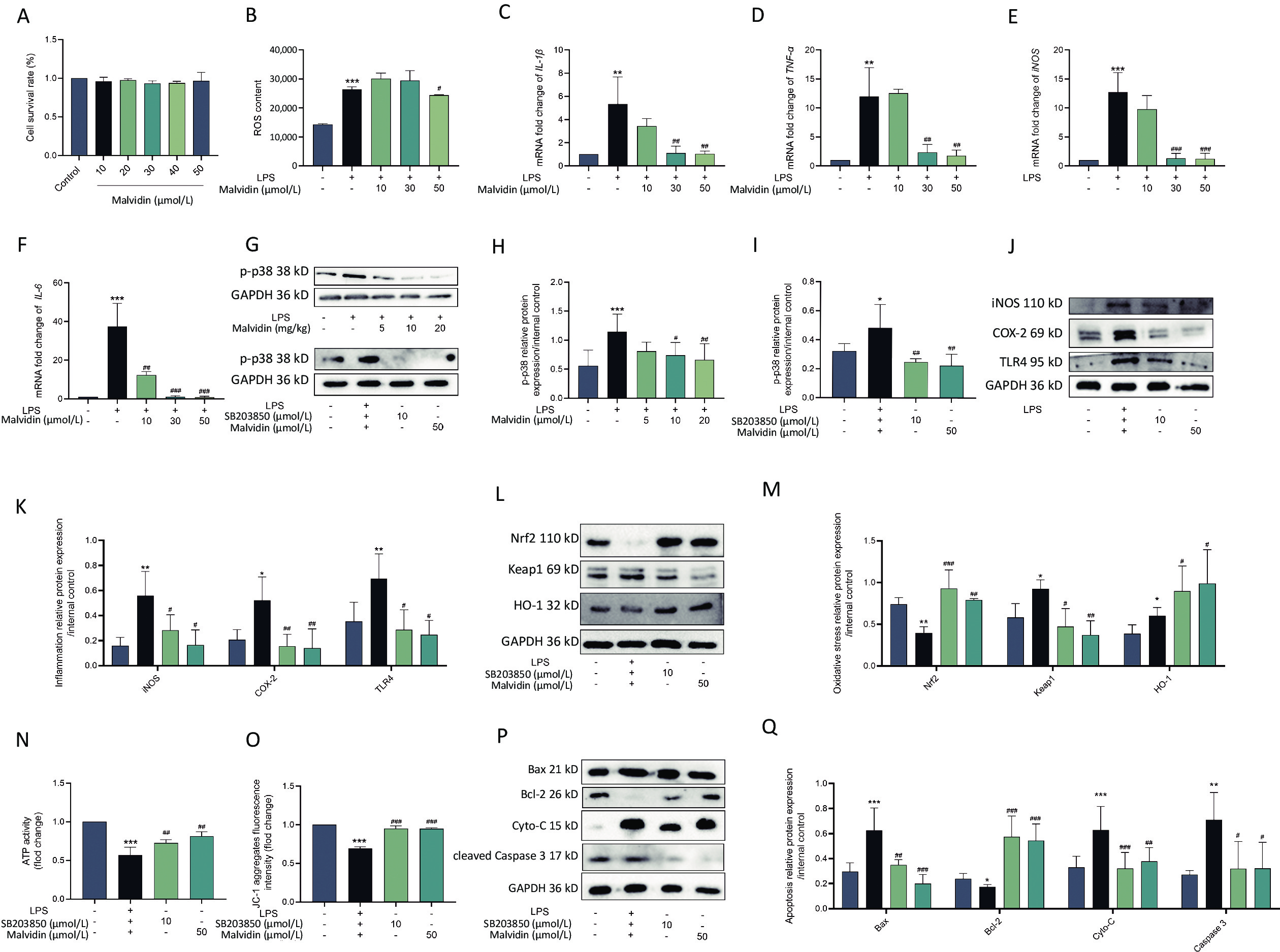
Through in vivo trials, we discovered that malvidin protects against heart injury. However, the precise mechanism remains unknown and requires further investigation. Macrophages, which activate several inflammatory signaling pathways, are primary innate inflammatory cells that respond to damage-associated molecular patterns (DAMP)[9]. Several studies have demonstrated that macrophages are the main participants in human heart disease and are associated with the recruitment of a large number of macrophages in the damaged myocardium, which is closely related to heart damage. Therefore, in the subsequent experiment, we used RAW 264.7 macrophages treated with H9C2 conditioned medium for further research. To ensure that malvidin alleviates LPS-induced RAW 264.7 macrophages due to drug efficacy rather than cell death, we determined the impact of malvidin treatment on RAW 264.7 cell viability. As shown in Figure 3A, 0–50 μmol/L malvidin is an effective and safe range for cells. We also found that the level of ROS was elevated after LPS stimulation and decreased under the treatment with 50 μmol/L malvidin (Figure 3B). The results in Figure 3C–F indicate that the expression levels of IL-1β, tumor necrosis factor-alpha (TNF-α), iNOS, and IL-6 were elevated under LPS stimulation. However, the expression levels of these genes decreased after malvidin treatment, suggesting that malvidin has a preliminary anti-inflammatory impact on LPS-induced RAW 264.7 macrophages, which can be studied further in future studies.
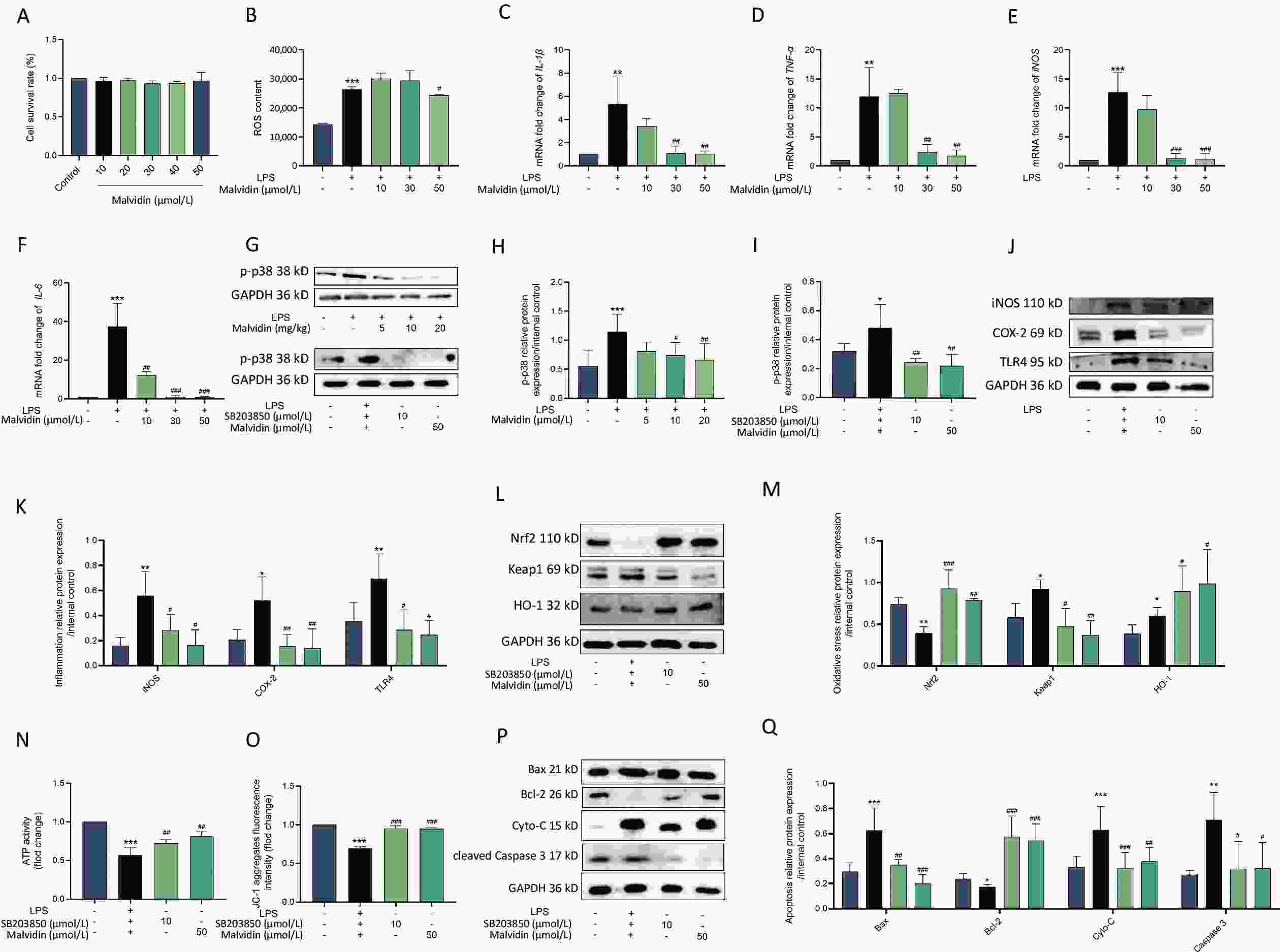
Figure 3. Malvidin reduces LPS-induced inflammation, oxidative stress, and apoptosis by preventing activation of the p38 mitogen-activated protein kinase (MAPK) pathway. RAW 264.7 cells are treated with malvidin (50 μmol/L) and SB203850 (10 μmol/L, p38 MAPK inhibitor) for 1 h and subsequently incubated with a conditioned medium of H9C2 to mimic LPS stimulation in the heart for 12 h. (A) Detection of the safe range of malvidin to RAW 264.7 using cell counting kit-8 (CCK8) (n = 3). (B) Detection of changes in reactive oxygen species (ROS) levels (n = 3). (C–F) The mRNA extracted from RAW 264.7 is reverse transcribed into cDNA, and the mRNA expression levels of interleukin (IL)-1β, tumor necrosis factor (TNF-α), inducible nitric oxide synthase (iNOS), and IL-6 are measured by quantitative real-time polymerase chain reaction (qRT-PCR) (n = 6). (G–I) The expression of p38 MAPK is detected within in vivo-heart tissue and in vitro RAW 264.7 cells, and analyzed for grayscale value (n = 3 mice per group /n = 3). (J–K) Inflammatory proteins iNOS, cyclooxygenase-2 (COX-2), and toll-like receptor (TLR)4 are measured in proteins extracted from RAW 264.7 (n = 3). (L–M) Expression levels of important proteins in the kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2/heme oxygenase-1 (Keap 1/Nrf2/HO-1) axis are assessed using proteins extracted from RAW 264.7 cells (n = 3). (N) ATP activity (n = 3). (O) The mitochondrial membrane potential (n = 3). (P–Q) Expression of the autophagy-related proteins autophagy-related gene 5 (ATG5), (ATG5), sequestosome 1 (SQSTM1) (P62), and microtubule-associated protein 1A/1B-light chain 3 (LC3) in proteins extracted from RAW 264.7 cells is examined by western blot analysis, and assessed using grey scale value analysis (n = 3), LPS, Lipopolysaccharide.
The p38 mitogen-activated protein kinase (MAPK) signaling pathway has been implicated in heart damage, which exerts its effect by modulating the generation of myocardial proinflammatory cytokines[10]. Therefore, understanding the alterations in p38 MAPK activity related to sepsis-induced cardiac damage by LPS is critical. As shown in Figure 3G–I, we observed a high expression level of p-p38 MAPK in the group exposed to LPS, which was reduced after malvidin administration. The elevated expression level of p-p38 MAPK following LPS stimulation was subsequently examined in RAW 264.7 cells. The cells were then treated with SB203580 and malvidin for further analysis. We discovered that these treatments reduced the expression of p-p38 MAPK, indicating that malvidin inhibited the LPS-induced activation of the p38 MAPK signaling pathway both in vivo and in vitro.
Ample evidence has indicated that the development of inflammatory responses depends on p38 MAPK activation. Our results demonstrate that inhibition of p38 MAPK activity significantly reduces the expression of LPS-induced inflammation-related proteins (iNOS, COX-2, TLR4) in RAW 264.7 cells, and this effect is also observed after malvidin treatment. These findings suggest that the LPS-induced inflammatory response is dependent on p38 MAPK and that malvidin reduces inflammation by inhibiting p-p38 MAPK activity (Figure 3J–K). Moreover, p38 MAPK has been implicated in the activation of Nrf2 and the induction of antioxidant enzymes. Our results showed that inhibition of p-p38 MAPK activity reduced the production of LPS-induced oxidative stress-related proteins, which is comparable to the outcomes following inhibitor and malvidin administration (Figure 3L–M). SB203580 decreased the activity of p-p38 MAPK, reduced cardiac apoptosis, and protected the heart. Mitochondria played a crucial role in maintaining normal physiological activities of cells and also in apoptosis, and closely related to apoptosis and cell death. ATP, the most important energy molecule, plays critical roles in various physiological and pathological cellular processes. ATP levels typically decrease when cells are in a state of apoptosis, indicating impaired mitochondrial function. Therefore, we measured changes in ATP levels in each group at the cellular level to demonstrate changes in the mitochondria in each group and found that there was a significant reduction in ATP after LPS stimulation, which was alleviated after the administration of malvidin and p38 MAPK inhibitors. In addition, the key index of mitochondrial activity level is the mitochondrial membrane potential, and we determined the characteristics of mitochondrial activity by measuring the changes in mitochondrial membrane potential in each group. The membrane potential decreased after LPS stimulation, indicating mitochondrial damage, which was reversed by malvidin administration. The p38 MAPK inhibitors exerted similar effects (Figure 3N–O). Detection of apoptosis-related proteins revealed that p38 MAPK, after regulation, could attenuate the expression levels of apoptosis-related proteins, and malvidin exerted a similar effect (Figure 3P–Q). Our findings suggest that malvidin can reduce LPS-induced oxidative stress, apoptosis, and inflammation by preventing activation of the p38 MAPK pathway.
Overall, our study demonstrated the pharmacological effects of malvidin on LPS-induced septic heart injury. In the in vivo experiments, heart injury was triggered by LPS injection and treated with three doses of malvidin. In in vitro experiments, RAW 264.7 was cultured in either LPS-containing or LPS-free H9C2 conditioned medium to simulate a realistic cardiac environment, followed by treatment with malvidin and inhibitors of p38 MAPK. Malvidin was found to have significant therapeutic effects against LPS-induced septic heart injury both in vivo and in vitro. These effects can be reasonably attributed to their strong anti-inflammatory, antioxidant, anti-apoptotic, and autophagy-enhancing properties as well as their inhibitory effects on p38 MAPK. Therefore, we hypothesized that malvidin alleviates LPS-induced sepsis following heart injury.
HTML
 23253Supplementary Materials.pdf
23253Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: