-
Inflammatory bowel disease (IBD) is characterized by chronic inflammation of the intestinal tract, and shows a high rate of recurrence. Clinicians have accumulated extensive experience in the treatment of IBD, with biologics and small molecule drugs having greatly expanded the therapeutic scope of IBD in recent years. Nonetheless, such treatments involve a high price and a single target of drug action. In contrast, Chinese herbs have multi-component and multi-target action characteristics. Lonicera japonica Caulis (LJC) is the dried flower buds of Lonicera japonica, which has been recorded in the Compendium of Materia Medica with functions such as clearing heat, removing toxins, and reducing inflammation. Moreover, the compound has been extensively used in many traditional Chinese medicine formulas due to its lack of obvious toxic side effects, easy accessibility, and low price. Multiple modern pharmacological studies have investigated the anti-inflammatory and injury repair effects of LJC[1]. However, LJC contains many components and corresponding targets of action, and which of these components act on IBD remains unknown. Therefore, this study screened the main acting components in LJC; in addition, bioinformatics analysis and in vitro experiments were carried out to explore their potential mechanism of action in the treatment of inflammatory bowel disease.
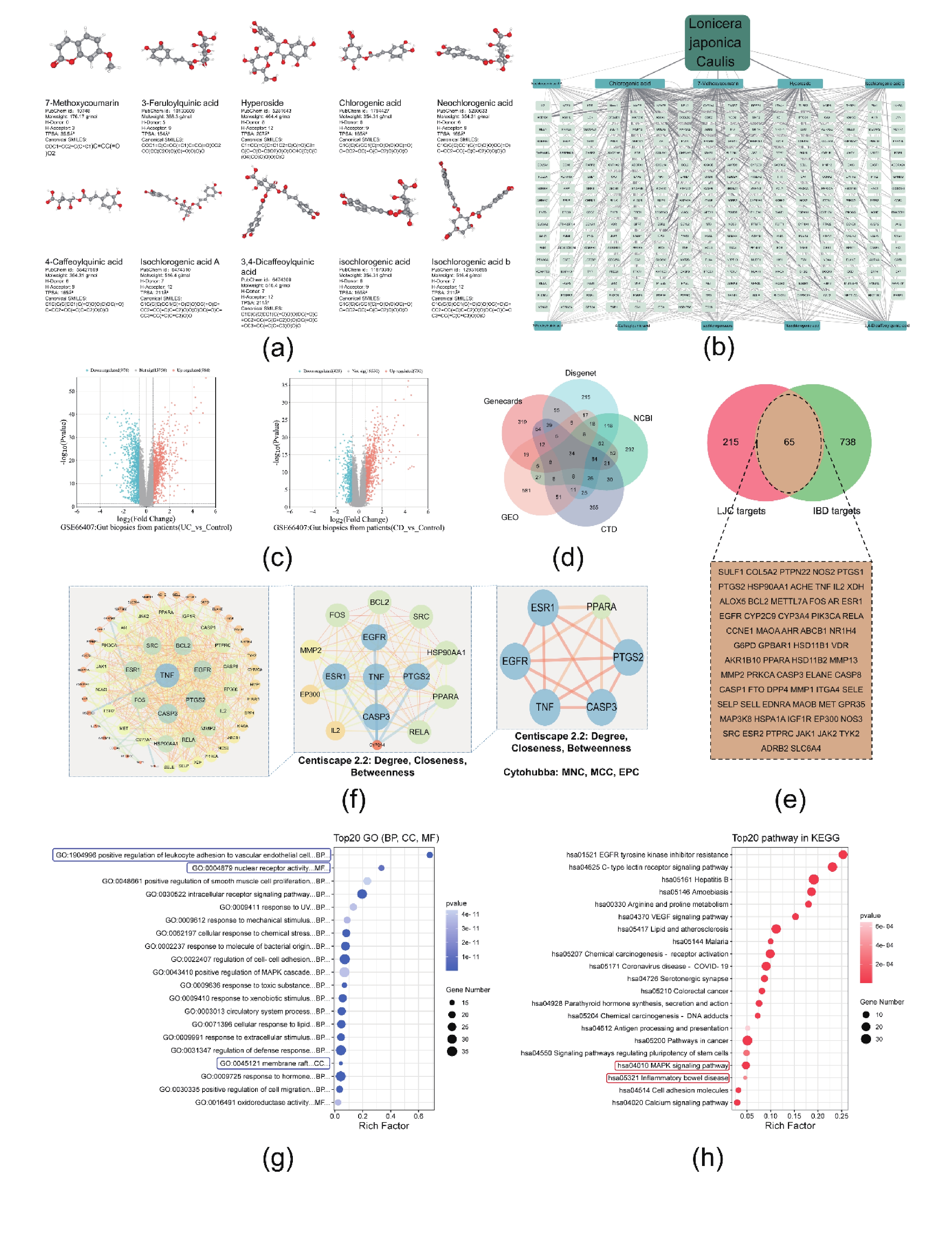
Firstly, the blood-entry components of Lonicera japonica reported by Zhou et al. were supplemented with “LJC” data from the ETCM database[2]. The data were analyzed to obtain the 10 main compounds of LJC [Figure 1A]. Furthermore, the ETCM database was utilized to identify the corresponding differential genes to the compounds. Finally, 280 corresponding target genes were predicted [Figure 1B]. Then, the data set GSE66407 related to "IBD" was downloaded from GEO, and the differential genes were screened according to P < 0.05 and |log2 foldchange| > 1 [Figure 1C]. Meanwhile, the Genecards, CTD, DisGeNET, and GEPIA databases were used to supplement and merge the potential targets, and the important targets related to IBD were finally obtained [Figure1D]. Using the jvenn tool, the targets of action of LJC were mapped and compared with IBD-related gene targets, and determine the potential targets of LJC in IBD [Figure1E]. These targets were imported into STRING to generate the PPI network. The degree, closeness, EPC, MCC, and MNC of each target were analyzed by using cytoHubba plug-in; finally, the results were sorted and visualized, and the targets with the highest weights in the network were identified, including TNF, CASP3, PTGS2, EGFR, ESR1, and PPARA [Figure1F]. Therefore, components in LJC could act on IBD through these six target genes, which are closely associated with cellular inhibition of oxidative stress, protection against injury, protection of the intestinal mucosal epithelial barrier, modulation of T-helper cells, and antimicrobial activity. Moreover, 65 targets related to LJC and IBD were imported into the Metascape database for GO annotation and KEGG signaling pathway enrichment analysis [Figure1g]. The KEGG enrichment analysis revealed that the relevant target genes were highly enriched in the inflammatory bowel disease pathway, suggesting the possible role of LJC in alleviating IBD. Predictions also showed that relevant targets were also enriched in the MAPK-signaling-pathway, C-type-lectin-receptor-pathway, and EGFR-pathway [Figure1H]. The relevant targets of these pathways were compared with those of LJC in IBD, finding that the relevant target proteins of the MAPK pathway were associated with all the core components of LJC. Besides, studies also shows the relationship between MAPK and IBD, so the MAPK-pathway was a potentially important mechanistic pathway in the treatment of IBD by LJC[3]. By comparing LJC targets with MAPK pathway, five closely related compounds were further screened, including 7-methoxycoumarin, hyperoside, chlorogenic acid, neochlorogenic acid, and 4-caffeoylquinic acid [Figure2A].
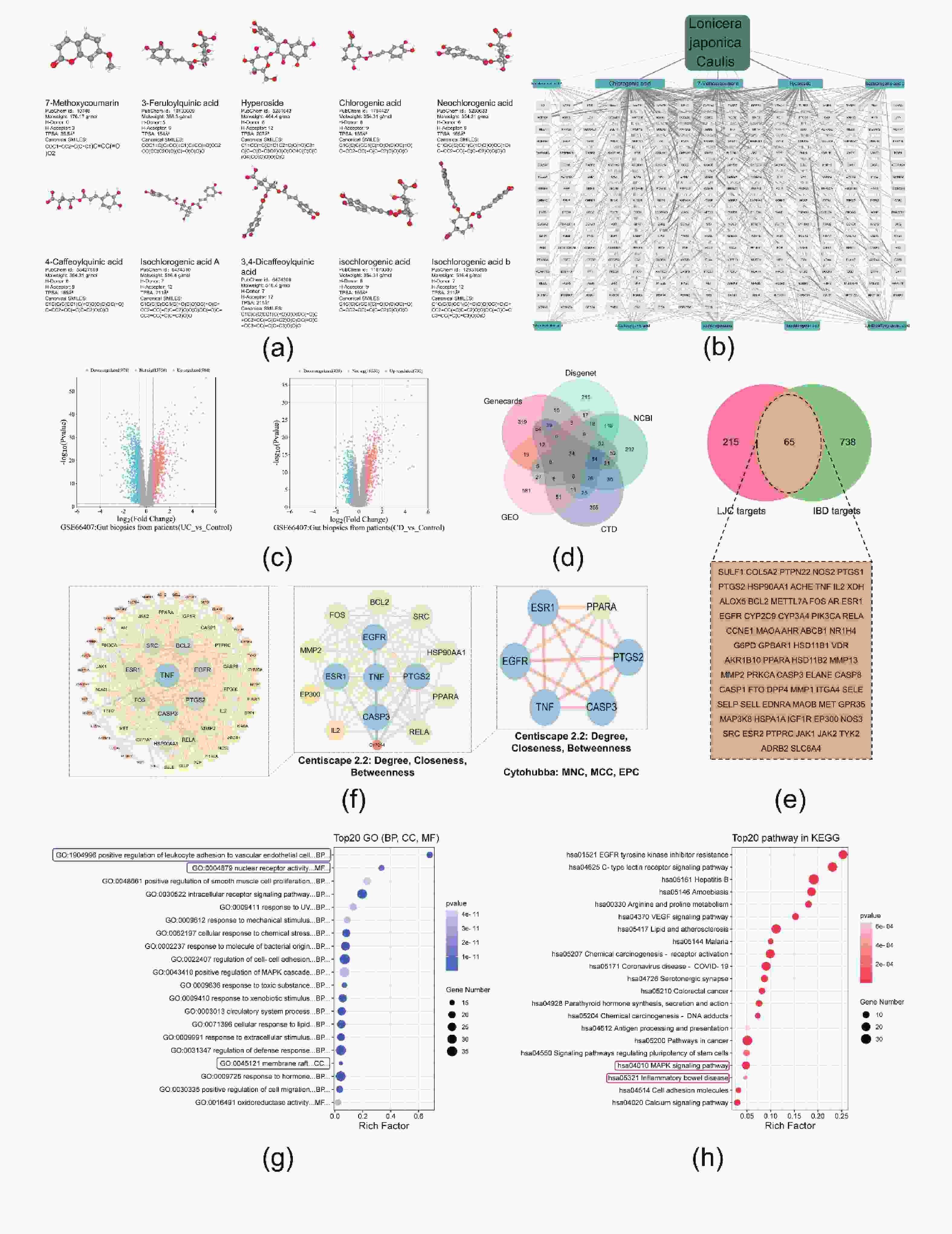
Figure 1. Prediction of the mechanism of LJC in alleviating IBD based on network pharmacology. (A) The 10 main compounds in LJC. (B) LJC-compounds-targets network. (C, D) Volcano map of differential genes in the GSE66407; meanwhile, the Genecards, CTD, DisGeNET, and GEPIA databases were used to supplement and merge the potential targets (E) Venn diagram of genes related to LJC's effects on IBD. (F) PPI analysis of genes related to LJC's effects on IBD, the identification of the highest-weighted targets in the network. (G, H) GO and KEGG enrichment analysis.

Figure 2. Preliminary validation of the predictions by molecular docking. (A) Schematic representation of LJC and MAPK pathway-related proteins. (B) Molecular docking of 5 major compounds in LJC with proteins related to the MAPK pathway. The lowest binding energy is indicated by the color, the lower energy represented by a darker color, the larger bubbles indicate a larger number of hydrogen bonds formed, reflecting stronger binding capability.
In order to preliminarily verify that LJC acts on IBD through the MAPK pathway, molecular docking was performed using the five core components as ligands and the relevant targets in the pathway as receptors. As shown in [Figure2B], the depth of color represents the binding ability and the size of the dots represents the number of hydrogen bonds formed. The docking results revealed a lowest binding energy of less than -1.2 kcal/mol, and all the results formed more than 2 hydrogen bonds. These findings preliminarily verified that the five components of LJC were associated with MAPK-related target proteins, and preliminarily indicated that LJC may play a protective role in IBD through the ERK, JN, and p38 branches of MAPK.
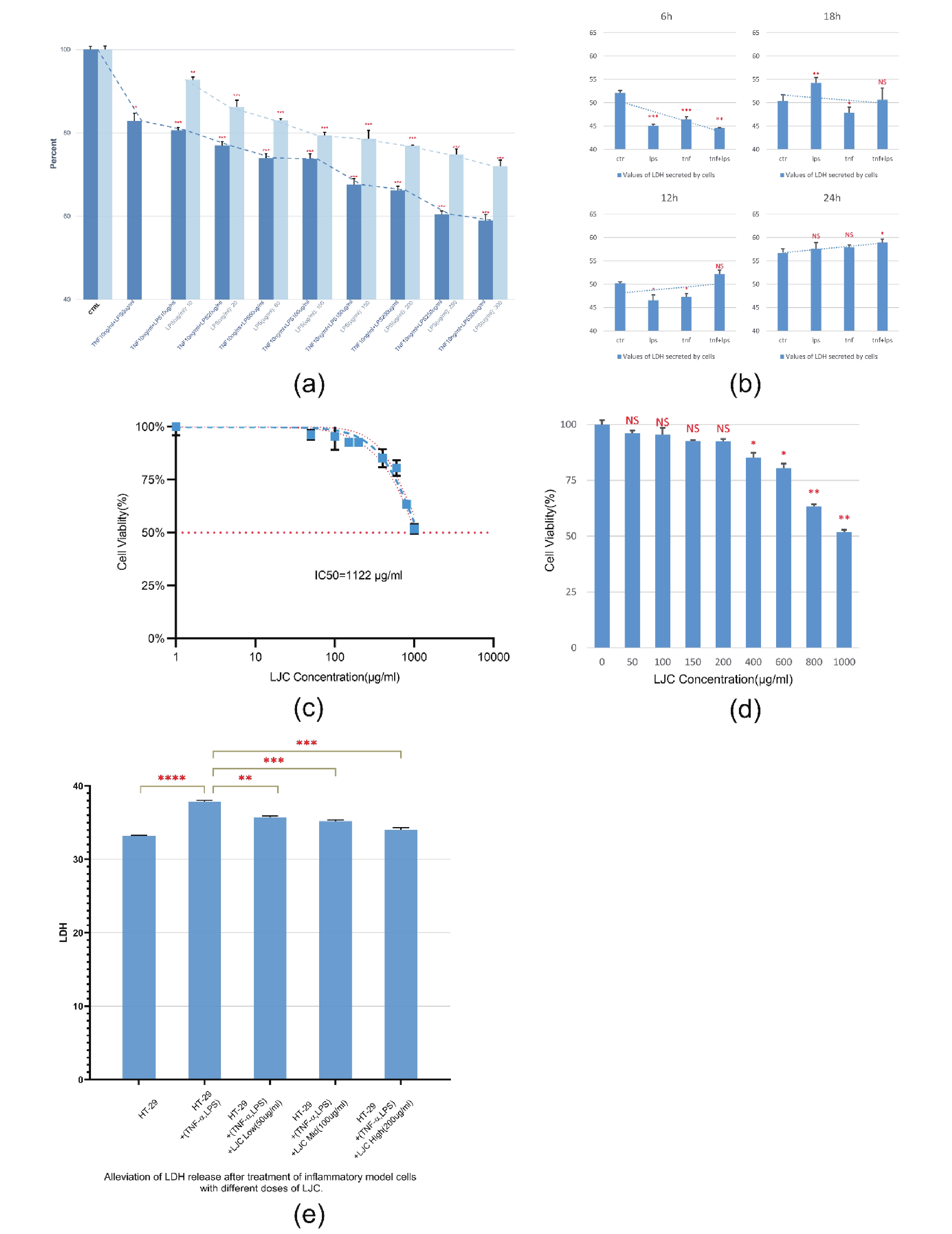
Subsequently, HT-29 cell models were constructed by co-treatment of TNF-α+ LPS. The cells were initially treated with LPS at a dose of 10-300 μg/mL for 24 h, and then treated with TNF-α 10 ng/mL combined with different doses of LPS for 24 h. The changes in cell viability were evaluated by CCK-8 analysis. We found, the combination of TNF-α and LPS to construct the intestinal model was significantly better than LPS alone (***P ˂ 0.001) [Supplementary S1A, available in www.besjournal.com]. Therefore, TNF-α 10 ng/mL+ LPS 50 μg/mL for 24 hours was selected for cell modeling. Co-treatment with TNF-α greatly reduced the number of agents used to construct the model and reduced the error of the final results caused by these agents.
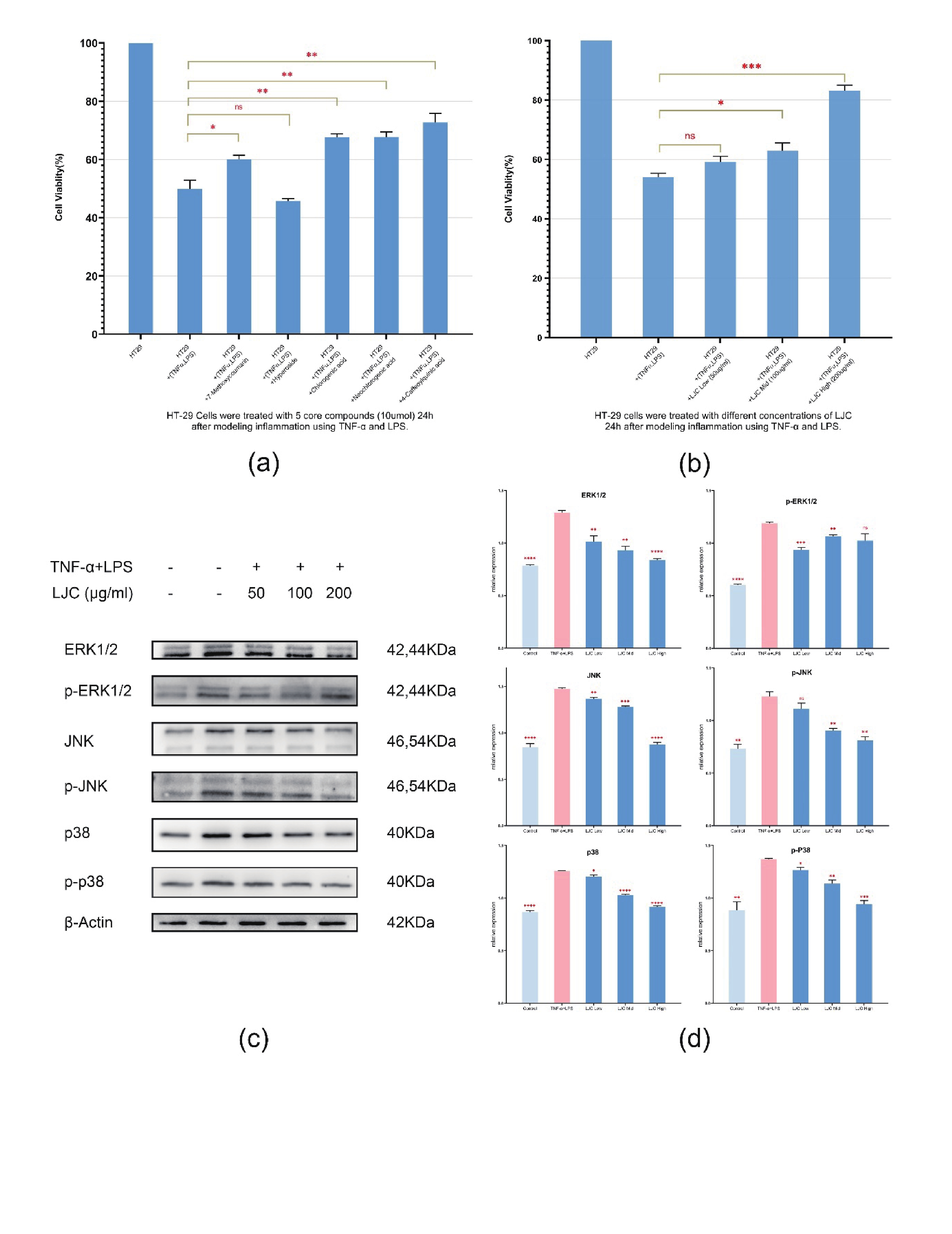
Additionally, the mitigating effect of LJC and its five components on the cellular model was evaluated. A toxicity assay was performed using a dose of LJC 50-800 μg/mL to treat HT-29 cells, revealing an IC50 of LJC of 1,122 μg/mL [Supplementary S1C]. When the dose was less than or equal to 200 μg/mL, the cell viability showed no significant difference from the control group [Supplementary S1D]. Hence, the experimental groups of LJC were categorized into the low-dose group (50 μg/mL, the medium-dose group (100 μg/mL), and the high-dose group (200 μg/mL). Then, the model cells were treated with five important components of LJC and three doses of LJC extracts. Among the five compounds, 7-methoxycoumarin, chlorogenic acid, neochlorogenic acid, and 4-caffeoylquinic acid attenuated the reduction of cell viability (*P ˂ 0.05) [Figure 3A]. Meanwhile, the reduction of cell viability was mildly alleviated in the middle-dose group of LJC (*P ˂ 0.05), while a more significant alleviation was obtained in the high-dose group (***P ˂ 0.001) [Figure 3B]. In addition, different doses of LJC attenuated the release of LDH from model cells (**P ˂ 0.01), which alleviated model cell damage [Supplementary S1E]. 7-methoxycoumarin belongs to the coumarin class, and its derivative 4-Hydroxy-7-methoxycoumarin was found to alleviate inflammation in LPS-activated RAW264.7 macrophages by inhibiting the activation of NF-κB and MAPK in a study by Kang et al[4]. Hyperoside's anti-inflammatory, antiviral, and neuroprotective effects have also been widely studied[5]; in experiments related to lung cancer cells, hyperoside was found to induce mitochondrial death by activating cellular p38 and JNK, thereby leading to tumor cell apoptosis[6]. Notably, hyperoside can induce apoptosis in HT-29 human colon cancer cells by disrupting the plasma membrane of mitochondria, activating caspase-9 and PARP, and increasing the expression of Bax[7], which may explain the role of hyperoside in alleviating IBD. Nonetheless, computer simulation to be important in the alleviating effects of hyperoside on the IBD cell model did not show an alleviation the reduction of cell viability, but rather a decrease, which may be related to its ability to damage HT-29 itself. On the other hand, chlorogenic acid and its derivatives neochlorogenic acid and 4-caffeoylquinic acid are considered to be the most important substances in LJC. They can attenuate intestinal mitochondrial damage by increasing the antioxidant effect and activity of respiratory complexes in the mitochondria of intestinal cells[8]. Our present experiments revealed that chlorogenic acid and its two derivatives could significantly enhance the viability of HT-29 inflammatory cells induced by TNF-α and LPS (P ˂ 0.01).

Figure 3. LJC extract and its five core substances alleviated model cell damage through the MAPK pathway. (A) The reduction of cell viability was alleviated by the treatment of model cells with the 5 core components. (B) Recovery of cell viability in the model cells treated with LJC: The medium-dose group showed a slight alleviation of injury, while the high-dose group exhibited more significant (*P ˂ 0.05, ** P ˂ 0.01, *** P ˂ 0.001). (C) Protein expression in HT-29 cells was determined by Western blot analysis. (D) LJC inhibits the activation of ERK, JNK, and p38 in the MAPK pathway in HT-29 cells.
Finally, to determine whether LJC exerts its effects by inhibiting the activation of MAPKs in HT-29 cells, the activation of related proteins was evaluated by western blot [Figure3C]. Erk1/2, JNK, and p38 proteins were significantly activated in the model cells (vs. control, P ˂ 0.01). Compared with the model group, the LJC extract effectively blocked the activation of Erk1/2, JNK, and p38, with the effect of the high dose group (200 μg/ml) being particularly pronounced (P ˂ 0.01) on Erk1/2, JNK and p38. The validated results were in accordance with the network pharmacology predictions [Figure3d]. MAPK is a serine/threonine-specific protein kinase that mainly consists of four branches. Among these, the function of Erk1/2 is mainly related to cell growth and differentiation and is also involved in apoptosis in conjunction with p38. JNK and p38 are mainly related to cell inflammation, proliferation, and apoptosis. The MAPK pathway plays an important role in inflammatory bowel disease, related studies have shown that MAPK can be activated in disease models of IBD[9]. Furthermore, reports on the proteomics of IBD patients have shown the relationship between MAPK, especially p38MAPK, and IBD[10]. Therefore, inhibiting the activation of MAPK may be an important means of controlling intestinal inflammation in inflammatory bowel disease. Our computer and cellular experiments revealed that a high dose of LJC (200 μg/mL) could effectively inhibit the proteins and their phosphorylation of Erk1/2, JNK, and p38 branches of the MAPK pathway. These findings suggested that LJC may play a role in alleviating the intestinal injury of IBD through the MAPK pathway.
In this study, LJC extract was found to alleviate the damage of intestinal model cells. 7-methoxycoumarin, hyperoside, chlorogenic acid, neochlorogenic acid, and 4-caffeoylquinic acid, were intimately involved in the beneficial effects against intestinal injury. LJC extract may decrease IBD intestinal damage by inhibiting Erk, JNK, and p38 and their phosphorylation in the MAPK pathway. These results may provide a foundation for the treatment of IBD with LJC.
No potential conflicts of interest were disclosed
GUO Qiang provided the concept. LU Hanxiu analyzed the data. YAN Wei and YUAN Xiao contributed to finding references. LIU Zhongjian reviewed the manuscript. All authors read and approved the final manuscript.

Figure . Supplemental Figure S1. (A) When cells were co-treated with TNF-α at 10 ng/mL and LPS at 50 µg/mL, the cellular changes were more pronounced and more effective than using LPS alone. (B) Validation of cellular model construction using cellular LDH release and selection of appropriate dosing times. (C, D) IC50 and dose testing of LJC. (E) LJC attenuated LDH release from model cells (*P < 0.05, **P < 0.01, ***P < 0.001).
Lonicera Japonica Caulis Ameliorates LPS and TNF-α-Induced HT-29 Cell Injury by Inhibiting the MAPK/ERK/JNK/p38 Pathway
doi: 10.3967/bes2024.091
- Received Date: 2023-11-30
- Accepted Date: 2024-05-06
| Citation: | LU Han Xiu, YAN Wei, YUAN Xiao, KANG Yong Bo, LIU Zhong Jian, GUO Qiang. Lonicera Japonica Caulis Ameliorates LPS and TNF-α-Induced HT-29 Cell Injury by Inhibiting the MAPK/ERK/JNK/p38 Pathway[J]. Biomedical and Environmental Sciences. doi: 10.3967/bes2024.091 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: