-
Theileria is a tick-borne intracellular protozoan that typically parasitizes erythrocytes, lymphocytes, and macrophages and causes acute clinical symptoms in host animals, including high fever, anemia, jaundice, and swelling of superficial lymph nodes[1]. Theileriosis can lead to high mortality rates, and its rising global prevalence has resulted in significant economic losses to livestock husbandry and the national economy[2]. Theileriosis is predominant in the western regions of China, particularly in Gansu, Ningxia, Inner Mongolia, Qinghai, Sichuan, and Shaanxi provinces[3]. Changzhi is located in Shanxi Province and is characterized by mountainous basin terrain and complex natural environments, making it more susceptible to zoonotic and vector-borne diseases. To date, there have been no reports on the prevalence of Theileria in Changzhi.
To investigate the distribution of Theileria in Changzhi, we collected ixodid ticks from five counties and performed polymerase chain reaction (PCR) for the molecular identification of Theileria. The isolated pathogens were sequenced to analyze the strains of Theileria. The findings of this study are expected to provide a scientific basis for the monitoring, prevention, and control of tick-borne diseases in Changzhi.
A total of 627 ticks were collected from eight sampling sites in five counties (districts) of Changzhi from May to July 2022. Parasitic ticks were collected from sheep, dogs, and rabbits using sterile tweezers, and free ticks were obtained from fields or forests via the tick-dragging method using a 1 m2 piece of felt cloth. Following collection, the ticks were transferred into centrifuge tubes and stored at −80 °C. Captured tick samples were first classified using taxonomic keys such as color, capitulum, body profile, enamel on the scutum, pale rings on legs, spiracular plate shape, and other morphological features. Female ticks, male ticks, and nymphs could be mainly differentiated on the basis of scutum, genital openings, body size, and blood-feeding characteristics. The tick samples were further classified based on the mitochondrial cytochrome C oxidase subunit I (COI) gene, which was sequenced by Sangon Biological Co., LTD (Shanghai, China).
Tick samples were prepared for molecular detection of Theileria using the 18S rRNA gene as the target fragment. They were initially immersed in 75% alcohol and rinsed thoroughly with phosphate buffered saline (PBS). Subsequently, each tick was transferred individually to a 2-mL grinding tube containing pre-cooled PBS and three stainless-steel iron beads with a diameter of 2 mm. The tick samples were then ground at 1,800 rpm for a total of six cycles, with each cycle consisting of 30 s of grinding followed by a 10-s interval using a grinder (JX-24, Shanghai JingXin Technology Co., Ltd). The genomic DNA of the ticks was extracted from the abrasive solution according to the instructions provided by the Tianlong Animal Tissue Genome DNA Extraction Kit (Xi'an Tianlong Science & Technology Co., Ltd, Shaanxi, China). PCR amplification was performed to detect Theileria spp. using the extracted genomic DNA as a template. The forward and reverse primers targeting the partial 18S rRNA gene (514 bp) of Theileria were 5ʹ-GTC TTG TAA TTG GAA TGA TGG-3ʹ and 5ʹ-TAG TTA TGG TTA GGA CTA CG-3ʹ, respectively. The PCR mixture comprised 10 µL PCR Master Mix (2× EasyTaq PCR SuperMix, TRANS), 0.8 µL (10 µmol/L) forward and reverse primers, and 3 µL DNA template, which were supplemented with nuclease-free water to adjust the final volume to 20 µL. The PCR conditions were set as follows: an initial denaturation at 95 °C for 5 minutes, 40 cycles of denaturation at 95 °C for 30 seconds, annealing at 55 °C for 30 seconds, extension at 72 °C for 45 seconds, and a final extension step at 72 °C for 10 minutes. Positive and negative controls were used in all experiments. The positive samples were sent to Sangon Biological Co., Ltd. for sequencing. After conducting a Basic Local Alignment Search Tool (BLAST) sequence comparison analysis with sequences published in GenBank and aligning the sequences using ClustalW and the maximum-likelihood algorithm, we used the neighbor-joining method (500 bootstrap replications) implemented in MEGA X to construct a phylogenetic tree.
Among the 627 tick samples collected, 518 were parasitic (432 parasitic sheep ticks, 55 parasitic dog ticks, and 31 parasitic rabbit ticks), and 109 were free ticks. Preliminary morphological analysis identified all ticks as Haemaphysalis. Further verification using COI analysis confirmed that all 20 representative samples were Haemaphysalis longicornis. Using the 18S rRNA PCR assay, Theileria spp. were primarily detected in parasitic sheep ticks (9.26% total prevalence, 40/432) (Table 1 and Supplementary Table S1, available in www.besjournal.com), female ticks (13.09% total prevalence, 39/298) (Table 1 and Supplementary Table S2, available in www.besjournal.com), and Pingshun County (6.86% total prevalence, 43/627) (Table 1). A small amount of Theileria was detected in free ticks (2.75% total prevalence, 3/109) (Table 1 and Supplementary Table S1), nymphs (4.60% total prevalence, 4/87) (Table 1 and Supplementary Table S2), and Dongsitou village in Pingshun County (5.26% total prevalence, 3/57) (Table 1). Theileria-positive free tick samples were found only in Zhuanghe village in Pingshun County, where there is a relatively high prevalence of Theileria (11.90% total prevalence, 40/336). No Theileria-positive dog or rabbit tick samples were found in the present study, which is consistent with previous reports that cattle and sheep are the main hosts of Theileria[4]. Additionally, no Theileria were detected in male ticks, possibly owing to their non-blood-feeding characteristics. With the exception of the samples collected from Pingshun County, all samples collected from other counties tested negative for Theileria spp. (Table 1).
Samples* n Positive no. Positive rate (%) Ticks from different areas Ticks parasitizing different
animals (n)Positive no. Ticks types and developmental
stage (n)Taling village of Luzhou district Parasitic dog ticks (55) 0 Female (0/21); male (0/24);
namphs (0/10)97 0 0.00 Parasitic rabbit ticks (31) 0 Female (0/16); male (0/11);
namphs (0/4)Free ticks (11) 0 Namphs (0/11) Siyuan village of Qinyuan county parasitic sheep ticks (44) 0 Female (0/44); male (0/1) 44 0 0.00 Xiwangyong village of Qinyuan county Parasitic sheep ticks (32) 0 Female (0/32) 32 0 0.00 Angou village of Xiangyuan county Parasitic sheep ticks (13) 0 Female (0/5); male (0/8) 13 0 0.00 Houbu town of Xiangyuan county Parasitic sheep ticks (16) 0 Female (0/8); male (0/8) 16 0 0.00 Shipan village of Wuxiang county Parasitic sheep ticks (32) 0 Female (0/24); male (0/8) 32 0 0.00 Dongsitou village of Pingshun county Parasitic sheep ticks (28) 3 Female (2/20); male (0/8) 57 3 5.26 Free ticks (29) 0 Female (0/11); male (0/5);
namphs (1/13)Zhuanghe village of Pingshun county Parasitic sheep ticks (267) 38 Female (34/110); male (3/156);
namphs (1/1)336 40 11.90 Free ticks (69) 2 Female (0/7); male (0/12);
namphs (2/50)Total 627 43 6.86 Note. *P < 0.001. Table 1. Detection of Theileria in ticks in Changzhi area, Shanxi Province
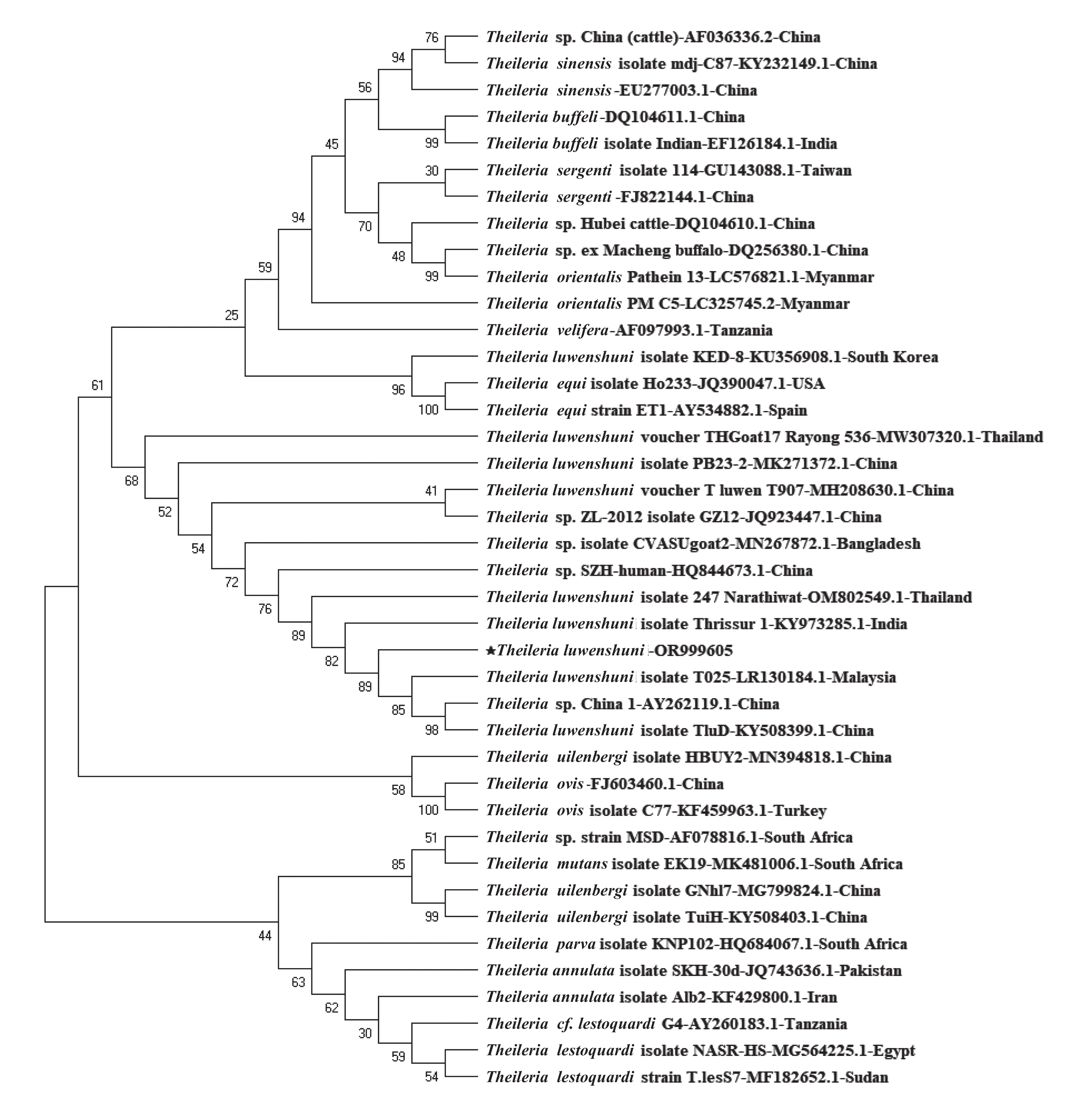
In this study, 43 samples tested Theileria-positive using the PCR method, of which 14 were sequenced successfully. BLAST analysis revealed 99% identity of the amplicon fragment with the corresponding segments of the T. luwenshuni isolate DRPB2 from Qinghai Province, China (GenBank accession no. OR104985), and the T. luwenshuni voucher T_luwen from Shandong Province, China (GenBank accession no. MH208630). All representative sequences exhibited a high similarity of approximately 99.8% and were registered in the GenBank database under the accession numbers OR999605–OR999618. A phylogenetic tree based on the 18S rRNA gene sequences of Theileria was constructed to analyze its genetics and evolution (Figure 1). The T. luwenshuni isolated in this study clustered into a separate lineage within the same clade as T. luwenshuni from China and Malaysia and unclassified Theileria species from China. This further demonstrates a clear genetic distinction between Theileria found in Changzhi and other Theileria spp.
Theileria are transmitted through ixodid ticks, with different species of Theileria having certain vector correspondence with a particular tick[5]. In the present study, Theileria-positive samples were exclusively identified in sheep ticks and specifically belonged to the genotype T. luwenshuni. This genotype is commonly found in parasitic sheep ticks, along with T. ovis and T. uilenbergi[6]. Additionally, our study demonstrated that Theileria detection rates were higher in samples with higher blood volumes, such as engorged female ticks. This finding can be attributed to the fact that Theileria is an obligate intracellular protozoan that resides abundantly in erythrocytes. Furthermore, no variations were observed in Theileria species across different geographic regions in this study, possibly because of the uniformity in the collected ticks. Notably, despite the absence of regional variations in Theileria species, our findings offer valuable insights into the hosts and vectors of Theileria and provide a foundation for further preventive investigations and prediction of livestock outbreaks in this area. A comprehensive investigation of various factors driving this high prevalence is crucial for tracking the dynamics of Theileria transmission in Changzhi, particularly in the village of Zhuanghe. These factors include tick population density, animal host composition, specific ecological influences, and anthropogenic activity, among others.
Changzhi has a temperate continental monsoon climate with four distinct seasons. The diverse ecological environment provides favorable conditions for the growth and reproduction of ixodid ticks. H. longicornis is widely distributed in China, commonly infesting sheep, goats, cattle, and deer, and can transmit various pathogens. T. luwenshuni is primarily transmitted by H. qinghaiensis and H. longicornis in China[7], with the latter being the predominant tick species in the Changzhi area, significantly increasing the risk of Theileria transmission within the region, particularly among free-ranging sheep in this mountainous area. T. luwenshuni, known for its high prevalence and strong virulence, poses a significant threat to the sheep industry in China, resulting in considerable losses owing to theileriosis and decreased productivity[8,9]. The wide host range and blood-feeding attributes of ixodid ticks facilitate the transmission of T. luwenshuni to various hosts. Therefore, further studies are necessary to identify the potential transmission of T. luwenshuni to other animals via ixodid ticks in this area. In addition, regarding the co-evolutionary relationship between parasites and their hosts[10], future investigations on T. luwenshuni and its associated ticks will contribute to identifying the unknown host of Theileria, as well as improving livestock farming practices and vector control efforts. According to our field investigation, no apparent cases of theileriosis were detected among the sheep herds in the surveyed counties; however, this study revealed the presence of a reservoir for Theileria in sheep in Pingshun County. Therefore, caution should be exercised regarding potential stressors (e.g., severe weather conditions, inadequate animal husbandry practices, and compromised animal immune systems) that could contribute to the development of symptomatic theileriosis.
Conclusively, in this study, we analyzed the prevalence of Theileria in H. longicornis in Changzhi and revealed that T. luwenshuni was the dominant species found in Theileria-positive samples. These findings augment the regional reports of H. longicornis carrying T. luwenshuni and serve as a crucial reference for the comprehensive prevention and treatment of the disease in Changzhi.
-
Host n Positive no. Positive rate (%) P Parasitic sheep ticks 432 40 9.26 < 0.001 Parasitic dog ticks 55 0 0.00 Parasitic rabbit ticks 31 0 0.00 Free ticks 109 3 2.75 Total 627 43 6.86 Table 1. Detection rate of Theileria in ticks parasitizing different animals
Type n Positive no. Positive rate (%) χ2 P Female 298 39 13.09 36.617 < 0.001 Male 242 0 0.00 Nymphs 87 4 4.60 Total 627 43 6.86 Table 2. Detection rate of Theileria in female, male ticks and nymphs in Changzhi
HTML
 24090+Supplementary Materials.pdf
24090+Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: