-
Diabetes during pregnancy, comprising primarily gestational diabetes mellitus (GDM) and pre-pregnancy diabetes mellitus (PGDM), is a prevalent complication during pregnancy. GDM is characterized by onset of diabetes mellitus with normal glucose metabolism before pregnancy, constituting over 90% of cases of diabetes in pregnancy[1,2]. Consequently, GDM is a major concern for obstetricians and maternal and child health care professionals, affecting an estimated 16% of pregnant women worldwide[3].
GDM increases immediate maternal and neonatal health risks, such as pregnancy-induced hypertension, macrosomia, dystocia, birth injury, cesarean delivery, and congenital anomalies[4,5], and also has a lasting negative impact on the health of mothers and their children[6,7]. Pregnant women with GDM face a higher risk of developing type 2 diabetes mellitus (T2DM), metabolic syndrome, and cardiovascular disease[8-11]. Their offspring are more susceptible to glucose irregularities and obesity from childhood through adolescence and adulthood[12,13]. The postponement of marriage and childbearing, together with an increasing rate of pre-pregnancy obesity and overweight linked to poor lifestyles and dietary patterns, signals a growing risk of GDM for an expanding population of pregnant women and their descendants. Therefore, investigating GDM risk factors and implementing early identification and intervention among high-risk populations are crucial to reducing its incidence and preventing both immediate and long-term adverse health outcomes for mothers and infants.
The ABO system antigens are complex carbohydrates found on the surface of erythrocytes. Located on chromosome 9 (9q34.1), the ABO gene encodes for ABO glycosyltransferase with three main alleles: A and B, codominant, and O, respectively. A and B alleles add N-acetyl galactosamine and D-galactose to the precursor substance H, forming A or B antigens, whereas the O allele’s mutation yields no functional enzyme[14]. In addition to red blood cells, A and B antigens are widely found in various tissues and secretions, including intestinal mucosa, vascular endothelium, kidneys, heart, and other organs[15]. Consequently, ABO blood types, essential in transfusion medicine, show a linkage to an increased susceptibility to certain infectious, neoplastic, and cardiovascular diseases, particularly type 2 diabetes[16-20]. The biological mechanisms underpinning the association between ABO groups and diabetes remain unclear. For instance, the ABO antigen may influence a range of biomarkers relevant to insulin resistance and type 2 diabetes, including E-selectin, P-selectin, tumor necrosis factor-alpha (TNF-α), soluble intercellular adhesion molecule-1 (sICAM-1), and interleukin-6 (IL-6)[21-23].
Globally, several studies have investigated the association between ABO blood types and GDM across diverse populations[24-36]. Studies conducted in France[32], Turkey[33] and Japan[27] have indicated that blood type AB is a risk factor for GDM. Conversely, research on pregnant women from Israel[30] and China[26] identified blood type AB as a protective factor for GDM, demonstrating a lower risk than the other three blood types. Additionally, a study by Sapanont et al. in Thailand[28] reported that blood type O independently increased the risk of GDM. However, other studies, such as recent study conducted in Mexican pregnant women[35], along with previous research on women in Saudi Arabia[36], Sudan[34], Pakistan[29], and Thailand[31] did not find a significant association between ABO blood types and the risk of GDM. Thus, the current evidence on the relationship between ABO blood types and GDM is inconsistent and varies across countries and regions. The reasons for these discrepancies may stem from genetic differences among ethnic groups, which affect the distribution of ABO blood types and susceptibility to GDM. Other factors, such as variations in study design, sample sizes, and adjustments for covariates, may also contribute to these inconsistencies.
To date, the body of literature examining the association between ABO blood types and GDM remains limited, with most studies being retrospective, small-sample analyses based on hospital or regional data. The collection and adjustment of risk factors for GDM is comparatively limited, which restricts the in-depth analysis of the relationship between ABO blood types and GDM. Given the current sparse and uncertain evidence, alongside ethnic and regional differences, it is crucial to gather more robust evidence from prospective, large-sample studies conducted across diverse populations. Therefore, this study, based on a prospective large-sample birth cohort in Beijing, China, aims to investigate the relationship between ABO blood types and the risk of GDM, thereby providing valuable birth cohort evidence from the Chinese population.
-
The China Birth Cohort Study (CBCS) was an ongoing multi-center prospective longitudinal cohort study initiated in November 2017 in China, which aimed to investigate the risk factors for congenital disabilities, as well as other maternal and child diseases including gestational diabetes, etc. The rationale and methodology were introduced previously[37]. Briefly, Pregnant women were enrolled in the CBCS study if they met the following criteria: (1) at 6-13+6 weeks of gestation, (2) living in the local area for more than one year, (3) plan to attend routine antenatal examination and deliver in the study site; (4) understand the study and provide informed, written consent.
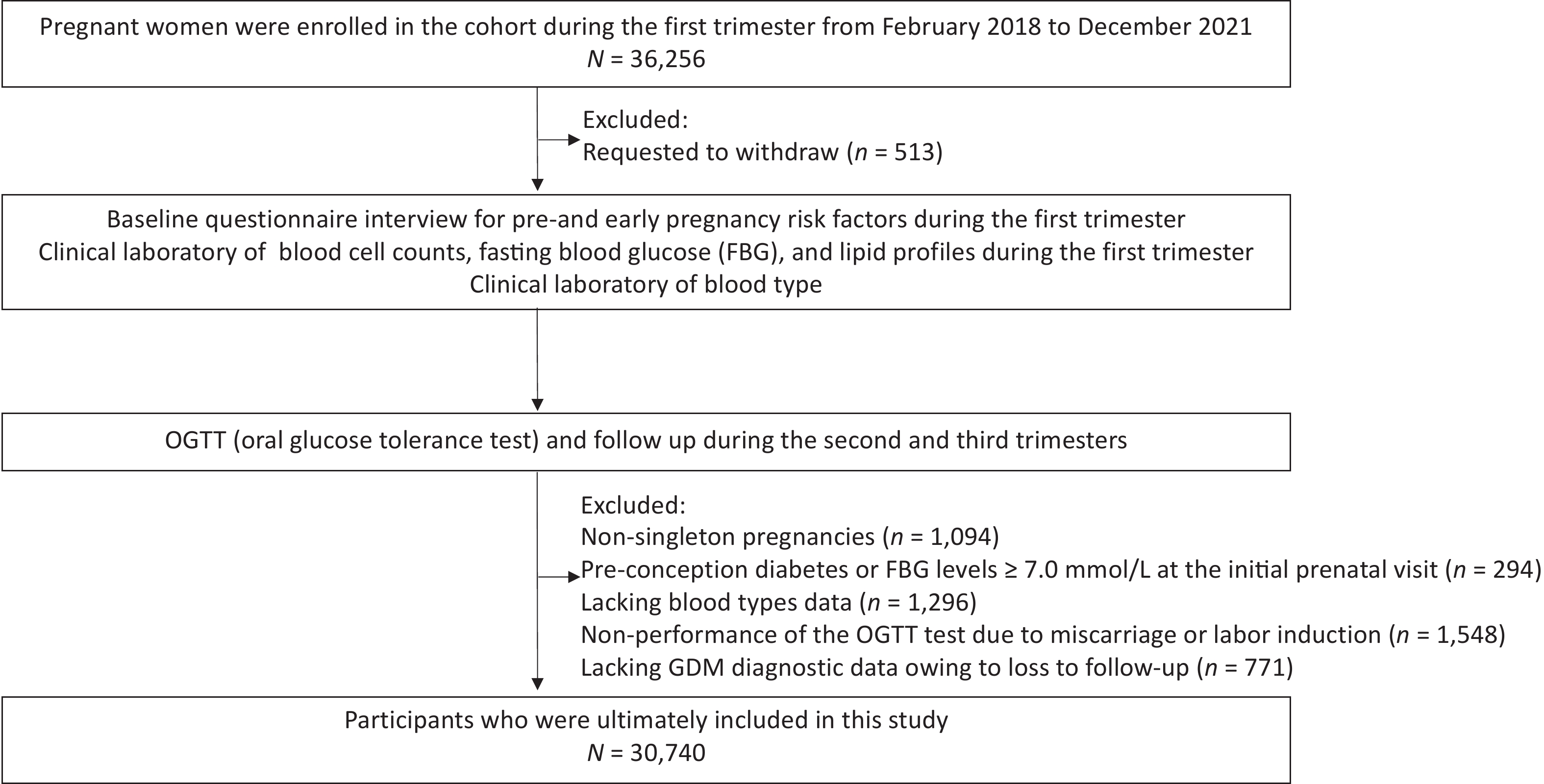
Pregnant women participating in the CBCS at the center of Beijing Obstetrics and Gynecology Hospital, Capital Medical University during November 2017 to December 2021 were selected for the present study (n = 36,256). Of the 36,256 pregnant women, participants were excluded for the following reasons: (1) Withdrawal from the study post-recruitment (n = 513); (2) Nonsingleton pregnancies confirmed via ultrasound during the first trimester (n = 1,094); (3) Pre-conception diabetes or fasting venous blood glucose levels ≥ 7.0 mmol/L at the initial prenatal visit (n = 294); (4) Lacking blood types data (n = 1,296); (5) Non-performance of the OGTT test due to miscarriage or labor induction (n = 1,548); (6) Lacking GDM diagnostic data owing to loss to follow-up (n = 771). Ultimately, 30,740 participants were included in the present study (Figure 1).
-
All eligible pregnant women completed a baseline questionnaire interview for pre- and early pregnancy risk factors, including demographic characteristics (date of birth, ethnicity), socioeconomic characteristics (education, income, etc.), previous and current pregnancy status (primigravida status, history of pregnancy complications, fertilization way, number of fetuses in utero, etc.), lifestyle factors (smoking, alcohol consumption), as well as nutritional status and vitamin intake (pre-pregnancy BMI, folic acid supplementation, multivitamin supplementation, etc.). Pre-pregnancy BMI was calculated by dividing weight in kilograms by the square of height in meters. Folic acid or multivitamin supplementation was defined as the intake of folic acid preparations, either alone or in combination, within three months before and during early pregnancy. This included continuous consumption for at least one week, at a minimum frequency of five days per week, with a dosage of no less than 400 units daily. The longitudinal follow-up visits were conducted at mid-pregnancy, late pregnancy, and delivery based on their routine prenatal examinations at Beijing Obstetrics and Gynecology Hospital.
-
After the sampling site on the pregnant woman has been sanitized, 5 mL of venous blood is drawn into a sterile, dry test tube without an anticoagulant. Blood types were determined by the slide method. Red blood cells (RBC) from the subjects tested were mixed with diagnostic serum containing anti-A and anti-B, and the erythrocyte agglutination reaction was evaluated to ascertain their blood types. RBC that contain the A antigen are classified as type A, those with the B antigen as type B, those with both A and B antigens as type AB, and those without either antigen as type O. Additional clinical laboratory parameters, including white blood cell (WBC) and RBC counts, hemoglobin (HGB) levels, triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and fasting blood glucose (FBG) concentrations, were assessed per standard operating procedures.
-
GDM diagnosis was based on a one-step oral glucose tolerance test (OGTT). A fasting blood glucose sample was obtained after 8–10 h of fasting; thereafter, the participants consumed 75g of glucose within 5 minutes (75g of anhydrous glucose powder dissolved in 300ml of pure water), and blood samples were collected at the 1st and 2nd hours post-ingestion. GDM diagnosis was established when one or more blood glucose readings surpassed the established OGTT threshold values: fasting blood glucose > 5.1 mmol/L, or blood glucose > 10.0 mmol/L at the 1st hour and > 8.5 mmol/L at the 2nd hour, as per the International Association of Diabetes and Pregnancy Study Groups (IADPSG) guidelines. Furthermore, data from the hospital's case diagnosis system was utilized to supplement and verify the clinical diagnoses of the cohort in terms of pregnancy comorbidities and outcomes. For those not recorded in the hospital’s case diagnosis system, telephone follow-up was employed to ascertain pregnancy comorbidities and outcomes.
-
Gestational age calculation was based on the date of the last menstruation or first-trimester ultrasonography. The subjects’ general characteristics were presented as mean ± standard deviation (SD) for continuous variables and as percentages for categorical variables. The normality test results on the distribution of continuous variables indicate that all continuous variables do not satisfy the normal distribution (Supplementary Table S1, S2). Characteristics of the study subjects between GDM and non-GDM groups were compared using the Chi-Square test for categorical variables and the Wilcoxon test for continuous variables. Baseline characteristics across different blood types were assessed using the Chi-Square test for categorical variables and the Kruskal-Wallis test for continuous variables. The odds ratios (ORs) and 95% confidence intervals (95% CIs) for developing GDM among various blood types were determined using logistic regression analysis, with the type O group as the reference. In the multivariable analyses, adjustments were made for known GDM risk factors and potential confounders such as age at pregnancy, ethnicity, education level, family annual income, pre-pregnancy BMI, menstrual regularity, primigravida status, history of GDM and adverse pregnancy outcomes, fertilization way, smoking, alcohol consumption, and supplementation with folic acid and multivitamins, along with adjustments for first-trimester laboratory measurements like WBC and RBC counts, HGB, TG, LDL-c, HDL-c, and FBG levels. Subgroup analyses focused on age at pregnancy (less than 35 years, 35 years or older)[38], pre-pregnancy BMI (< 24 kg/m2, ≥ 24 kg/m2)[39], education level (below college, undergraduate/College, postgraduate or higher), menstrual status (regularity, irregularity), fertilization way (natural pregnancy, assisted reproductive techniques), and blood lipid profiles in the first trimester (normal vs. abnormal). The age threshold of 35 is considered because it is widely recognized as a high-risk threshold in both clinical practice and previous research[38]. Dyslipidemia was defined as TC greater than or equal to 6.2 mmol/L, LDL-C greater than or equal to 4.1 mmol/L, HDL-C less than 1.0 mmol/L, or TG greater than or equal to 2.3 mmol/L, per the 2016 Chinese Adult Dyslipidemia Prevention Guideline. Sensitivity analysis of blood types and GDM risk was conducted as follows: participants with a history of GDM were excluded due to a higher likelihood of recurrent glucose metabolism disorders; Mean imputation, deletion of missing data, and multiple imputation methods were employed to handle missing data, and analyzed the relationship between ABO blood type and GDM. For ease of interpretation, the results of this study present analyses using mean imputation, the results from direct deletion and multiple imputation methods are presented in the sensitivity results. Data management and analyses were performed using the SAS software, version 9.4 (SAS Institute Inc.). All statistical tests were two-tailed, and a P-value of < 0.05 was considered statistically significant.
-
A total of 30,740 pregnant women were enrolled in this study, having a mean age of 31.81 ± 3.87 years. Among these participants, 5,563 were aged ≥ 35, comprising 18.10% of the group. The average preconception BMI was 21.81 ± 3.51 kg/m2, with an overweight rate of 15.34% (4,717 cases) and an obesity rate of 4.74% (1,457 cases). Of the total, 53.03% (16,300 cases) represented primigravida status, and 6.03% (1,853 cases) involved assisted reproductive techniques. This cohort generally had higher educational levels, with 54.54% (16,765 cases) holding bachelor’s degrees and 24.38% (7,494 cases) possessing postgraduate education or higher. The rates of smoking, alcohol consumption, menstrual irregularity, previous gestational diabetes, previous adverse pregnancy outcomes, and supplementation with folic acid and multivitamins were 3.10%, 4.98%, 17.45%, 2.35%, 16.23%, 88.99%, and 77.26%, respectively (Table 1).
Characteristics Total cohort GDM χ2 or Z value P value Yes No Total, N 30,740 4,439 26,301 Age at pregnancy (years) 532.831 < 0.001 < 35 25177 (81.90) 3088 (69.57) 22089 (83.99) ≥ 35 5563 (18.10) 1351 (30.43) 4212 (16.01) Ethnicity 1.582 0.209 Han 28374 (92.30) 4118 (92.77) 24256 (92.22) Others 2366 (7.70) 321 (7.23) 2045 (7.78) Education level 54.106 < 0.001 Below college 6481 (21.08) 1082 (24.37) 5399 (20.53) Undergraduate/College 16765 (54.54) 2434 (54.83) 14331 (54.49) Postgraduate or higher 7494 (24.38) 923 (20.80) 6571 (24.98) Family annual income (CNY) 15.534 0.001 < 100,000 3500 (11.39) 572 (12.89) 2928 (11.13) 100,000-200,000 8405 (27.34) 1189 (26.79) 7216 (27.44) 200,000-400,000 11204 (36.45) 1546 (34.82) 9658 (36.72) > 400,000 7631 (24.82) 1132 (25.50) 6499 (24.71) Pre-pregnancy BMI (kg/m2) 781.756 < 0.001 < 18.5 3731 (12.14) 266 (5.99) 3465 (13.17) 18.5–24 20835 (67.78) 2640 (59.47) 18195 (69.18) 24–28 4717 (15.34) 1109 (24.98) 3608 (13.72) ≥ 28 1457 (4.74) 424 (9.56) 1033 (3.93) Smoking 23.140 < 0.001 No 29787 (96.90) 4250 (95.74) 25537 (97.10) Yes 953 (3.10) 189 (4.26) 764 (2.90) Alcohol consumption 1.082 0.298 No 29210 (95.02) 4232 (95.34) 24978 (94.97) Yes 1530 (4.98) 207 (4.66) 1323 (5.03) Menstrual status 22.689 < 0.001 Regularity 25376 (82.55) 3553 (80.04) 21823 (82.97) Irregularity 5364 (17.45) 886 (19.96) 4478 (17.03) Primigravida status 91.864 < 0.001 No 14440 (46.97) 2380 (53.62) 12060 (45.85) Yes 16300 (53.03) 2059 (46.38) 14241 (54.15) History of GDM 643.395 < 0.001 No 30018 (97.65) 4098 (92.32) 25920 (98.55) Yes 722 (2.35) 341 (7.68) 381 (1.45) History of adverse pregnancy outcomes 93.363 < 0.001 No 25751 (83.77) 3499 (78.82) 22252 (84.61) Yes 4989 (16.23) 940 (21.18) 4049 (15.39) Fertilization way 98.291 < 0.001 Natural pregnancy 28887 (93.97) 4026 (90.70) 24861 (94.52) Assisted reproductive techniques 1853 (6.03) 413 (9.30) 1440 (5.48) Folic acid supplementation 0.193 0.66 No 3383 (11.01) 497 (11.20) 2886 (10.97) Yes 27357 (88.99) 3942 (88.80) 23415 (89.03) Multivitamin supplementation 40.995 < 0.001 No 6990 (22.74) 844 (19.01) 6146 (23.37) Yes 23750 (77.26) 3595 (80.99) 20155 (76.63) Blood count parameters WBC (109/L) 8.35 ± 1.94 8.91 ± 2.05 8.26 ± 1.91 20.323 < 0.001 RBC (1012/L) 4.29 ± 0.33 4.35 ± 0.34 4.28 ± 0.33 14.466 < 0.001 HGB (g/L) 129.74 ± 8.96 131.24 ± 9.14 129.48 ± 8.9 12.985 < 0.001 First-trimester FBG (mmol/L) 4.65 ± 0.36 4.82 ± 0.42 4.62 ± 0.34 28.490 < 0.001 First-trimester blood lipid profiles (mmol/L) TC 4.22 ± 0.69 4.38 ± 0.73 4.19 ± 0.68 16.158 < 0.001 TG 1.08 ± 0.48 1.30 ± 0.60 1.05 ± 0.45 30.755 < 0.001 HDL-c 1.51 ± 0.29 1.46 ± 0.29 1.52 ± 0.29 −12.646 < 0.001 LDL-c 2.22 ± 0.60 2.39 ± 0.63 2.20 ± 0.58 19.944 < 0.001 Note. Continuous variables were summarized as mean (standard deviations), categorical variables were displayed as a percentage (%). GDM: gestational diabetes mellitus, CNY: Chinese Yuan, BMI: body mass index, WBC: white blood cell, RBC: red blood cell, HGB: hemoglobin, TC: total cholesterol, TG: triglyceride, LDL-c: low-density lipoproteins cholesterol, HDL-c: high-density lipoproteins cholesterol, FBG: fasting blood glucose. Table 1. Baseline characteristics of participants according to occurrence of gestational diabetes mellitus among 30,740 pregnant women
During the follow-up, the OGTT results showed that among 30,740 participants, 4,439 developed GDM, resulting in an incidence rate of 14.44%. Pregnant women diagnosed with GDM, compared to those without, tended to be older (≥ 35 years), have a relatively lower level of education, be more susceptible to pre-pregnancy overweight and obesity, and more frequently reported smoking, irregular menstruation, non-primigravida status, previous GDM and adverse pregnancy outcomes, assisted reproduction, and multivitamin supplementation (P < 0.05). There were no significant differences in ethnicity, alcohol consumption, and folic acid supplementation rates between the two groups (P > 0.05). Significantly higher counts of WBC, RBC, and higher levels of HGB, FPG, TC, TG, and LDL-c were detected in the GDM group, whereas HDL-c levels were considerably lower (P < 0.05) (Table 1).
-
Baseline characteristics according to blood types were shown in Table 2. Among 30,740 pregnant women, there were 9,524 cases (30.98%) of type O, 8,172 cases (26.58%) of type A, 9,899 cases (32.20%) of type B, and 3,145 cases (10.23%) of type AB. Significant differences were observed in the distribution of education level, pre-pregnancy BMI, menstrual status, and fertilization way among the four blood types (P < 0.05). There are significant differences in the levels of RBC, HGB, FBG, TC, HDL-c, and LDL-c among pregnant women with different blood types (P < 0.05). However, other baseline characteristics, including age at pregnancy, ethnicity, family annual income, smoking, alcohol consumption, primigravida status, history of GDM, adverse pregnancy outcomes, and supplementation with folic acid and multivitamins, showed no significant differences in distribution across the ABO blood type phenotypes (P > 0.05) (Table 2).
Characteristics ABO blood types χ2 value P value O A B AB Total, N 9,524 8,172 9,899 3,145 Age at pregnancy (years) < 35 7794 (81.84) 6673 (81.66) 8118 (82.01) 2592 (82.42) 0.997 0.802 ≥ 35 1730 (18.16) 1499 (18.34) 1781 (17.99) 553 (17.58) Ethnicity 3.457 0.326 Han 8752 (91.89) 7560 (92.51) 9148 (92.41) 2914 (92.66) Others 772 (8.11) 612 (7.49) 751 (7.59) 231 (7.34) Education level 16.405 0.012 Below college 1914 (20.10) 1758 (21.51) 2098 (21.19) 711 (22.61) Undergraduate/College 5295 (55.60) 4380 (53.60) 5372 (54.27) 1718 (54.63) Postgraduate or higher 2315 (24.30) 2034 (24.89) 2429 (24.54) 716 (22.76) Family annual income (CNY) 15.427 0.080 < 100,000 1089 (11.43) 903 (11.05) 1144 (11.56) 364 (11.57) 100,000-200,000 2551 (26.78) 2257 (27.62) 2739 (27.67) 858 (27.28) 200,000-400,000 3431 (36.02) 2945 (36.04) 3636 (36.73) 1192 (37.91) > 400,000 2453 (25.77) 2067 (25.29) 2380 (24.04) 731 (23.24) Pre-pregnancy BMI (kg/m2) 21.886 0.009 < 18.5 1155 (12.13) 1005 (12.30) 1198 (12.10) 373 (11.86) 18.5–24 6571 (68.99) 5461 (66.83) 6724 (67.93) 2079 (66.10) 24–28 1379 (14.48) 1308 (16.01) 1513 (15.28) 517 (16.44) ≥ 28 419 (4.40) 398 (4.86) 464 (4.69) 176 (5.60) Smoking 2.946 0.400 No 9252 (97.14) 7911 (96.81) 9584 (96.82) 3040 (96.66) Yes 272 (2.86) 261 (3.19) 315 (3.18) 105 (3.34) Alcohol consumption 2.577 0.462 No 9043 (94.95) 7783 (95.24) 9411 (95.07) 2973 (94.53) Yes 481 (5.05) 389 (4.76) 488 (4.93) 172 (5.47) Menstrual status 8.368 0.039 Regularity 7901 (82.96) 6703 (82.02) 8219 (83.03) 2553 (81.18) Irregularity 1623 (17.04) 1469 (17.98) 1680 (16.97) 592 (18.82) Primigravida status 0.179 0.981 No 4476 (47.00) 3839 (46.98) 4638 (46.85) 1487 (47.28) Yes 5048 (53.00) 4333 (53.02) 5261 (53.15) 1658 (52.72) History of GDM 0.800 0.849 No 9292 (97.56) 7978 (97.63) 9672 (97.71) 3076 (97.81) Yes 232 (2.44) 194 (2.37) 227 (2.29) 69 (2.19) History of adverse pregnancy outcomes 1.325 0.723 No 8001 (84.01) 6820 (83.46) 8305 (83.90) 2625 (83.47) Yes 1523 (15.99) 1352 (16.54) 1594 (16.10) 520 (16.53) Fertilization way 11.451 0.010 Natural pregnancy 8986 (94.35) 7644 (93.54) 9331 (94.26) 2926 (93.04) Assisted reproductive techniques 538 (5.65) 528 (6.46) 568 (5.74) 219 (6.96) Folic acid supplementation 4.903 0.179 No 1018 (10.69) 947 (11.59) 1062 (10.73) 356 (11.32) Yes 8506 (89.31) 7225 (88.41) 8837 (89.27) 2789 (88.68) Multivitamin supplementation 6.685 0.083 No 2226 (23.37) 1862 (22.79) 2236 (22.59) 666 (21.18) Yes 7298 (76.63) 6310 (77.21) 7663 (77.41) 2479 (78.82) Blood count parameters WBC (109/L) 8.38 ± 1.96 8.32 ± 1.89 8.36 ± 1.96 8.33 ± 1.96 4.105 0.250 RBC (1012/L) 4.29 ± 0.34 4.25 ± 0.33 4.32 ± 0.33 4.28 ± 0.33 180.077 < 0.001 HGB (g/L) 129.78 ± 8.95 128.88 ± 8.87 130.46 ± 9.00 129.56 ± 8.89 154.816 < 0.001 First-trimester FBG (mmol/L) 4.64 ± 0.36 4.65 ± 0.36 4.64 ± 0.36 4.67 ± 0.37 11.936 0.008 First-trimester blood lipid profiles (mmol/L) TC 4.18 ± 0.68 4.27 ± 0.71 4.21 ± 0.68 4.25 ± 0.70 72.312 < 0.001 TG 1.09 ± 0.48 1.08 ± 0.49 1.08 ± 0.47 1.09 ± 0.48 3.848 0.278 HDL-c 1.50 ± 0.29 1.51 ± 0.29 1.51 ± 0.29 1.50 ± 0.29 9.228 0.026 LDL-c 2.19 ± 0.59 2.27 ± 0.61 2.21 ± 0.59 2.25 ± 0.60 85.821 < 0.001 Note. Continuous variables were summarized as mean (standard deviations), categorical variables were displayed as a percentage (%). GDM: gestational diabetes mellitus, CNY: Chinese Yuan, BMI: body mass index, WBC: white blood cell, RBC: red blood cell, HGB: hemoglobin, TC: total cholesterol, TG: triglyceride, LDL-c: low-density lipoproteins cholesterol, HDL-c: high-density lipoproteins cholesterol, FBG: fasting blood glucose Table 2. Baseline characteristics of participants by ABO blood types among 30,740 pregnant women
-
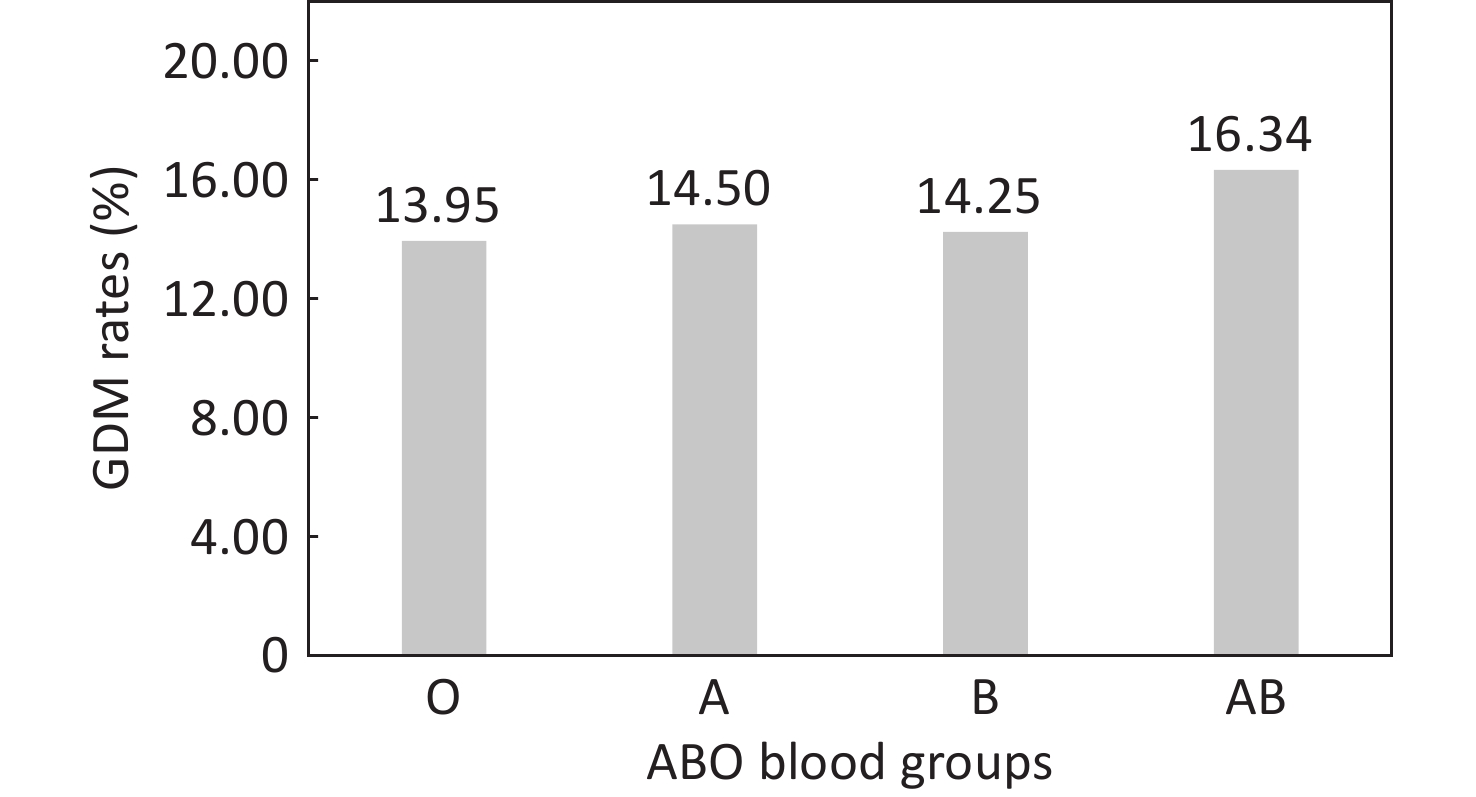
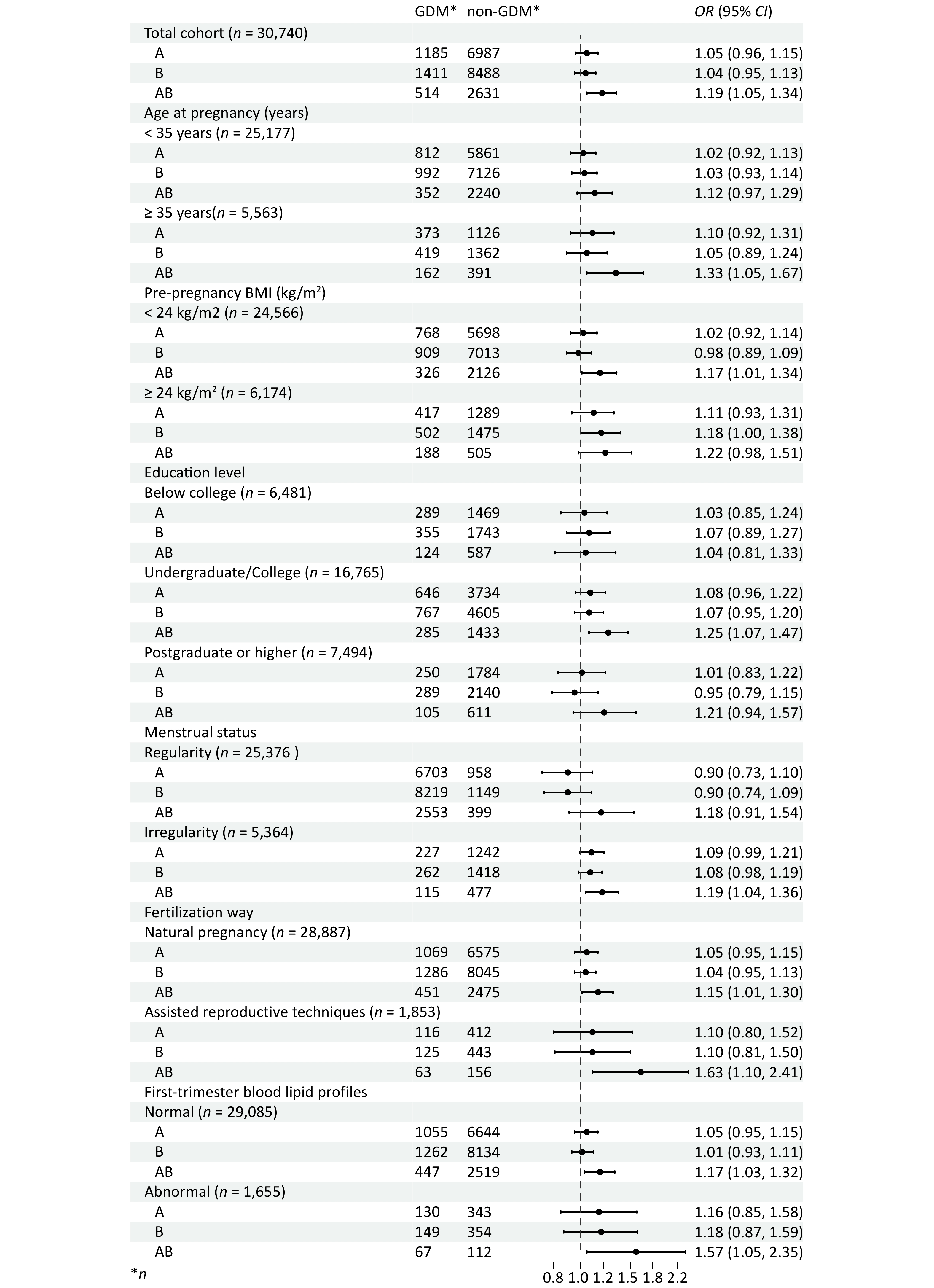
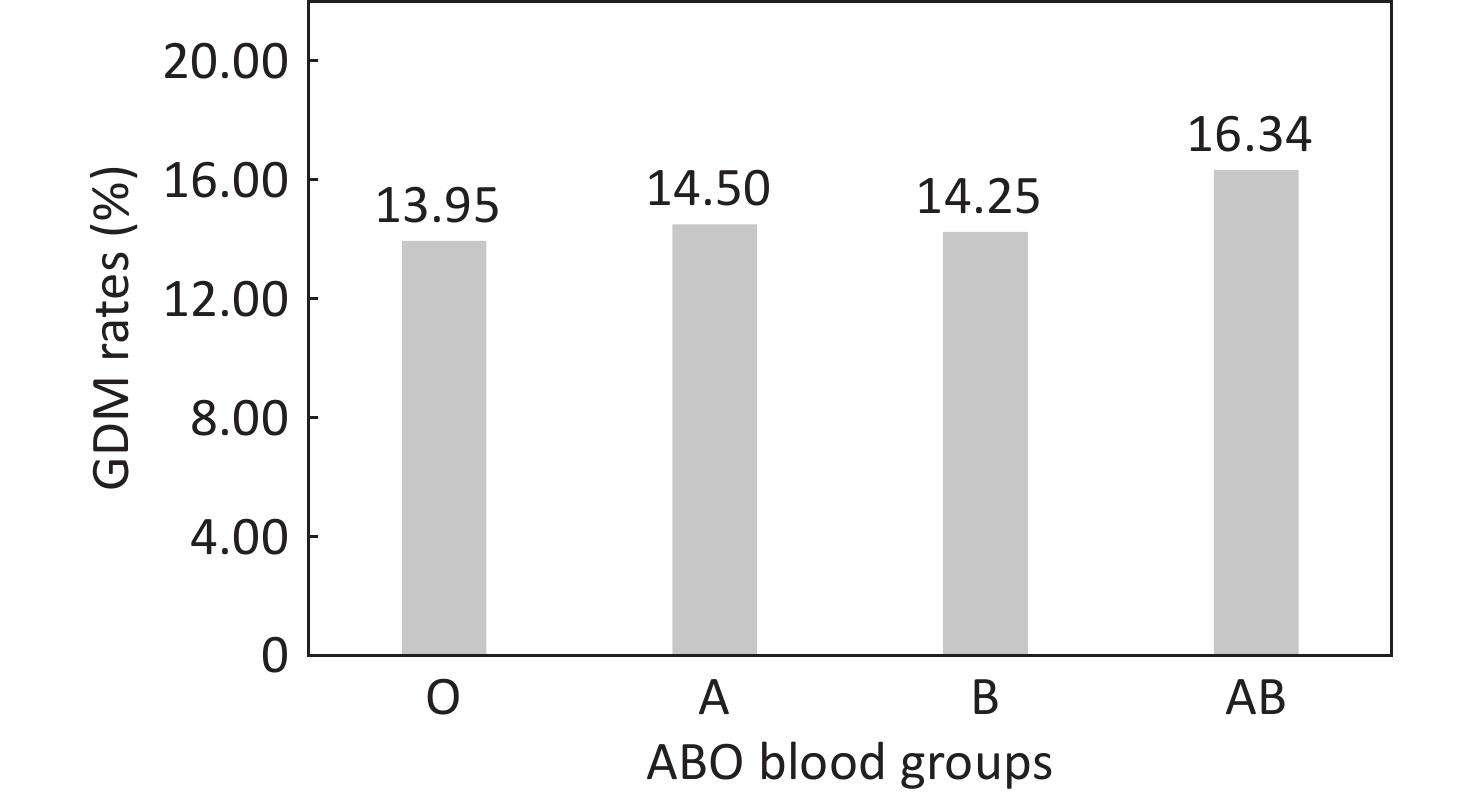
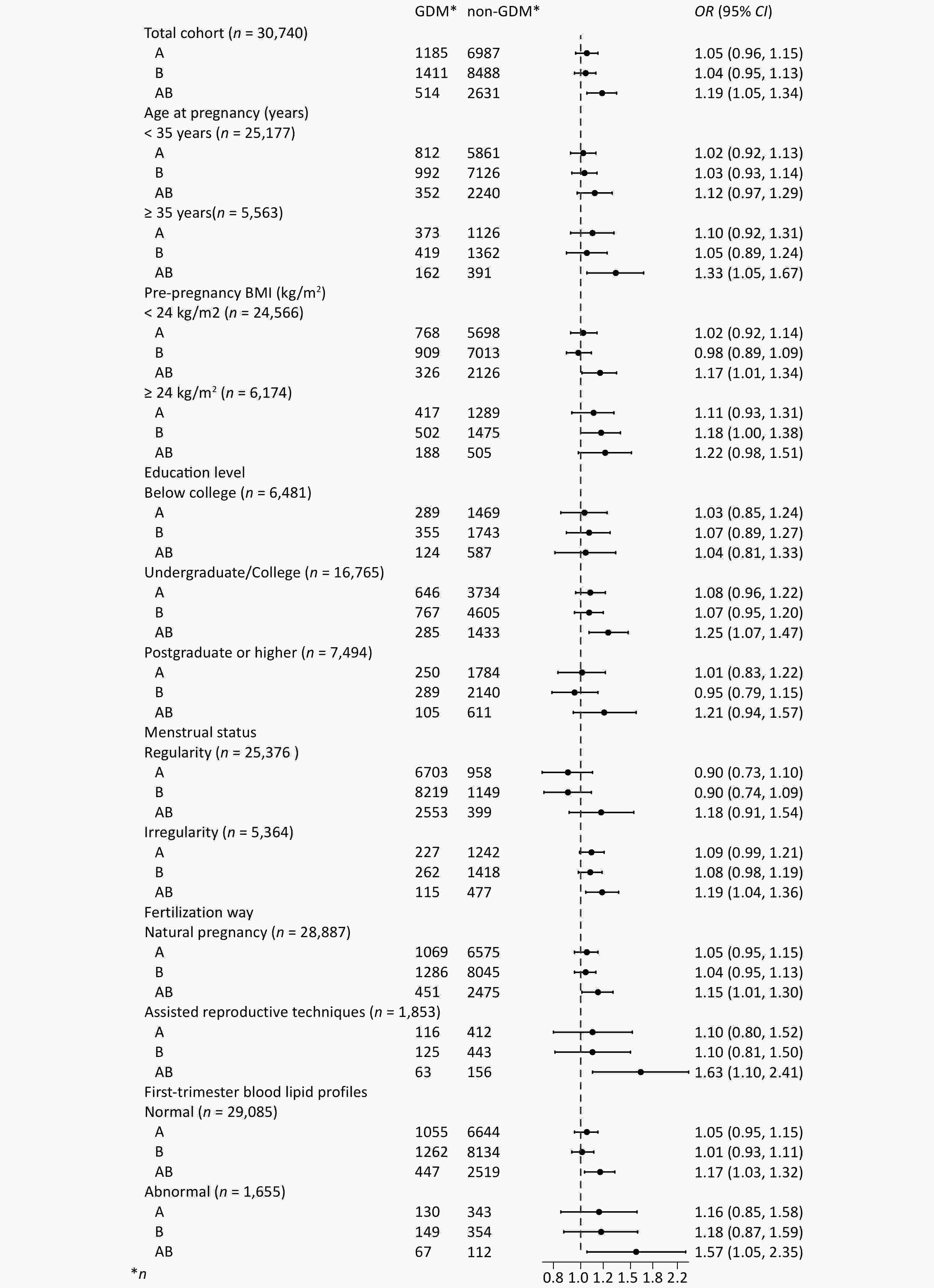
Pregnant women bearing the AB blood type experienced increased incidence rates of GDM, compared to those with type O (Figure 2). The univariate analysis results revealed that the ORs (95% CIs) for GDM in pregnant women with blood types A and B were 1.05 (0.96-1.14) and 1.03 (0.95-1.11) respectively, demonstrating no statistical significance (P > 0.05). Pregnant women with the AB blood type were found to have a 21% increased risk of GDM compared to those with the O blood type (OR = 1.21, 95% CI = 1.08-1.35) (P < 0.05) (Supplementary Table S3). After adjusting for factors such as age at pregnancy, education level, family annual income, pre-pregnancy BMI, smoking, menstrual status, primigravida status, history of GDM, history of adverse pregnancy outcomes, fertilization way, multivitamin supplementation, as well as early pregnancy laboratory measurements including WBC and RBC counts, HGB, TG, LDL-c, HDL-c, and FBG levels, the risk of GDM in pregnant women with the AB blood type was still 19% higher than in those with the O blood type (OR = 1.19, 95% CI = 1.05-1.34). (Figure 3, Supplementary Table S3).
The results of the age subgroup analysis showed that among pregnant women aged < 35 years, the risk of GDM in women with blood type AB was 12% higher than in those with blood type O (95% CI = 0.97-1.29). Among pregnant women aged ≥ 35 years, the risk of developing GDM was 33% higher in those with the AB blood type than in those with the O blood type (95% CI = 1.05-1.67). For pregnant women with either a normal or abnormal pre-pregnancy BMI, as well as those with natural pregnancy or assisted reproductive techniques, those with normal or abnormal early pregnancy blood lipids, the AB blood type all emerged as a risk factor for the occurrence of GDM. When compared to pregnant women with blood type O, the ORs (95% CIs) for GDM in pregnant women with the AB blood type, for a pre-pregnancy BMI of < 24 kg/m2 and ≥ 24 kg/m2, were 1.17 (1.01-1.34) and 1.22 (0.98-1.51), respectively. For pregnant women with natural pregnancy and assisted reproductive techniques, the ORs (95% CIs) were 1.15 (1.01-1.30) and 1.63 (1.10-2.41), respectively. In the case of the AB blood type with normal and abnormal early pregnancy blood lipids, the ORs (95% CIs) for GDM were 1.17 (1.03-1.32) and 1.57 (1.05-2.35), respectively. (Figure 3, Supplementary Table S3).
The figure demonstrates that pregnant women bearing the AB blood type had an increased risk of GDM, compared to those with type O. The increased risk with type AB also remained consistent in subgroups.GDM: gestational diabetes mellitus, CNY: Chinese Yuan, BMI: body mass index, WBC: white blood cell, RBC: red blood cell, HGB: hemoglobin, TC: total cholesterol, TG: triglyceride, LDL-c: low-density lipoproteins cholesterol, HDL-c: high-density lipoproteins cholesterol, FBG: fasting blood glucose, OR odds ratio, CI confidence interval. Adjusted for age at pregnancy, education level, family annual income, pre-pregnancy BMI, smoking, menstrual status, primigravida status, history of GDM, history of adverse pregnancy outcomes, fertilization way, multivitamin supplementation, and first-trimester laboratory measurements including WBC and RBC counts, HGB, TG, LDL-c, HDL-c, and FBG levels.
-
Participants who with a history of GDM (n = 722) were further excluded from the analysis, due to an increased risk of recurrence. The results showed pregnant women with the AB blood type retained an increased risk of developing GDM, the ORs (95% CIs) were 1.18 (1.04-1.33), respectively (Supplementary Table S4). Among the 30,740 subjects included in this study, covariates data on blood glucose and blood lipid measurements were incomplete (10.7%, n = 3292). The missing data may potentially affect the analysis of the relationship between blood types and GDM (Supplementary Table S5). Thus we employed deletion of missing data and multiple imputation methods to handle missing data. After direct deletion, the ORs (95% CIs) for the risk of GDM in pregnant women with blood types O, A, B, and AB were 1.05 (0.95-1.15), 1.03 (0.94-1.13), and 1.18 (1.04-1.34), respectively. After multiple imputation, the ORs (95% CIs) for the risk of GDM in pregnant women with blood types A, B, and AB were 1.05 (0.96-1.15), 1.04 (0.95-1.13), and 1.19 (1.05-1.34), respectively (Supplementary Table S6).
-
Based on a prospective birth cohort, this study found that ABO blood types have a correlation with the risk of GDM. Pregnant women bearing the AB blood type were found to have an increased risk of developing GDM. The associations remained stable following comprehensive adjustments for confounding factors, as well as subgroup and sensitivity analyses. This prospective birth cohort study encompassed over 30,000 pregnant women to thoroughly evaluate the association between ABO blood types and the risk of GDM. The findings are poised to offer robust epidemiological evidence regarding the relationship between ABO blood types and GDM.
Globally, the incidence of GDM varies depending on the study population, screening methods used, or diagnostic criteria. The incidence of GDM among pregnant women in this study cohort was 14.4%, which was basically consistent with the previously reported GDM incidence in Chinese population (14.8%)[40]. This study found that, in addition to ethnicity, alcohol consumption and folic acid supplementation, several macro-level traditional risk factors and clinical blood detection of molecular factors were associated with GDM. The traditional risk factors included age at pregnancy, education level, family annual income, pre-pregnancy BMI, smoking status, menstrual history, primigravida status, history of GDM, history of adverse pregnancy outcomes, fertilization way, and multivitamin supplementation. Molecular factors included first-trimester laboratory measurements such as WBC and RBC counts, HGB, TG, LDL-c, HDL-c, and FBG levels. This is also consistent with the results of previous studies on the risk factors for GDM[41]. ABO blood type is the most important blood type system in humans. The distribution of ABO phenotypes varies by racial/ethnic origin and geographic region. Our survey of over 30,000 pregnant women revealed that the distribution frequencies of blood types O, A, B, and AB were 31.0%, 26.6%, 32.2%, and 10.2%, respectively. The frequency was similar to that previously reported in the Chinese population (29.4% for type A, 26.8% for type B, 33.7% for type O, 10.1% for type AB)[26]. Among the baseline influencing factors, education level, menstrual status, fertilization way, WBC, HGB, FBG, TC, HDL-c, LDL-c were correlated with blood type distribution. Therefore, when analyzing the relationship of ABO blood types and GDM, these factors were included as covariates in the multivariate model. After adjusting for these factors, the results still indicated that AB blood type was an independent risk factor for GDM. Furthermore, subgroup analyses also suggested that the AB blood type was associated with an increased risk of GDM across various subgroups.
The results regarding the relationship between ABO blood type phenotypes and the GDM risk have been inconsistent[24,42]. Chen et al. have reviewed and summarized studies concerning the relationship between ABO blood types and gestational complications, such as GDM, finding that existing evidence supports an increased risk of preeclampsia (PE) associated with the AB blood type. The O or AB blood types may be correlated with the incidence of GDM; however, the evidence is limited due to a paucity of epidemiological studies with consistent outcomes[42,43]. In this prospective birth cohort study involving Chinese pregnant women, it was found that ABO blood types are associated with the onset of GDM, with the AB blood type presenting as a risk factor for GDM. In this study, 2.35% of pregnant women (n = 722) had a history of GDM. Therefore, we excluded these pregnant women from the sensitivity analysis. The OR for the relationship between AB blood type and GDM risk remained largely unchanged (1.19 vs. 1.18), suggesting that AB blood type is associated with an increased risk of GDM. This consistency may be attributed to the relatively small number of women with a history of GDM in this study population. A case-control study by Karagoz Hatice et al. in Turkey demonstrated that the proportion of pregnant women with the AB blood type was significantly higher in the GDM group compared to the control group (12% vs. 8%, P = 0.029)[33]. Another retrospective case-control study by Shimodaira Masanori et al. in Japan, which included 5424 pregnant women (149 with GDM)[27], also identified AB blood type as a risk factor for GDM (OR 2.73, 95% CI: 1.64-4.57), corroborating the findings of this study. In a cohort study by Lemaitre. et al. in France involving 1194 pregnant women (1069 with GDM), it was found that pregnant women with the AB blood type had a higher likelihood of developing GDM compared to those with type O (OR 2.50, 95% CI 1.43-4.36)[32], which also aligns with our findings.
However, some studies found no significant association between ABO blood types and GDM, including relatively small retrospective analyses conducted in Mexico[35], Saudi Arabia[36], Sudan[34], and Pakistan[29]. These studies had sample sizes ranging from 253 to 5320 cases, with the number of GDM cases varying between 17 and 333. The reasons for the inconsistency between these studies and our study may be related to factors such as retrospective design, small sample size, limited covariate adjustments, or genetic variations across different populations.
Conversely, two studies identified a decreased risk with group AB[26,30]. An Israeli retrospective cohort study by Rom et al.[30]using hospital records found that blood type AB was associated with a lower risk of GDM compared to other blood types, after adjusting for three confounding factors: maternal age, parity, and number of pregnancies. The reasons for the inconsistent results may stem from ethnic differences between Israelis and Chinese, as well as variations in the diagnostic methods and criteria for GDM. In the study by Rom et al., the distribution percentages of blood types O, A, B, and AB in pregnant women were 36.9%, 36.5%, 19.6%, and 7%, respectively. In contrast, in our study population, the corresponding percentages for each blood types were 31.0%, 26.6%, 32.2%, and 10.2%, respectively. Ethnic differences may contribute to variations in ABO blood type distribution and genetic susceptibility to GDM. Additionally, Rom et al.’s study employed a two-step approach to diagnose GDM. The initial screening involved a 50-g, 1-h glucose screening test, where pregnant women with plasma glucose levels ≥130 mg/dl (or whole blood ≥119 mg/dl) proceeded to a 100-g, 3-h OGTT. GDM was diagnosed based on the Carpenter and Coustan criteria. In our study, a one-step diagnosis of GDM was adopted, using the 75-g, 2-h OGTT, according to the IADPSG criteria. The different testing methods and diagnostic criteria resulted in significant differences in the prevalence of GDM between the study populations (10.7% vs. 14.4%), which may explain the differences between our study and Rom et al.’s study.
Interestingly, in a study of 14,198 pregnant women conducted in China, Zhang et al.[26] found that blood type AB was associated with a lower risk of GDM compared to non-AB blood types. A comparison of the study designs suggests that the differences in results may be attributed to variations in diagnostic methods, GDM incidence, and demographic characteristics. The study by Zhang et al. was conducted in Tianjin, China, from 2010 to 2012. All pregnant women initially underwent a non-fasting 50-g, 1-h glucose challenge test (GCT) at approximately 65 primary hospitals in Tianjin. Pregnant women with 1-h blood glucose ≥ 7.8 mmol/L were referred to a tertiary hospital for a standard 75-g, 2-h OGTT. Our study was conducted in Beijing, China, from 2019 to 2021. All pregnant women were enrolled in a large tertiary hospital during the first trimester, where they underwent baseline epidemiological investigation, early trimester blood biochemical testing, follow-up OGTT test, and other routine prenatal examinations. Due to differences in GDM screening strategies and methods, the incidence of GDM differed significantly between the two populations (7.5% in Zhang et al.’s study vs. 14.4% in ours), nearly twice as high in our study. This may be one of the main reasons for the inconsistent conclusions between the two studies.
Overall, evidence for an association between ABO blood types and GDM remains limited and inconsistent. Compared to previous studies, our study is based on a larger birth cohort with relatively more comprehensive information. In addition to adjusting for macro-level risk factors, we further accounted for first-trimester molecular indicators such as WBC and RBC counts, HGB, TG, LDL-c, HDL-c, and FBG levels. We also conducted detailed subgroup analyses. The findings of this study provide new evidence from a birth cohort regarding the relationship between ABO blood types and GDM. However, given the inconsistent results in current studies, further validation in larger, multicenter cohorts is warranted. If our current findings can be replicated, the ABO blood type could potentially be included as a risk factor for GDM. Incorporating the ABO blood type phenotype—either as a single risk factor or in combination with other known factors—could facilitate the identification of individuals at risk for GDM before or during early pregnancy. This would enable healthcare professionals and obstetricians to implement timely screening and management measures, thereby reducing the incidence and adverse outcomes of GDM within the population.
The current understanding of the role of ABO blood types in the context of GDM is primarily based on observational studies, providing limited insight into the underlying biological mechanisms. Existing research indicates that the association between ABO blood types and GDM may be mediated through various biological mechanisms. These mechanisms include various genetic variants, molecular markers linked to inflammation and endothelial dysfunction, the composition of gut microbiota, and potentially other as-yet-unidentified factors[1]. First of all, GDM, akin to type II diabetes, is postulated to be a multifaceted disease influenced by polygenic inheritance, environmental factors, and their interplay[1]. Research has revealed that certain genetic variants, which play a role in the regulation of insulin secretion, are implicated in the susceptibility to GDM that is linked to insulin resistance[41]. The differentiation of ABO blood types is a result of the glycosylation patterns of the H antigen, a product of the FUT1 gene, orchestrated by the ABO gene. Despite the four primary ABO phenotypes—O, A, B, and AB—there is a considerable genetic diversity with around 100 mutations underlying these categories. Typically, the AB blood type exhibits two distinct glycosylations corresponding to A and B antigens, that is, variations of the H antigen. It is posited that the variation in glycosylation of targets beyond the H antigen may contribute to the correlation between ABO blood types and the incidence of GDM[22,44]. Secondly, research has also indicated that ABO antigens can influence various inflammatory markers (such as TNF-α and IL-6) and endothelial function-related molecular markers (such as E-selectin and sICAM-1)[1,15]. These biomarkers have been strongly linked to the pathogenesis of insulin resistance and type 2 diabetes and could also be implicated in the development of GDM, owing to the similar etiological pathways[19,23,44,45]. Thirdly, genes associated with the ABO blood types may influence the composition of the gut microbiota, thereby affecting energy homeostasis, glucose metabolism, and inflammatory responses[46-48]. In addition, this study found that among pregnant women undergoing assisted reproductive technology (ART), those with an AB blood type had a higher risk of developing GDM compared to those who conceived naturally. ART typically involves the use of a variety of hormones, such as follicle-stimulating hormone (FSH) and human chorionic gonadotropin (HCG), to stimulate follicle development. These hormones can reduce insulin sensitivity, leading to increased insulin resistance and a higher risk of developing GDM. Besides, pregnant women undergoing ART are often older and may have pre-existing health conditions, such as infertility. The emotional stress and anxiety associated with infertility could also impact the endocrine system, increasing levels of stress hormones like cortisol. These elevated stress hormones may affect insulin sensitivity, further raising the risk of GDM.
In this research, we explored the association between ABO blood type and GDM risk utilizing a prospective birth cohort approach, thereby contributing evidence to the link between ABO blood type phenotypes and GDM. Based on the abundant risk factor information collected in the cohort, multi-factor adjustment regression models were employed in the analysis to adjust for confounders, including first-trimester RBC count, blood glucose, and lipid levels, elucidating ABO blood type’s impact on GDM. The cohort study was designed to avoid the influence of recall bias on the results.
However, this study also has certain limitations. Firstly, of the initial cohort of pregnant women who met the inclusion criteria, some pregnant women did not undergo ABO blood types testing or GDM screening in the hospital, leading to partial sample loss (n = 2,067). These missing sample could impact the study’s findings. We conducted a comparative analysis of the baseline epidemiological characteristics between pregnant women included in this study (Group A, 30,740 cases) and those with missing ABO blood types data or GDM follow-up losses (Group B, 2,067 cases) (Supplementary Table S7). The results show no significant differences in most of the covariates. For the covariates with statistical differences, the impact on results was minimal, as their actual distribution values were not significantly different. Thus the impact on the results might be minimal. Secondly, physical activity, nutritional status, and weight gain during pregnancy are significant factors influencing the occurrence of GDM. However, data on these three variables were not collected in our cohort. This represents one of the limitations of the study. However, we collected factors correlated with the clinical physiology of these factors, including pre-pregnancy BMI, early pregnancy hemoglobin, lipids, and blood glucose, and included them as adjustment factors in the analysis. The results indicated that blood type AB remained associated with GDM after adjusting for baseline macro risk factors and micro-indicators such as WBC, RBC, HGB, blood lipids and glucose. Thirdly, the absence of blood glucose (11%, n = 3271) and lipid profiles (4%, n = 1301) information may have influenced the results. We used direct deletion and multiple imputation for missing data in sensitivity analysis. The results showed that after direct deletion and multiple imputation, the relationship between ABO blood types and GDM remained consistent. Lastly, although it is a large sample cohort study with more than 30,000 subjects, being a single-center cohort study limits the generalizability of our findings. Further multicenter, large-scale, prospective cohort studies are warranted to examine the relationship between ABO blood type phenotypes and GDM risk.
-
In conclusion, this prospective birth cohort study suggests ABO blood types may serve as a novel risk factor for GDM. Specifically, the AB blood type is associated with a higher risk of developing GDM. Early screening and prevention of GDM are of significant clinical importance. Identifying risk factors during prenatal care and early pregnancy may aid in the early prediction and prevention of GDM. Blood types, a low-cost and convenient test with stable phenotypes throughout an individual's life, is essential in assessing its association with GDM risk. If our current findings can be replicated in studies with larger and more diverse populations across different countries, the ABO blood types could potentially be included as a risk factor for GDM. Incorporating the ABO blood type phenotype—either as a single risk factor or in combination with other known factors—could facilitate the identification of individuals at risk for GDM before or during early pregnancy. This would allow healthcare professionals or obstetricians to implement timely screening and management measures, thereby reducing the incidence and adverse outcomes of GDM within the population.
Association between ABO Blood Types and the Risk of Gestational Diabetes Mellitus: A Prospective Cohort Study
doi: 10.3967/bes2025.046
- Received Date: 2024-11-06
- Accepted Date: 2025-03-04
-
Key words:
- ABO blood type phenotype /
- Cohort study /
- Risk factors /
- Gestational diabetes mellitus
Abstract:
All authors have stated that they have no competing interest.
The study protocol was approved by the Ethics Committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University (approval number: 2018-KY-003-02). All individual participants gave their informed consent according to the guidelines of the Helsinki declaration.
&These authors contributed equally to this work.
| Citation: | Shuanghua Xie, Shuangying Li, Shaofei Su, Enjie Zhang, Shen Gao, Yue Zhang, Jianhui Liu, Minhui Hu, Ruixia Liu, Wentao Yue, Chenghong Yin. Association between ABO Blood Types and the Risk of Gestational Diabetes Mellitus: A Prospective Cohort Study[J]. Biomedical and Environmental Sciences. doi: 10.3967/bes2025.046 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: