-
Preterm birth (PTB), defined as delivery before 37 weeks of gestation, is the most common adverse pregnancy outcome[1]. PTB is a global health concern, with an estimated 13.4 million cases in 20201, accounting for more than one in 10 births worldwide. Compared to full-term births, PTBs are associated with a higher risk of short- and long-term complications, including bronchopulmonary dysplasia, necrotizing enterocolitis, visual impairment, and cerebral injuries[2]. Despite substantial research efforts to prevent PTB, the global PTB rate has shown little improvement over the past decade1. Therefore, identifying additional risk factors remains a critical goal in preventing PTB.
Air pollutants exert harmful effects on human health[3], and recognized as significant environmental risk factor for adverse pregnancy outcomes[4]. Among these pollutants, fine particulate matter (PM2.5) is a major contributor to adverse pregnancy outcomes, including PTB. PM2.5 comprises various chemical constituents—black carbon (BC), organic matter (OM), nitrate (NO3−), ammonium (NH4+), and sulfate (SO42−)—which differ in their toxicity levels. A limited number of studies have investigated the relationship between specific PM2.5 constituents and PTB risk[4]. Although some studies have identified BC, NO3−, SO42−, and NH4+ as key contributors to PTB[4], gene-environment interactions suggest that the effects of these constituents may be more complicated than previously understood.
The H19 imprinted gene plays a critical role in fetal development. Research has shown that the rs2107425 polymorphism in H19 is associated with H19 mRNA expression[5]. Reduced expression of H19 has been linked to decreased in vitro migration and invasion of extravillous trophoblasts, ultimately affecting embryonic growth[6]. Additionally, the rs2067051 polymorphism has been associated with birth weight defects[7]. However, only a few studies have investigated the impact of maternal preconception H19 gene variation on the risk of PTB.
Given this, the present study focuses on two maternal single-nucleotide polymorphisms (SNPs), rs2067051 and rs2107425, with the hypothesis that preconception H19 gene variation is associated with the risk of PTB and may modify the relationship between exposure to PM2.5 chemical constituents and the risk of PTB. Understanding the complex patterns of gene-environment interactions associated with PTB risk may provide important insights for genetic counseling and targeted public health interventions.
A total of 6,000 women planning pregnancies (1,000 per county) residing in six districts (Supplementary Table S1) were recruited using successive sampling. With the support from local Maternal and Child Health Hospitals, China, professional nursing staff conducted preconception examinations, collected fasting peripheral blood samples, and performed genotyping. Ultrasound imaging was performed to check pregnancy status. Among the participants, 1,874 women who conceived within one year of their preconception examination and subsequently delivered live singleton infants were included in the study. These pregnancies occurred between June 2016 and June 2018. Demographic and clinical data collected from preconception examinations and birth records included mother’s age, preconception body mass index (BMI, kg/m2), passive exposure to smoke (defined as exposure for at least 15 minutes per day over a period of at least one month), education level (middle school or below, and high school or above), season of conception (warm season: April–September; cold season: October–March), residential address, date of the last menstrual period, parity (primiparous and multiparous), date of delivery, gestational age, and infant sex (boy or girl). After excluding participants who were not genotyped due to insufficient DNA sample size or genotyping failure (n = 25), urban residents (n = 1), and women with gestational ages outside the viable range (< 20 or > 44 weeks, n = 2), a total of 1,846 mother-infant pairs were included in the study (Supplementary Table S1).
Daily average estimates of PM2.5, BC, OM, NO3−, NH4+, and SO42− were obtained at a 10 km2 resolution using data from the Tracking Air Pollution, China (TAP) (http://tapdata.org.cn)[8,9]. The concentrations of PM2.5 constituents from TAP were highly consistent with the observed measurements, with correlation coefficients exceeding 0.67 for all species[8,9]. To assess maternal exposure, the geographical coordinates (latitude and longitude) of the mothers’ residential addresses were extracted using Baidu Maps API (https://lbsyun.baidu.com/). Meteorological data, including ambient temperature (T, °C), relative humidity (RH, %), and concentrations of other air pollutants (PM10, inhalable particles; CO, carbon monoxide; SO2, sulfur dioxide; NO2, nitrogen dioxide; O3, ozone), were obtained as described in a previous study.10 Exposure windows were calculated based on the last menstrual period and the date of delivery. Four exposure periods were defined: first trimester (Trimester 1, weeks 1–12), second trimester (Trimester 2, weeks 13–27), third trimester (Trimester 3, ≥28 weeks), and the entire pregnancy.
Two SNPs in the H19 gene (rs2067051 and rs2107425, Supplementary Table S2) were selected using Haploview software, with inclusion criteria of minor allele frequency > 0.05 and low linkage imbalance (LD, r2 < 0.8). Genomic DNA was extracted from maternal peripheral blood samples using the Blood Genomic DNA Extraction Kit (Shanghai Generay Biotechnology Corporation, Shanghai, China). Genotypes of the selected SNPs were determined using the Improved Multiple Ligase Detection Reaction (iMLDRTM) technique (Shanghai Genesky Biotechnologies Inc., Shanghai, China), which utilizes double ligation and multiplex fluorescence Polymerase Chain Reaction (PCR). Ligation reactions were conducted using an ABI3730XL thermal cycler (Supplementary Tables S3–S4). All samples met quality control standards, with SNP call rates > 95%, Hardy-Weinberg equilibrium test P > 0.05, and no amplification was observed in negative controls.
Descriptive statistics (percentages, means, and standard deviations) were calculated for the mother-infant pairs. Chi-square (χ2) test and Student’s t-test were used to compare differences between PTB and term birth groups. Pearson’s correlation analysis was used to assess the correlations among T, RH, PM2.5, and its constituents. Logistic regression models were used to evaluate the association between PM2.5, its constituents, H19 gene variants (rs2067051 and rs2107425), and PTB risk. Additionally, a weighted quantile sum (WQS) regression model was employed to estimate the overall combined impact of PM2.5 constituents on PTB risk and identify the key contributing pollutants. Subgroup analyses were conducted based on the SNP genotypes to examine the potential effects of modifications by H19 variants. Interaction terms (Pollutants * SNP) were included in the logistic regression models assessing PM2.5 constituents and PTB associations. Furthermore, generalized multifactor dimensionality reduction (GMDR) was used to investigate gene-environment interactions between H19 variants and PM2.5 constituents concerning PTB risk, using 10-fold cross-validation (http://ibi.zju.edu.cn/software/). Binary variables for PM2.5 and its constituents were created using their medians as cutoff points. Model performance was assessed by testing the balanced accuracy (TBA), cross-validation consistency (CVC), and P-values. TBA measured the accuracy of interaction models in predicting PTB status; CVC-reflected model stability and P-values assessed statistical significance. Known risk factors and potential confounders were adjusted for as covariates in the models, including maternal age, preconception BMI, season of conception, education level, exposure to smoke, parity, infant sex, T, and RH. Variable assignments are provided in Supplementary Table S5.
To assess the robustness of findings, a dual-pollutant model (“PM2.5 constituent + co-pollutant”) was applied to examine the stability of the associations between PM2.5 constituents and PTB risk. Sensitivity analyses were conducted by restricting the population to women aged 18–35 (excluding adolescents and older mothers) and excluding parity as a covariate due to missing data (≈15%).
GMDR analyses were conducted using GMDR software version 1.0, and all other statistical analyses were performed using R software version 4.3.3. Interaction effects were considered statistically significant at P < 0.10, while P < 0.05 was considered statistically significant for all other analyses.
Among the 1,846 mother-infant pairs enrolled in this study, 94 infants were considered with PTB. The mean maternal age and preconception BMI were 26.4 ± 3.66 years and 22.3 ± 3.50 kg/m2, respectively. The PTB group had a higher proportion of male infants compared to the term birth group (P < 0.05) (Supplementary Table S1). Additionally, the frequency of the T allele of rs2107425 was significantly higher among mothers in the PTB group (P < 0.05) (Supplementary Table S1).
The average concentrations of PM2.5 and its constituents during the entire pregnancy were as follows: BC, OM, NO3−, NH4+, and SO42− were 67.34, 2.78, 14.69, 16.20, 10.45, and 11.88 μg/m3, respectively (Supplementary Figure S1). Furthermore, the average RH and T were 62.94% and 15.40°C, respectively. At all pregnancy stages, PM2.5 and its constituents showed high positive correlations with each other and were negatively correlated with RH and T (P < 0.05) (Supplementary Figure S1).
Overall, exposure to PM2.5 and its constituents was associated with an increased risk of PTB (Table 1). Adjusted model estimates indicated that each 10.0 μg/m3 increase in PM2.5 concentration was associated with a 0.47-fold increase in PTB risk (95% confidence interval [CI]: 1.19–1.83). For the entire pregnancy, exposure to BC (2.57, 95% CI: 1.52–4.43), OM (1.20, 95% CI: 1.08–1.33), NO3− (1.15, 95% CI: 1.06–1.26), NH4+ (1.26, 95% CI: 1.10–1.46), and SO42− (1.28, 95% CI: 1.13–1.46) was significantly associated with increased PTB risk. Further, these associations remained stable in the dual-pollutant model (Supplementary Table S6).
Crude Adjusted OR (95% CI) P OR (95% CI) P PM2.5 Entire pregnancy 1.09 (0.94, 1.27) 0.270 1.47 (1.19, 1.83) < 0.001 Trimester 1 1.05 (0.96, 1.13) 0.281 1.23 (1.07, 1.41) 0.003 Trimester 2 1.01 (0.91, 1.11) 0.878 1.16 (0.98, 1.37) 0.079 Trimester 3 1.07 (0.97, 1.18) 0.164 1.29 (1.10, 1.52) 0.002 BC Entire pregnancy 1.17 (0.81, 1.71) 0.400 2.57 (1.52, 4.43) < 0.001 Trimester 1 1.12 (0.92, 1.33) 0.243 1.62 (1.19, 2.20) 0.002 Trimester 2 0.97 (0.76, 1.21) 0.762 1.19 (0.80, 1.75) 0.393 Trimester 3 1.13 (0.92, 1.38) 0.242 1.66 (1.16, 2.36) 0.006 OM Entire pregnancy 1.03 (0.96, 1.11) 0.460 1.20 (1.08, 1.33) < 0.001 Trimester 1 1.01 (0.98, 1.06) 0.321 1.10 (1.03, 1.17) 0.003 Trimester 2 0.99 (0.95, 1.04) 0.871 1.05 (0.97, 1.13) 0.266 Trimester 3 1.02 (0.98, 1.07) 0.343 1.10 (1.02, 1.18) 0.015 NO3− Entire pregnancy 1.02 (0.96, 1.08) 0.531 1.15 (1.06, 1.26) 0.001 Trimester 1 1.01 (0.98, 1.04) 0.451 1.07 (1.02, 1.13) 0.008 Trimester 2 0.99 (0.97, 1.03) 0.960 1.05 (0.99,1.12) 0.118 Trimester 3 1.02 (0.99, 1.06) 0.253 1.09 (1.02, 1.16) 0.007 NH4+ Entire pregnancy 1.03 (0.94, 1.14) 0.513 1.26 (1.10, 1.46) 0.001 Trimester 1 1.02 (0.97, 1.07) 0.416 1.12 (1.03, 1.21) 0.009 Trimester 2 0.99 (0.94, 1.06) 0.894 1.08 (0.97, 1.20) 0.164 Trimester 3 1.03 (0.97, 1.09) 0.283 1.14 (1.03, 1.26) 0.010 SO42− Entire pregnancy 1.09 (0.99, 1.21) 0.089 1.28 (1.13, 1.46) < 0.001 Trimester 1 1.05 (0.98, 1.11) 0.149 1.15 (1.05, 1.25) 0.002 Trimester 2 1.02 (0.95, 1.10) 0.592 1.11 (1.01, 1.23) 0.042 Trimester 3 1.07 (0.99, 1.15) 0.058 1.19 (1.08, 1.32) < 0.001 Note. Odds ratio (OR) and 95% confidence interval (95% CI)was estimated per 10.0 ug/m3 in PM2.5 and 1.0 ug/m3 increment in BC, OM, NO3−, NH4+, and SO42−. PTB, preterm birth; PM2.5, fine particulate matter; BC, black carbon; OM, organic matter; NO3−, nitrate; NH4+, ammonium; SO42−, sulfate. Table 1. Association between risk of PTB and exposure to PM2.5 constituents.
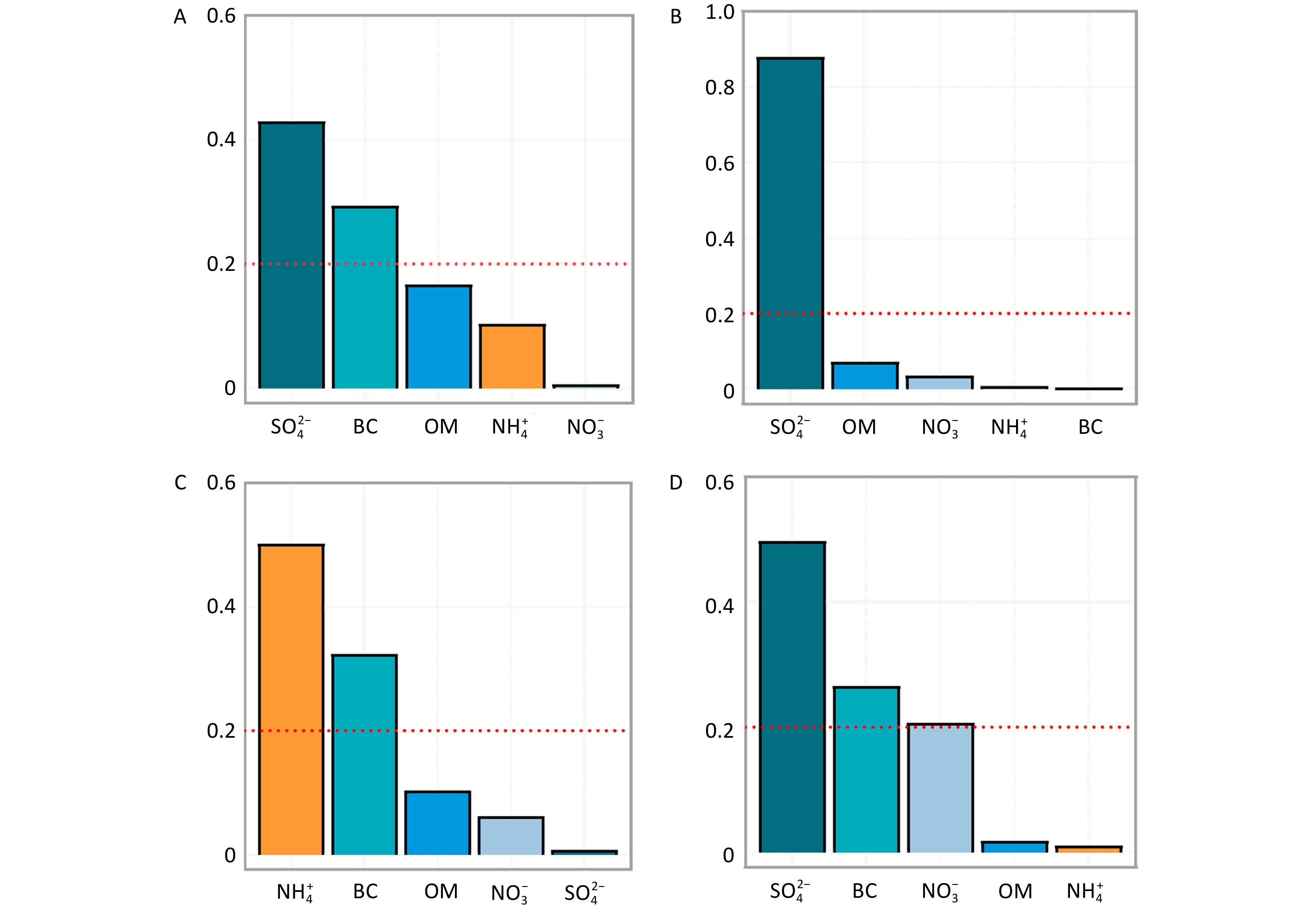
The results of the WQS regression analysis further supported the contribution of five constituents (BC, OM, NO3−, NH4+, and SO42−) to the risk of PTB (entire pregnancy: 1.68, 95% CI: 1.19–2.36; trimester 1: 1.51, 95% CI: 1.07–2.14; trimester 3: 1.98, 95% CI: 1.25–3.12) (Supplementary Table S7). Among these, SO42− and BC were identified as the major contributors, with SO42− playing a particularly significant role (Figure 1). SO42− is a secondary pollutant primarily originating from industrial activities, power generation, and vehicular emissions[4], while BC results mainly from gasoline combustion, such as traffic exhaust emissions[4]. These findings provide pertinent evidence supporting PTB prevention through traffic pollution control and reduction of coal combustion in factories.
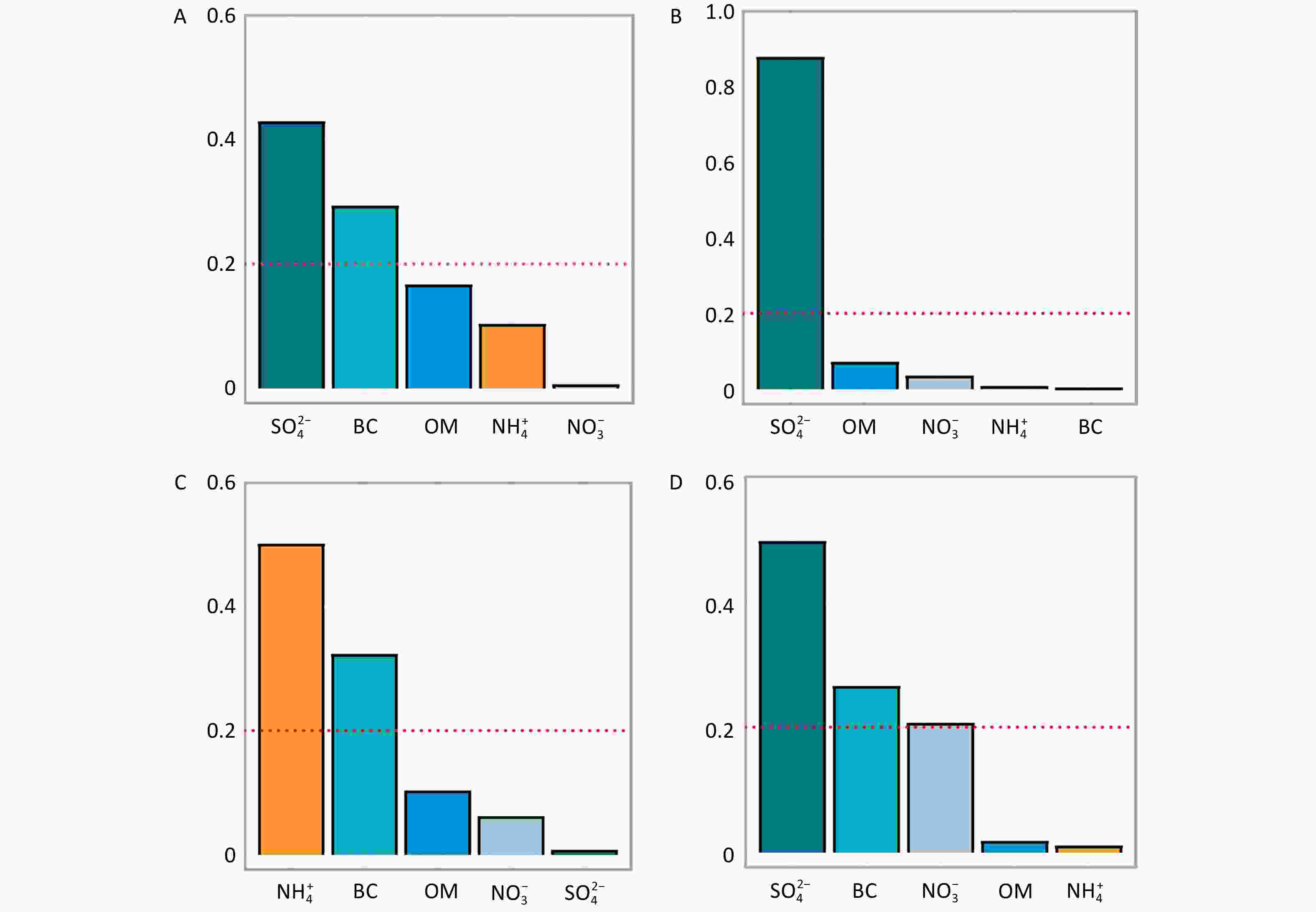
Figure 1. Weighted Quantile Sum (WQS) regression–Estimated weights of PM₂.₅ constituents associated with preterm birth (PTB). PM2.5, fine particulate matter; BC, black carbon; OM, organic matter; NO3−, nitrate; NH4+, ammonium; SO42−, sulfate. A-D represented the entire pregnancy, trimester 1, trimester 2, and trimester 3, respectively.
Logistic regression analysis showed a significantly increased risk of PTB among mothers carrying the rs2107425 CT genotype (2.60, 95% CI: 1.50–4.81) or CT+TT genotypes (2.42, 95% CI: 1.42–4.42), compared to those with the CC genotype (Supplementary Table S8). However, rs2067051 was not significantly associated with the risk of PTB (Supplementary Table S8).
Furthermore, rs2107425 significantly interacted with PM2.5 and its constituents (BC, OM, NO3−, NH4+, and SO42−) (Figure 2 and Supplementary Figure S2). Mothers carrying the rs2107425 CT genotype were more susceptible to the effects of PM2.5 constituent exposure throughout pregnancy than those carrying the CC genotype (P-interaction < 0.10). Stratified analysis based on maternal genotypes revealed that mothers carrying the rs2067051 C allele or rs2107425 T allele had an increased risk of PTB associated with PM2.5 and its constituents, particularly among those with the rs2067051 CC or rs2107425 CT genotypes (P<0.05). Sensitivity analyses that excluded adolescents and older mothers (Supplementary Tables S9 and Supplementary Figure S3) or did not adjust for parity (Supplementary Tables S9 and Supplementary Figure S4) yielded consistent results.
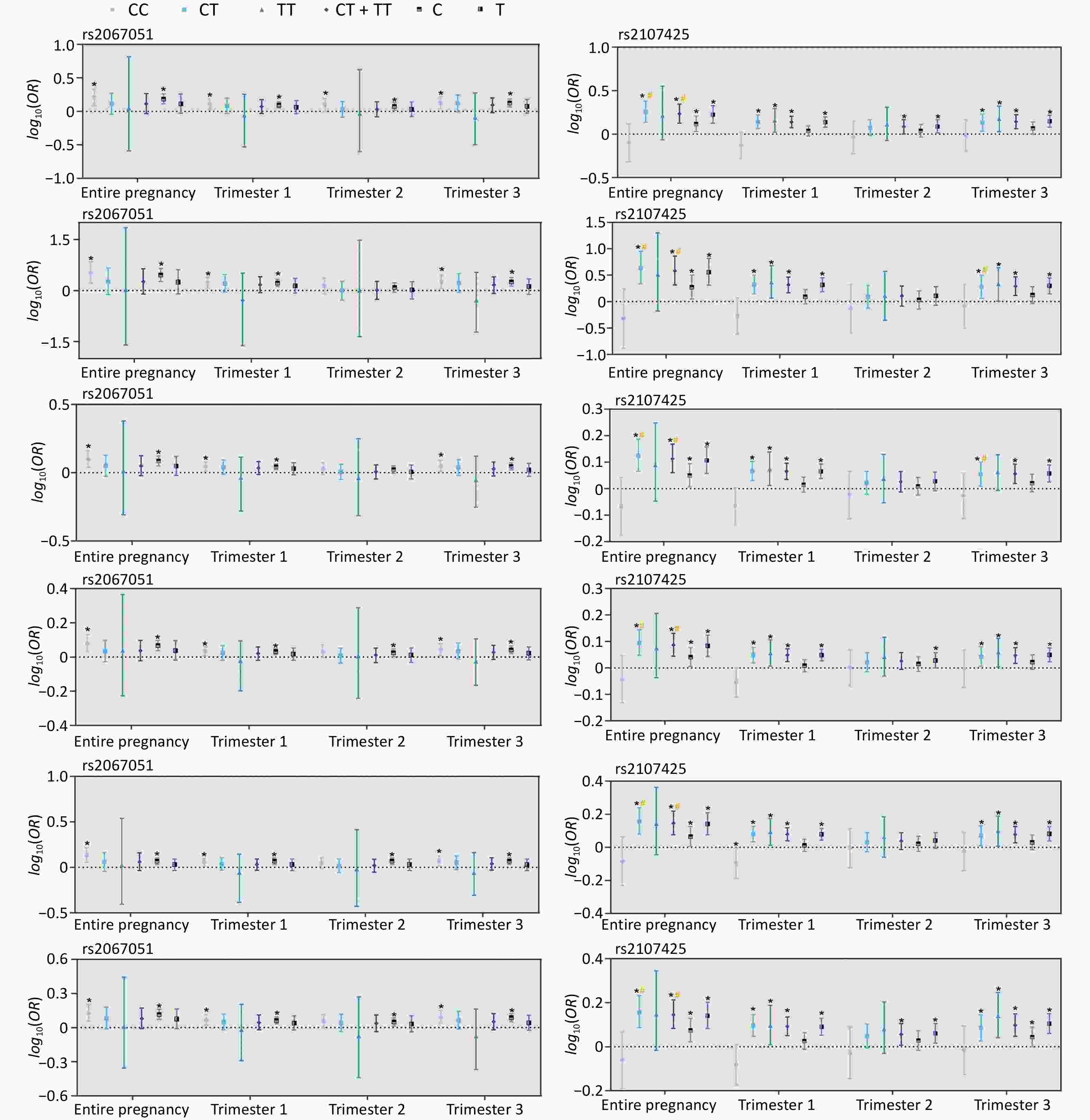
Figure 2. Association between preterm birth (PTB) risk and constituents of PM2.5 stratified by maternal genotypes (rs2067051, rs2107425). * denoted a P < 0.05. # is P-interaction < 0.10, compared with the CC genotype or C allele in rs2067051 or rs2107025. PM2.5, fine particulate matter; BC, black carbon; OM, organic matter; NO3−, nitrate; NH4+, ammonium; SO42−, sulfate.
A GMDR analysis was performed to further evaluate gene-environment interactions. Interaction models, such as PM2.5*rs2107425*rs2067051 and SO42−*rs2107425, showed significant differentiation (all P = 0.0547), indicating potential gene-environment interactions between PM2.5 constituents and these two SNPs. Based on TBA and CVC, the PM2.5*rs2107425*rs2067051 model was identified as optimal (TBA, 58.15%; CVC, 10/10) (Supplementary Table S10). The risk distributions of the Pollutants*rs2107425*rs2067051 models are shown in Supplementary Figure S5. Particularly, mothers carrying the rs2107425 CT genotype, exposed to PM2.5 concentrations above the median (>50th percentile), and carrying the rs2067051 CC genotype had the highest PTB risk and the highest summed score in the optimal model. Similarly, the SO42−*rs2107425 model showed the highest PTB risk when high SO42− exposure was combined with the rs2107425 CT genotype (Supplementary Figure S5). Based on the interaction analysis, we speculate that mothers carrying the rs2107425 CT and rs2067051 CC genotypes may be more vulnerable to the effects of PM2.5, ultimately leading to impaired fetal development and an increased risk of PTB. However, further experimental studies are required to explore the mechanisms underlying this interaction.
This study had several limitations. Although the five constituents analyzed in this study do not represent the entirety of PM2.5, their significant contribution to the total PM2.5-mass supports the relevance of our findings. The exposure assessment was solely based on the maternal residential address during pregnancy, without accounting for maternal mobility, which may have introduced potential exposure misclassification. Given the regional and population-specific limitations of this study, future studies should aim to validate these findings in a more diverse population. Additionally, we could not adjust for socioeconomic factors and the health status of a broader cohort of pregnant women due to data access restrictions. It is also important to consider that individuals with higher socioeconomic status may have greater health awareness and, consequently, might be more likely to participate in preconception health examinations, potentially introducing a selection bias. However, the biological mechanisms underlying these associations remain unclear. Further animal studies are required to elucidate these mechanistic pathways and validate our findings. Additionally, there are inherent biases in calculating gestational age based on the last menstrual period; a more accurate method, such as ultrasound imaging, will be employed in future studies. Finally, this study did not explore the critical windows of vulnerability during pregnancy, which will be addressed in future studies.
In summary, exposure to PM2.5 and its constituents—particularly SO42− and BC—is associated with an increased risk of PTB. In addition, mothers carrying the rs2107425 CT genotype were more susceptible to PM2.5 and its constituents compared to those with the CC genotype. These findings underscore the importance of PM2.5 control measures and highlight the gene-environment interaction pathway involving H19 gene polymorphisms.
Association between PM2.5 Chemical Constituents and Preterm Birth: The Undeniable Role of Preconception H19 Gene Variation
doi: 10.3967/bes2025.076
- Received Date: 2025-02-09
- Accepted Date: 2025-05-20
The authors declare that they have no conflict of interest.
All participants were thoroughly briefed on the research protocol and provided written informed consent. Health examinations and the collection of biological samples were approved by the Ethics Committee of the Henan Academy of Population and Family Planning Science and Technology. The use of data and detection of biomarkers were approved by the Ethical Committees of the Henan Institute of Reproductive Health Science and Technology (HIRHST-IRB2020-02) and Zhengzhou University (ZZUIRB2021-67).
&These authors contributed equally to this work.
| Citation: | Yalong Wang, Panpan Sun, Xinying Wang, Junxi Zhang, Xiangyu Yu, Jian Chai, Ruo Du, Wenyi Liu, Fangfang Yu, Yue Ba, Guo Yu Zhou. Association between PM2.5 Chemical Constituents and Preterm Birth: The Undeniable Role of Preconception H19 Gene Variation[J]. Biomedical and Environmental Sciences. doi: 10.3967/bes2025.076 |







 Quick Links
Quick Links
 DownLoad:
DownLoad:
