-
Cardiopulmonary diseases, including cardiovascular diseases (CVDs) and chronic obstructive pulmonary disease (COPD), are chronic conditions with complex etiologies that pose significant health risks[1]. Currently, CVDs are the leading contributors to the global disease burden[2], and respiratory diseases account for over four million premature deaths annually[3]. Blood pressure plays a crucial role in the prevalence of CVDs: diastolic blood pressure (DBP) is independently associated with the occurrence of stroke and coronary heart disease (CHD), while systolic blood pressure (SBP) is strongly correlated with the overall prevalence of CVDs[4]. Evidence is emerging regarding the critical role of environmental factors in the onset and progression of CVDs. Exposure to environmental pollutants significantly increases the risk of CHD and chronic respiratory diseases[5]. Overall, we applied two selection principles: first, pollutants should be ubiquitous, exhibiting high concentrations across various environmental media and human biological samples; second, they must have demonstrated a relatively high toxic potential based on previous studies. For example, polycyclic aromatic hydrocarbons (PAHs), which are ubiquitous in the environment and among the most common pollutants, are primarily ingested via dietary intake, inhalation, and skin contact[6]. Exposure to PAHs is strongly associated with an increased risk of various CVDs[7]. Furthermore, exposure to PAHs has been linked to adverse respiratory outcomes, particularly among occupational groups wherein toxic effects are most pronounced[8]. Bisphenol A (BPA) is the most widely used bisphenol compound; it is primarily used in the synthesis of plastics and resins. By 2018, the global production of BPA had reached approximately eight million tons per year[9], with China being a leading producer[10]. Animal experiments have demonstrated that BPA exhibits endocrine-disrupting activity and poses non-mutagenic and noncarcinogenic risks to humans. Phthalates (PAEs), a group of endocrine-disrupting chemicals (EDCs) widely used as plasticizers[11], represent another class of environmental pollutants associated with metabolic disorders, reproductive toxicity, and endocrine disruption[12]. Phthalates are widely present in the atmosphere, soil, sediments, and other environments because of their extensive production and use. Their teratogenicity and carcinogenicity have been demonstrated[13]. Urban areas, especially indoor environments, typically exhibit higher PAE concentrations than rural regions[14]. Parabens (PBs), which share toxicological characteristics with EDCs, are commonly used as preservatives in food and personal care products[15]. They pose potential risks to the endocrine system and may exacerbate cardiopulmonary diseases[16]. Therefore, addressing cardiopulmonary health issues induced by various environmental pollutants is critically important.
Oxidative stress is a well-recognized mediator of cardiopulmonary damage and serves as a critical link between environmental pollution and vascular endothelial dysfunction, atherosclerosis, and other CVDs[17]. Reactive oxygen species (ROS), generated under oxidative stress, can cause oxidative damage to DNA, proteins, and lipids, leading to cellular dysfunction and the onset of various diseases[18]. In addition, oxidative stress contributes to airway inflammation and impaired lung function[19]. Elevated oxidative stress levels are observed in patients with asthma, for whom oxidants play a role in pathogenesis and antioxidants exhibit therapeutic potential. Reduced antioxidant capacity and increased oxidative stress are common pathways linking exposure to organic pollutants with CVDs, such as hypertension, atherosclerosis, and respiratory diseases (including COPD[20]). Focusing on pollutant exposure’s effects on oxidative stress-related biomarkers can reveal the potential pathways through which pollutants induce cardiorespiratory impairment. For example, PAHs are metabolically activated in vivo to produce reactive oxygen and nitrogen species, elevate oxidative stress, and induce DNA mutations, inflammatory responses, and tissue damage[21]. A study measuring urinary 1-hydroxypyrene (1-OH-PYR) in 120 children from China and South Korea found a positive correlation between urinary hydroxy polycyclic aromatic hydrocarbons OH-PAHs and concentrations of the oxidative stress biomarker malondialdehyde (MDA)[18]. Similarly, a cross-sectional study reported that PAH exposure was associated with elevated MDA levels and oxidative DNA damage, as indicated by increased 8-hydroxy-2'-deoxyguanosine (8-OHdG) concentrations[22]. Significantly higher levels of oxidative stress biomarkers, including 8-iso-prostaglandin F2α (8-isoPGF2α) and 8-OHdG, have been observed among coke oven workers occupationally exposed to PAHs[23]. Hence, investigating the adverse effects of environmental pollutants on cardiopulmonary health and the mediating role of oxidative stress are crucial for safeguarding public health.
Previous studies have focused predominantly on the adverse effects of individual pollutants on cardiopulmonary health. However, for the general urban population, exposure levels to most pollutants are typically well controlled and often remain below occupational exposure thresholds. Given that humans are exposed to environmental pollutants through multiple pathways, examining the health effects of combined exposure routes is a pressing research need. The study design is illustrated in Figure 1. We conducted a repeated-measures randomized crossover study among healthy adults in Beijing, China, and comprehensively investigated the association between exposure to typical environmental organic pollutants and cardiopulmonary health by examining the potential mediating role of oxidative stress in a mixed-exposure scenario.
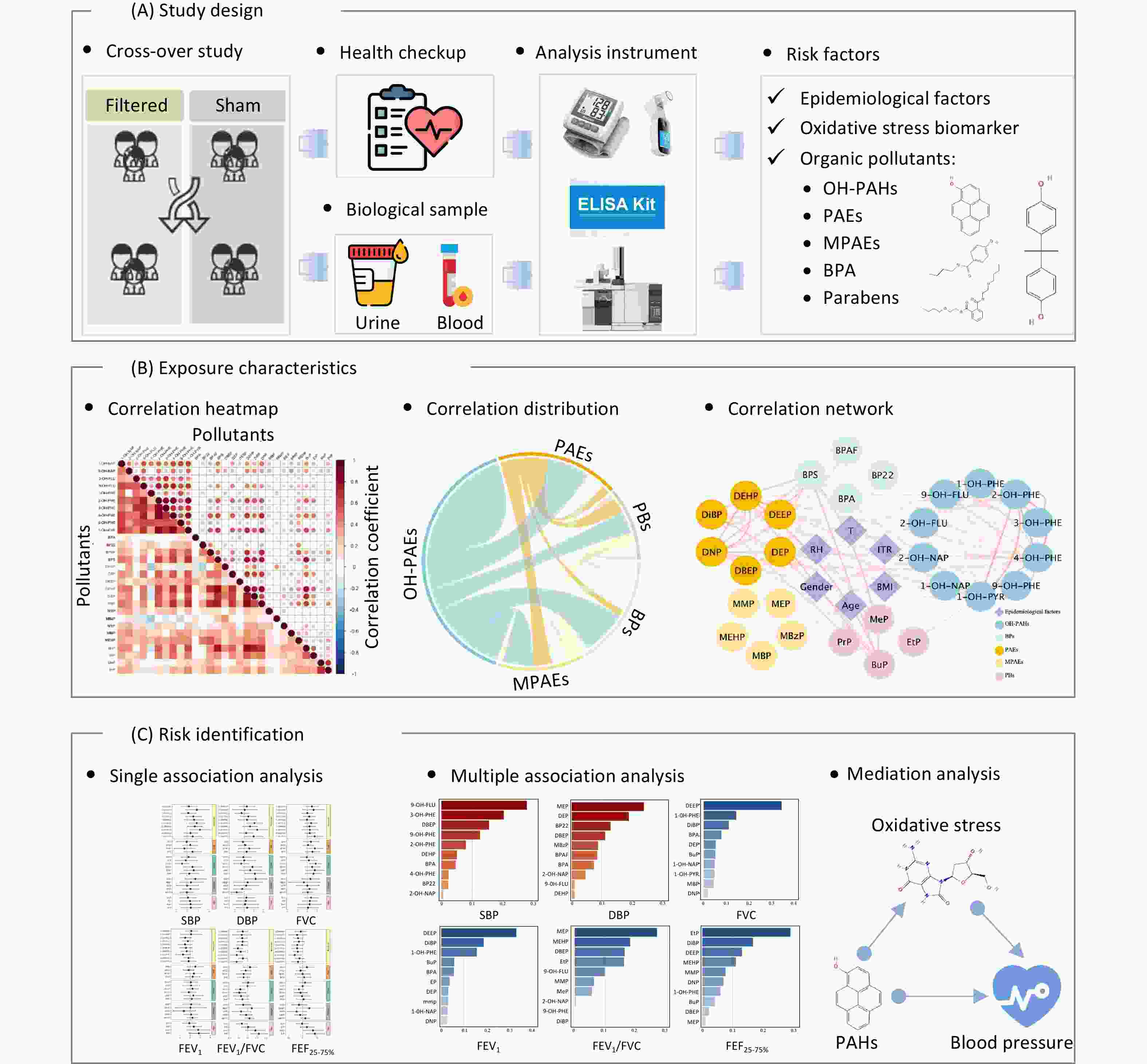
Figure 1. Overview of the study. (A) Study design: we conducted a repeated-measures, randomized crossover design; 46 healthy college students were recruited and randomly assigned to either the active air purifier intervention group (Filtered) or the sham air purifier control group (Sham). The epidemiological factors, oxidative stress biomarkers, and organic pollutants (OPs) in the biological samples were measured using various analytical instruments. (B) Exposure characteristics: interconnectivity between environmental exposures measured in urine samples, which were described using a correlation heatmap, circular correlation distribution, and correlation network. (C) Risk identification: investigating the effects of exposure to multiple environmental OPs on cardiopulmonary health—including diastolic blood pressure (DBP), systolic blood pressure (SBP), forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), FEV1/FVC, and forced expiratory flow between 25% and 75% of vital capacity (FEF25-75%)—using an association analysis and focusing on the potential mediating role of oxidative stress (e.g., mediation role of oxidative stress in the association between PAH exposure and blood pressure). OH-PAHs, monohydroxy-metabolites of polycyclic aromatic hydrocarbons; BPs, bisphenol A and substitutes; PAEs, phthalates; MPAEs, phthalates metabolites; PBs, parabens. Other abbreviations are given in Supplementary Table S1.
-
This study was based on a repeated-measures randomized crossover design conducted between December 2017 and April 2018. The participant recruitment flowchart is shown in Supplementary Figure S1. The overall study protocol has been described in detail in a previous study[24]. Briefly, 46 healthy college students from Beijing were recruited and randomly assigned to either the active air purifier intervention group or sham air purifier control group. Following a seven-day exposure period and subsequent one-week washout phase, participants crossed over to the alternative group using the same protocol. At the conclusion of each intervention phase, the participants completed health examinations. First-morning urine and fasting venous blood samples were collected simultaneously. First-morning urine samples were collected between 8:00 a.m. and 8:30 a.m. after fasting for at least 8 h. Urine samples were immediately centrifuged at 3000 rpm for 10 min and subpacked into polypropylene tubes. All samples were transported at 4 °C and frozen at -80 °C until the analysis was conducted. All participants provided written informed consent; the study was approved by the Biomedical Ethics Committee of Peking University.
-
Our study focused on several classes of typical organic pollutants (OPs), including parent compounds and their metabolites, such as hydroxylated polycyclic aromatic hydrocarbons (OH-PAHs), PAEs and their metabolites (MPAEs), BPA and its substitutes (BPs), and PBs. A total of 29 environmental exposures were assessed, and morning urine samples were collected from the study participants to measure their concentrations. The details are presented in Supplementary Table S1.
-
On the morning after each intervention period, the participants underwent a comprehensive health examination that included seated blood pressure measurements and pulmonary function testing. Pulmonary function parameters, including forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), the ratio of FEV1 to FVC (FEV1/FVC), and forced expiratory flow between 25% and 75% of vital capacity (FEF25–75%), characterize lung health and are frequently used to diagnose and monitor lung or airway injuries[1]. We measured the oxidative DNA damage biomarker (i.e., 8-OHdG) and a lipid peroxidation marker (i.e., MDA) in urine samples, another lipid peroxidation marker (i.e., 8-isoPGF2α), and two antioxidant enzymes (i.e., glutathione peroxidase 1 (GPx1), extracellular superoxide dismutase (EC-SOD)) in blood samples.
-
The detailed extraction procedures of the urinary pollutants are provided in the supplementary section, “Section I: Urinary Extraction Protocol of Organic Pollutants” (SM). Briefly, urine samples were buffered with acetic acid, followed by hydrolysis. The target chemical mixture was extracted, cleaned, and derivatized. Twenty-nine OPs were quantified by gas chromatography (GC, 7890 B; Agilent, Santa Clara, CA, US) coupled with triple-quadrupole tandem mass spectrometry (MS/MS, 7010 B; Agilent Co., USA). The GC parameters are shown in the supplementary section, “Section II: GC Parameters” (SM). Quantification- and qualification-related information, as well as the limit of quantification (LOQ), for the target pollutants are provided in Supplementary Table S2. The concentration was calibrated by the urine creatine concentration with the unit “μg/g crea.” The collected blood samples were analyzed using commercial assay kits to quantify the biomarkers related to systemic oxidative stress. 8-isoPGF2α, GPx1, and EC-SOD were each measured with an enzyme-linked immunosorbent assay (ELISA). The MDA levels were assessed using the thiobarbituric acid derivatization method. The reagent kits used to measure GPx1 and EC-SOD were sourced from Cusabio (China). Kits for 8-isoPGF2α and MDA were obtained from Cayman (USA) and Cell Biolabs (USA), respectively. The pulmonary function parameters FVC, FEV1, FEV1/FVC, and FEF25–75% were measured using a portable Pony FX spirometer (COSMED, Italy) following the standardized procedures in American Thoracic Society (ATS) and European Respiratory Society (ERS) guidelines. Seated blood pressure was measured on the upper arm using an Omron cuff-based electronic sphygmomanometer. Blood pressure was measured at least three times during each examination, with a minimum interval of one minute between each measurement. The average of the second and third measurements was used as the final blood pressure value.
-
Categorical variables were described using frequencies and proportions; continuous variables were expressed as the mean ± standard deviation (SD). The median (interquartile range (IQR), 25%–75% quartiles) was reported for non-normally distributed data. Associations between urinary pollutant concentrations and cardiopulmonary health indicators were analyzed using linear mixed-effects (LME) models. Standardized pollutant concentrations were included as independent variables along with age, sex, body mass index (BMI), proportion of indoor activity time, indoor temperature, and relative humidity (fixed effects). The identity and dormitory of each participant were treated as random variables (i.e., random effects). Considering the reality of mixed environmental pollution exposure, the effects of co-exposure to multiple environmental pollutants on cardiopulmonary health indicators were further analyzed using a weighted quantile sum (WQS) regression model. WQS indices (ranging from 0 to 1) were calculated based on the quartiles of environmental exposure to identify the relative importance of each environmental exposure to the overall combined effect[25]. The model-fitting process performed 1,000 bootstrap iterations, splitting the full set of samples into training (40%) and validation (60%) subsets for each iteration. A mediation analysis within the mixed-effects model was conducted according to the previous method[26]. Descriptive statistics, association analyses, and mediation analyses were performed using R software (version 4.3). Statistical tests were two-tailed, with a significance level of α = 0.05. The LME models were implemented using the package “lmer,” and WQS models were implemented using the package “gWQS.” Mediation analyses were conducted using the mediation package.
-
Our study ultimately included 46 healthy college students from Beijing (40 males); their basic characteristics are shown in Table 1. Both the values of mean ± SD and median (IQR) are provided in the table. The average age of the participants was 21.3 years, and their average BMI was 21.6 kg/m². No significant difference in average indoor activity time between the intervention and nonintervention periods was observed. The participants’ average blood pressure levels were 109.12 mmHg for SBP and 62.01 mmHg for DBP. Pulmonary function indicators included an average FVC of 4.29 L, FEV1 of 3.69 L, FEV1/FVC of 86.47%, and FEF25-75% of 4.02 L/s.
Characteristics Mean ± SD Median (IQR) Age, year 21.3 ± 2.09 21 (20, 22) BMI, kg/m2 21.6 ± 2.56 21.7 (19.9, 23.3) Indoor activity time proportion, % Sham 73.7 ± 7.05 73 (69, 78.75) Filtered 74.28 ± 9.08 73.5 (69, 80.75) Temperature, °C 26.63 ± 1.18 26.61 (25.62, 27.31) Relative humidity, % 27.98 ± 6.95 28.83 (22.44, 34.56) Blood pressure, mmHg SBP 109.12 ± 8.88 108.25 (103.38, 115.08) DBP 62.01 ± 7.29 60.59 (57, 65.54) Pulmonary function parameters FVC, L 4.29 ± 0.73 4.22 (3.91, 4.77) FEV1, L 3.69 ± 0.59 3.69 (3.34, 4.05) FEV1/FVC, % 86.47 ± 5.71 87 (84, 90) FEF25−75%, L/s 4.02 ± 0.78 4 (3.52, 4.31) Note. Mean ± SD, mean ± standard deviation; median (IQR), interquartile range (25–75% quartiles); BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; FEV1/FVC, the ratio of FEV1 to FVC; FEF25−75%, forced expiratory flow between 25% and 75% of vital capacity. Table 1. Population characteristics
-
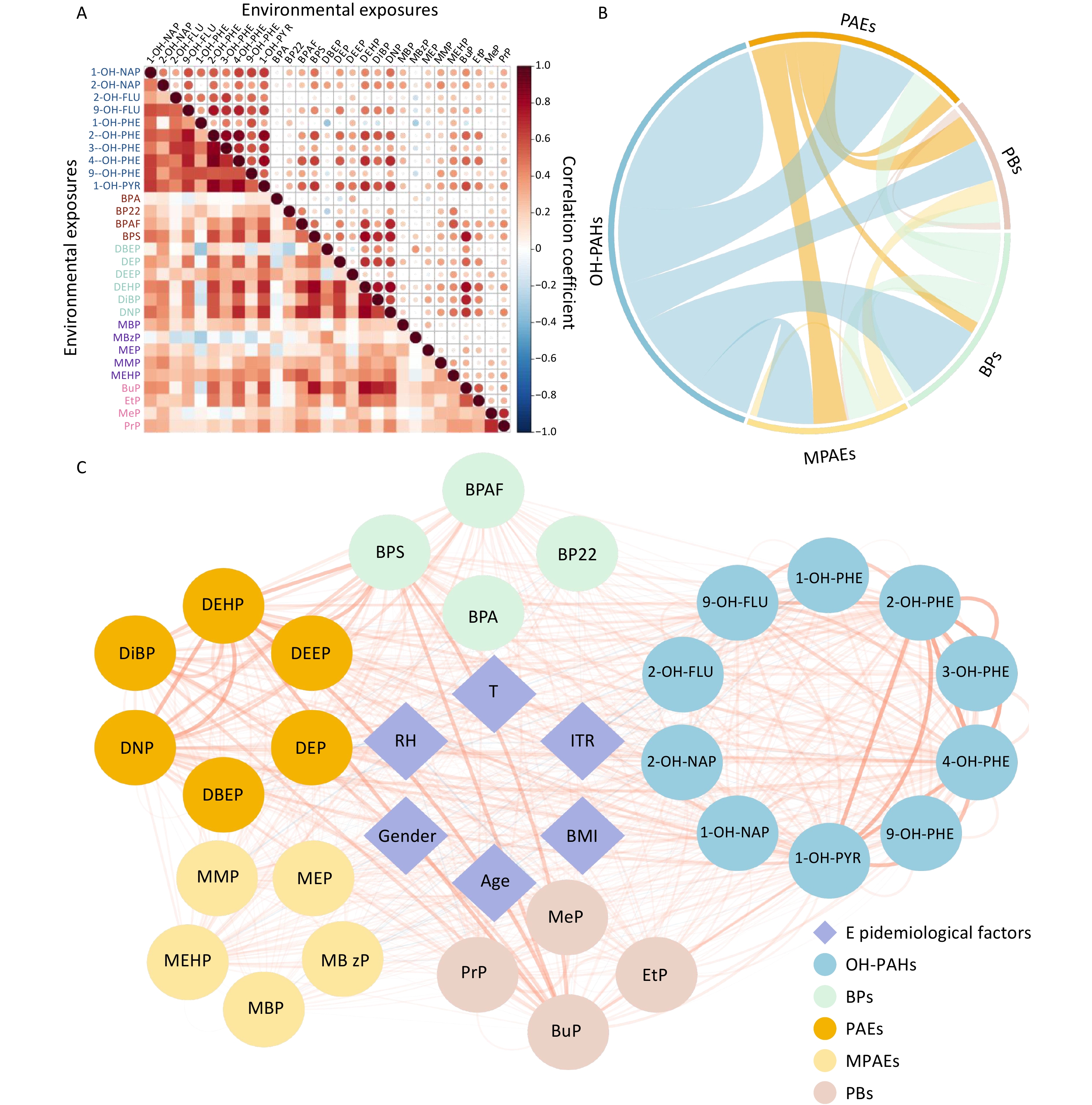
The concentrations of environmental pollutants in urine are presented in Table 2, and the correlation analysis results are shown in Figure 2. No statistically significant differences were observed between the intervention and nonintervention groups for any of the urinary pollutant concentrations, although some pollutants showed a decreasing trend in the intervention group. The correlations among urinary pollutant concentrations varied widely; Spearman’s rank correlation coefficients ranged from −0.33 to 0.88, and the majority were positive (Figure 2A). Correlation analyses were conducted both within and between exposure groups (Figure 2B). Intragroup correlations were notably stronger than intergroup correlations, with the highest intergroup correlation (79.54%) observed between OH-PAHs and BPs. Additionally, the correlations between urinary pollutant concentrations and epidemiological factors were analyzed (Figure 2C). Although significant associations were identified, the correlation coefficients were notably lower than those observed within exposure groups.
Exposure Sham Filtered Pa OH-PAHs 1-OH-NAP 0.699 ± 0.71b 0.599 ± 0.629 0.59 2-OH-NAP 1.35 ± 2.21 1.40 ± 1.35 0.13 2-OH-FLU 0.249 ± 0.126 0.239 ± 0.111 0.52 9-OH-FLU 0.678 ± 0.824 0.56 ± 0.485 0.57 1-OH-PHE 0.144 ± 0.134 0.125 ± 0.0825 0.66 2-OH-PHE 0.278 ± 0.189 0.252 ± 0.102 0.71 3-OH-PHE 0.11 ± 0.0734 0.096 ± 0.058 0.51 4-OH-PHE 0.172 ± 0.192 0.136 ± 0.0636 0.8 9-OH-PHE 0.0236 ± 0.0234 0.0198 ± 0.0181 0.21 1-OH-PYR 0.305 ± 0.19 0.28 ± 0.125 0.9 BPAs BPA 1.21 ± 2.69 1.64 ± 4.82 0.3 BP22 0.189 ± 0.169 0.181 ± 0.188 0.48 BPAF 0.27 ± 0.595 0.186 ± 0.165 0.77 BPS 0.405 ± 0.344 0.449 ± 0.257 0.1 PAEs and MPAEs DBEP 1.87 ± 1.39 2.17 ± 1.46 0.15 DEP 0.178 ± 0.387 0.108 ± 0.0655 0.74 DEEP 2.69 ± 1.85 2.47 ± 1.62 0.62 DEHP 29.7 ± 28.2 28.4 ± 16 0.11 DiBP 7.04 ± 7.55 7.21 ± 4.55 0.14 DNP 0.101 ± 0.0946 0.0893 ± 0.044 0.49 MBP 93.1 ± 122 85.4 ± 68.2 0.83 MBzP 0.876 ± 1.24 0.995 ± 1.23 0.1 MEP 19.5 ± 30.3 15.7 ± 21 0.54 MMP 24 ± 20.6 24.3 ± 15.9 0.51 MEHP 0.733 ± 0.504 0.787 ± 0.576 0.97 PBs BuP 2.34 ± 1.75 2.43 ± 1.36 0.31 EtP 11.9 ± 17.3 13.7 ± 26.6 0.62 MeP 59.6 ± 143 50.1 ± 79.8 0.46 PrP 22.6 ± 75.5 29.1 ± 76.5 0.32 Note.a The Wilcoxon rank-sum test was used to compare differences in urinary pollutants between nonintervention and intervention states. b Mean ± standard deviation, concentration unit: μg/g creatine. OH-PAHs, monohydroxy-metabolites of polycyclic aromatic hydrocarbons; BPs, bisphenol A and substitutes; PAEs, phthalates; MPAEs, phthalate metabolites; PBs, parabens. Other abbreviations are listed in Supplementary Table S1. Table 2. Urinary pollutant concentrations under different conditions
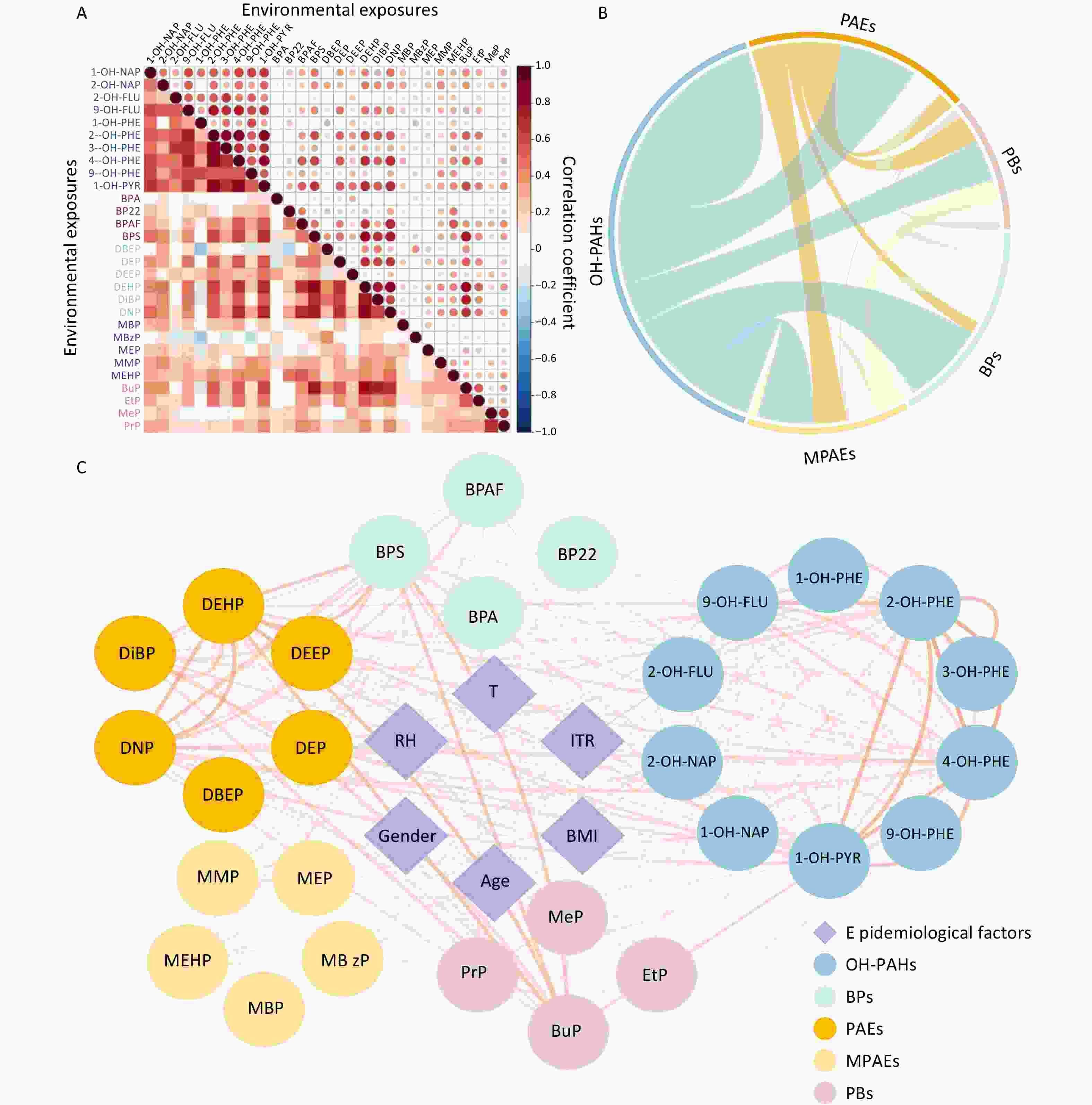
Figure 2. Interconnectivity between environmental pollutant cncentrations measured in urine samples. (A) Heatmap of the correlation coefficients for the concentrations of different pollutants. The colors in the legend represent Spearman’s rank correlation coefficients. (B) Summary of the intercorrelations of exposures across various groups. (C) Correlation network between urinary pollutant concentrations and epidemiological factors. The nodes represent exposure features and epidemiological factors. Node colors represent different groups of features. The edges represent the absolute Spearman’s rank correlation coefficients between features. Red lines represent a positive correlation; blue lines represent a negative correlation. OH-PAHs, monohydroxy-metabolites of polycyclic aromatic hydrocarbons; BPs, bisphenol A and substitutes; PAEs, phthalates; MPAEs, phthalates metabolites; PBs, parabens. Other abbreviations are given in Supplementary Table S1.
-
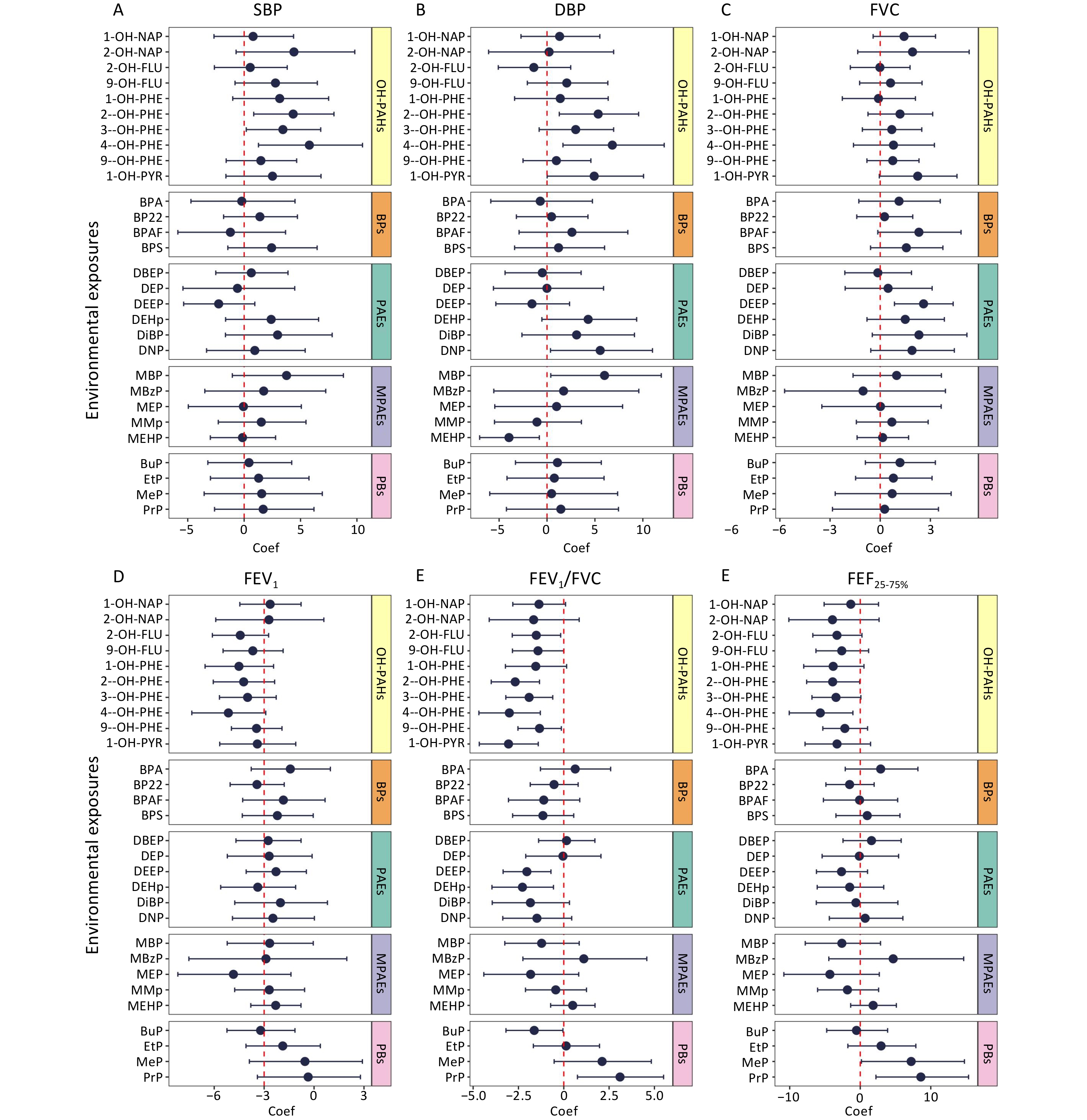
Associations between urinary pollutant concentrations and cardiopulmonary health indicators, including blood pressure and pulmonary function, were analyzed using LME models. The associations are shown in Figure 3, and detailed data are provided in Supplementary Table S3. Several urinary pollutants were significantly and positively associated with blood pressure. Specifically, each unit increase in the natural log-transformed urinary concentrations of 2-hydroxyphenanthrene (2-OH-PHE), 3-hydroxyphenanthrene (3-OH-PHE), and 4-hydroxyphenanthrene (4-OH-PHE) was associated with a 4.35% (95% CI: 0.85%, 7.97%), 3.44% (95% CI: 0.19%, 6.79%), and 5.78% (95% CI: 1.27%, 10.5%) increase in SBP, respectively. Similarly, each unit increase in the natural log-transformed urinary concentrations of 2-OH-PHE, 4-OH-PHE, monobutyl phthalate (MBP), and dinonyl phthalate (DNP) was associated with increases in DBP of 5.34% (95% CI: 1.28%, 9.56%), 6.82% (95% CI: 1.66%, 12.2%), 6% (95% CI: 0.39%, 11.9%), and 5.55% (95% CI: 0.36%, 11%), respectively.
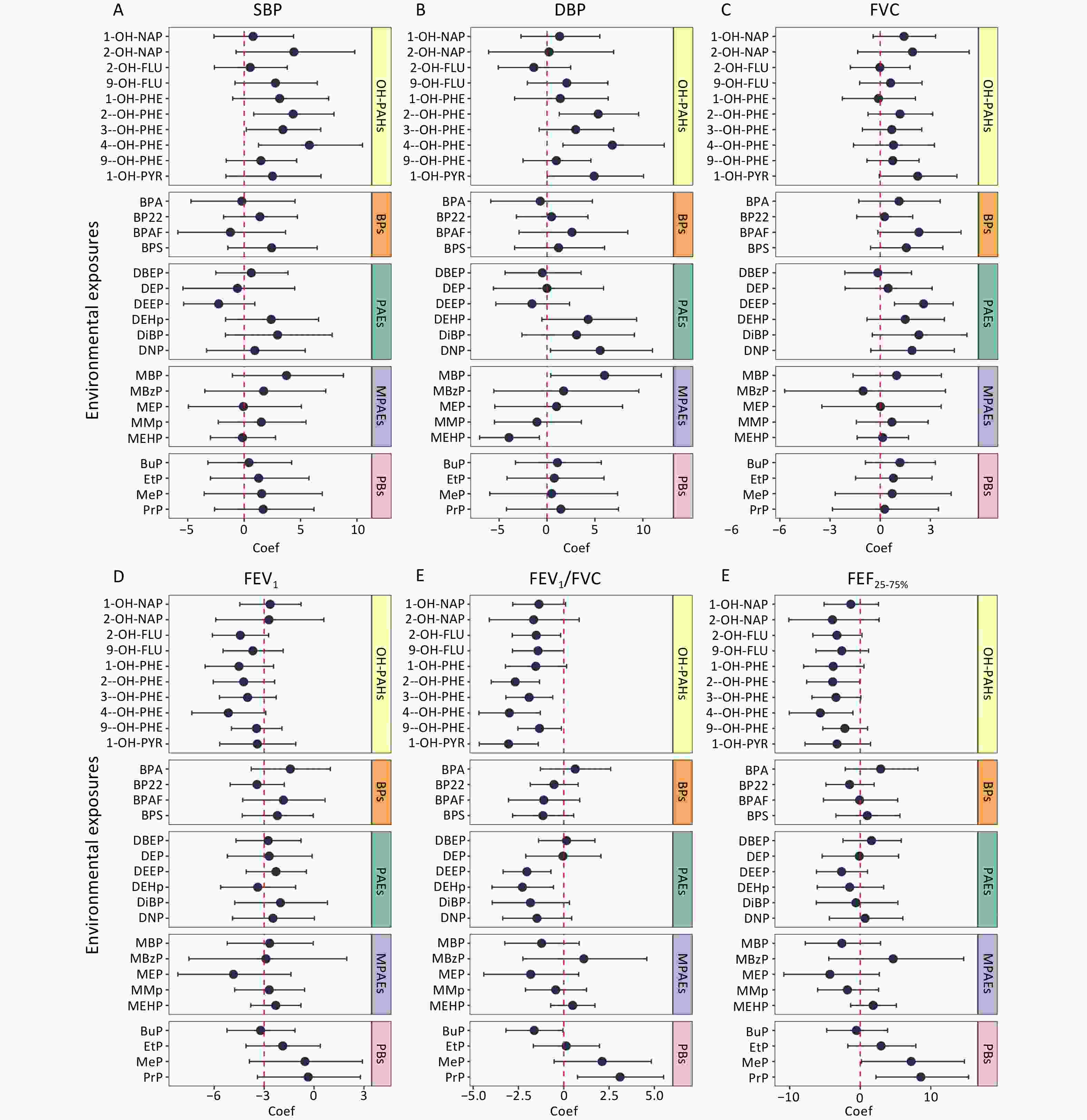
Figure 3. Associations between urinary pollutant concentrations and cardiopulmonary health indicators. Based on the LME models, age, gender, BMI, percentage of time spent in indoor activities, indoor temperature, and relative humidity were adjusted as fixed effects, and the number of study participants and dormitories in which they lived were adjusted as random effects. The association coefficients were expressed as percentage changes in blood pressure and lung function induced by each unit increase in the urinary pollutant concentration transformed by the natural logarithm, with 95% confidence intervals. Coef, coefficient; SBP, systolic blood pressure; DBP, diastolic blood pressure; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; FEV1/FVC, the ratio of FEV1 to FVC; FEF25-75%, forced expiratory flow between 25% and 75% of vital capacity. Other abbreviations are given in Supplementary Table S1.
Additionally, significant associations were observed between multiple urinary pollutant concentrations and pulmonary function indicators. For each unit increase in the natural log-transformed urinary concentrations of 1-OH-PYR, 2-hydroxyfluorene (2-OH-FLU), 2-OH-PHE, 3-OH-PHE, 4-OH-PHE, 9-hydroxyphenanthrene (9-OH-PHE), butyl paraben (BuP), bis(2-ethoxyethyl) phthalate (DEEP), and bis(2-ethylhexyl) phthalate (DEHP), the FEV1/FVC decreased by 3.05% (95% CI: −4.66%, −1.41%), 1.52% (95% CI: −2.84%, −0.17%), 2.68% (95% CI: −4%, −1.34%), 1.91% (95% CI: −3.2%, −0.61%), 3% (95% CI: −4.68%, −1.29%), 1.34% (95% CI: −2.53%, −0.13%), 1.63% (95% CI: −3.19%, −0.05%), 2.04% (95% CI: −3.35%, −0.71%), and 2.28% (95% CI: −3.96%, −0.57%), respectively. Furthermore, each unit increase in the natural log-transformed urinary concentration of 4-OH-PHE was associated with a significant decrease of 5.66% (95% CI: −10.1%, −1.04%) decrease in FEF25–75%.
-
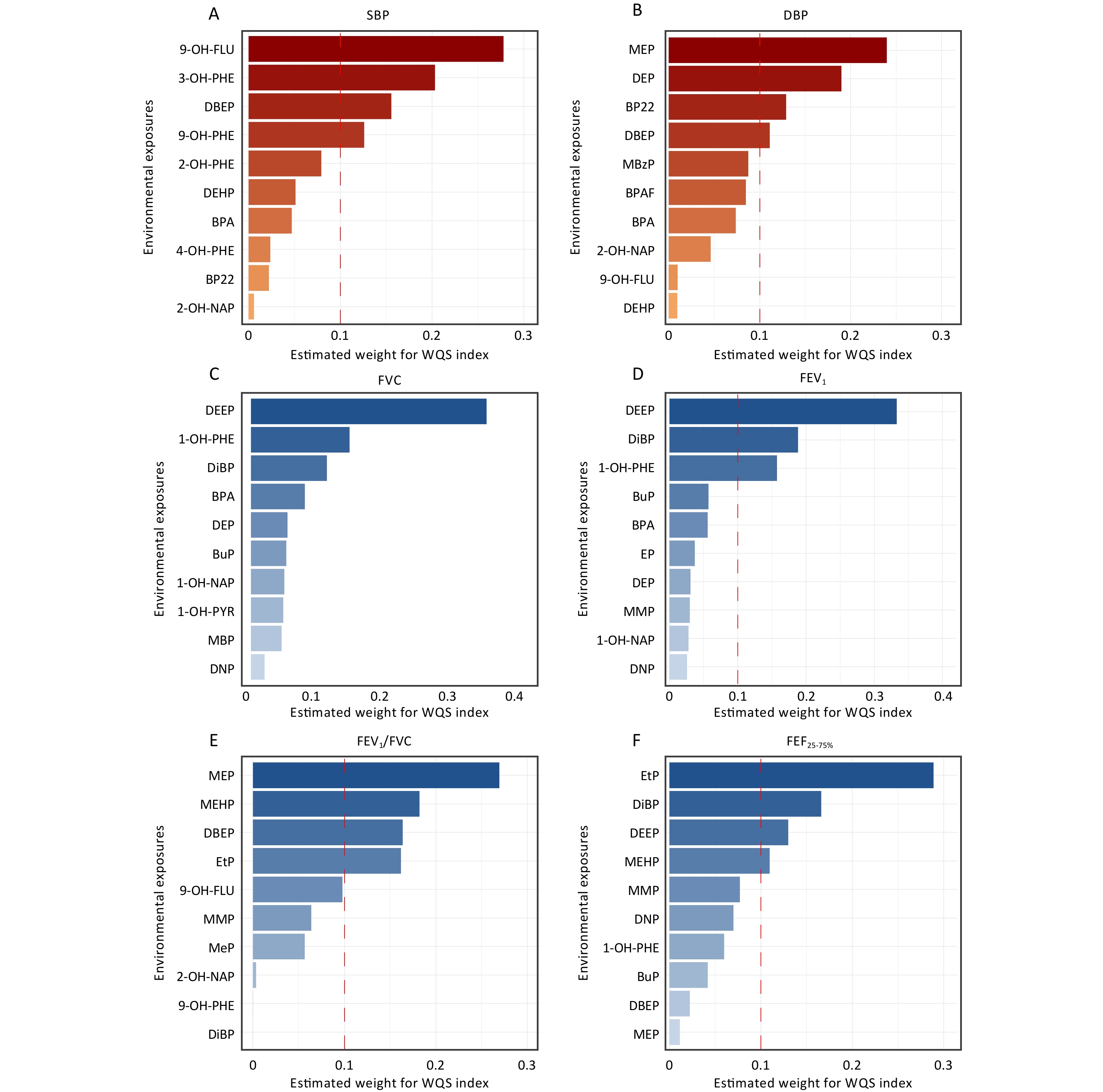
Based on the WQS regression models, the composite index for combined exposure (i.e., WQS index) was calculated (Table 3). Figure 4 shows the top 10 contributing factors affecting cardiopulmonary health indicators under mixed environmental pollutant exposure. Our findings suggested a potential trend wherein the mixed environmental pollutant exposure was associated with increased SBP and decreased FEV1/FVC. However, these associations were not statistically significant (all P-values > 0.05).
Outcome Coefficient (95% CI) P SBP 1.3 × 10−3 (−9.68 × 10−3, 1.23 × 10−2) 0.82 DBP −1.05 × 10−3 (−2.65 × 10−2, 2.44 × 10−2) 0.94 FVC 1.95 × 10−2 (−4.66 × 10−3, 4.37 × 10−2) 0.12 FEV1 1.34 × 10−2 (−1.01 × 10−2, 3.69 × 10−2) 0.27 FEV1/FVC −6.95 × 10−4 (−1.42 × 10−2, 1.28 × 10−2) 0.92 FEF25−75% 3.92 × 10−3 (−3.12 × 10−2, 3.9 × 10−2) 0.83 Note. SBP, systolic blood pressure; DBP, diastolic blood pressure; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; FEV1/FVC, ratio of FEV1 to FVC; FEF25–75%, forced expiratory flow between 25% and 75% of vital capacity; CI, confidence interval. Table 3. Associations between the weighted quantile sum (WQS) index and cardiopulmonary health indicators
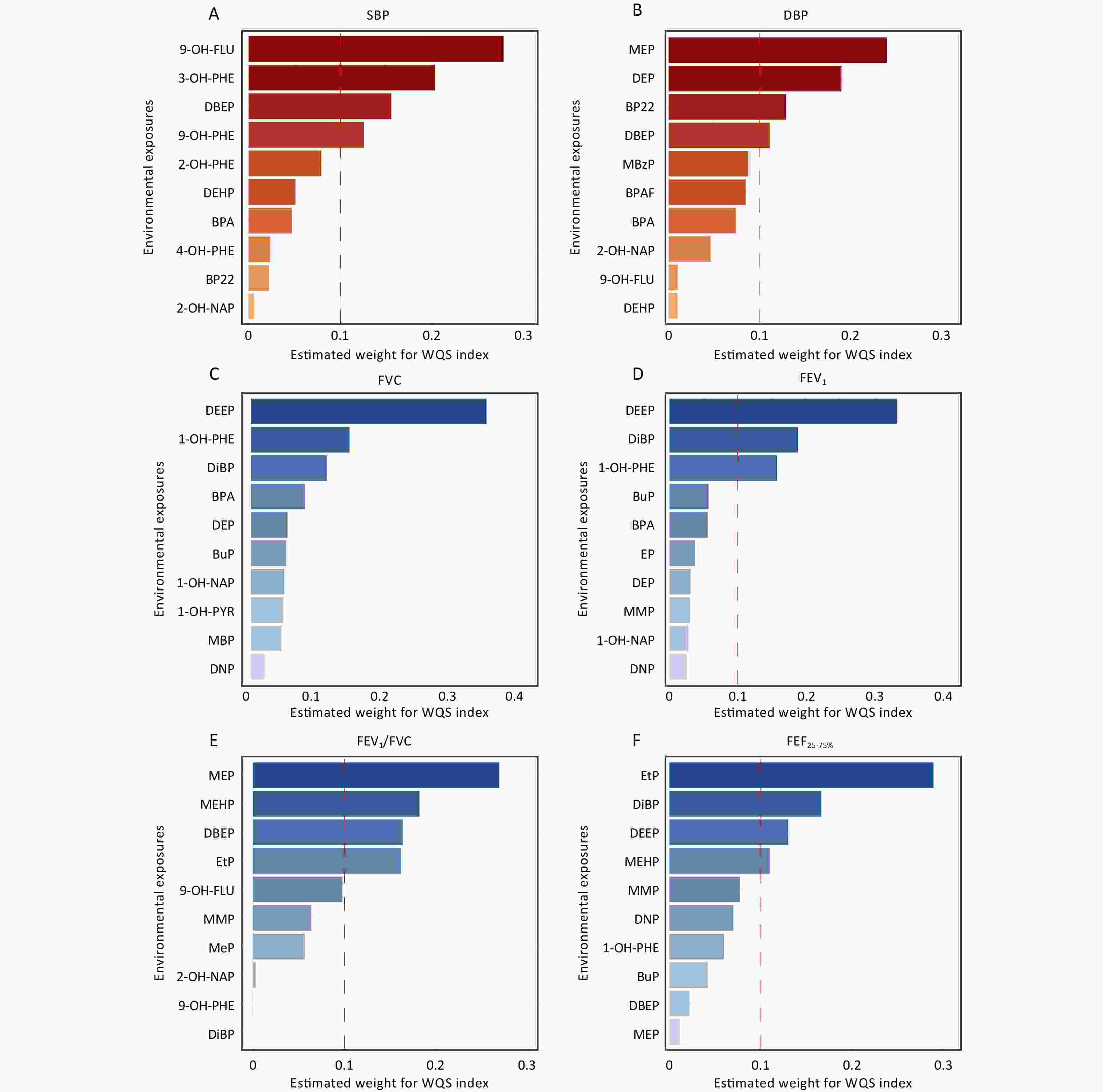
Figure 4. The top 10 index weights from the weighted quantile sum (WQS) model regression of the associations between environmental exposures and cardiopulmonary health indices. All WQS models were adjusted for age, gender, body mass index (BMI), percentage of time spent in indoor activities, indoor temperature and relative humidity. SBP, systolic blood pressure; DBP, diastolic blood pressure; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; FEV1/FVC, the ratio of FEV1 to FVC; FEF25–75%, forced expiratory flow between 25% and 75% of vital capacity. Other abbreviations can be found in Supplementary Table S1.
-
We analyzed the associations between environmental exposure and oxidative stress biomarkers as well as between biomarkers and cardiopulmonary health indicators, respectively (Supplementary Table S4–S5). Then, we conducted a comprehensive analysis to examine the mediating effects of various biomarkers on the association between environmental exposure and cardiopulmonary health indicators. Significant results are shown in Table 4, and detailed results are provided in Supplementary Table S6. Notably, 8-OHdG was identified as a significant mediator of the effects of 2-OH-PHE, 3-OH-PHE, 4-OH-PHE, and 1-OH-PYR on SBP, with mediation proportions of 18.4%, 13.8%, 12.5%, and 53.8%, respectively. Furthermore, 8-OHdG mediated the associations between 1-OH-PYR, 2-OH-PHE, and 4-OH-PHE with FEV1/FVC. We found that MDA was identified as a significant mediator of the effects of MBP on FEF25–75%, accounting for 13.4% of the overall mediation. In contrast, no mediating effects of oxidative stress biomarkers were observed for other exposures in relation to cardiopulmonary health indicators. However, this situation did not preclude the possibility of alternative mechanisms. Environmental exposure may influence cardiopulmonary health through various pathways, such as inflammation, immune dysregulation, and oxidative stress biomarkers, which were not examined in the present study. Additionally, the characteristics and dosages of specific exposures may modulate their capacity to induce oxidative stress and the associated health outcomes.
Pathways 8-isoPGF2α 8-OHdG EC-SOD GPx1 MDA Exposure-SBP 2-OH-NAP ADE a −0.30 (−2.42, 0.68) 16.54 (4.43, 38.58) −5.79 (−39.1, −0.02) −3.51 (−19.55, 1.63) 1.31 (−6.63, 8.17) ACMEb 44.73 (18.29, 133.24) 27.89 (7.31, 105.51) 50.22 (22.79, 156.28) 47.94 (18.73, 142.83) 43.12 (15.16, 118.72) Propc −0.68 37.23 −13.03 −7.91 2.94 2-OH-PHE ADE 0.3 (−0.74, 1.52) 8.54 (1.33, 20.75) −3.78 (−11.74, −0.07) −2.36 (−9.2, 1.61) 0.73 (−1.97, 4.5) ACME 46.23 (16.98, 75.91) 37.98 (6.79, 69.48) 50.31 (21.61, 81.38) 48.89 (20.96, 79.9) 45.8 (17.49, 76.42) Prop 0.65 18.36 −8.13 −5.07 1.57 3-OH-PHE ADE −0.04 (−3.72, 3.92) 6.08 (1.16, 15.38) 0.15 (−2.98, 3.19) −0.69 (−5.15, 1.41) −0.48 (−3.59, 2.51) ACME 44.2 (18.36, 68.58) 38.07 (10.19, 62.55) 44.01 (18.29, 69.5) 44.85 (19.2, 72.47) 44.64 (16.97, 70.49) Prop −0.09 13.78 0.34 −1.56 −1.09 4-OH-PHE ADE 0.36 (−1.29, 1.86) 8.3 (1.72, 34.87) −2.76 (−16.58, 1.1) −1.41 (−8.97, 3.47) 0.87 (−4.74, 7.23) ACME 66 (16.49, 97.5) 58.07 (−3.64, 83.68) 69.12 (25.33, 101.13) 67.78 (17.4, 94.31) 65.5 (18.64, 95.08) Prop 0.55 12.51 −4.15 −2.12 1.3 1-OH-PYR ADE 0.05 (−0.78, 0.84) 11.64 (0.46, 26.21) −6.19 (−19.07, −0.57) −2.5 (−12.98, 3.29) 1.54 (−3.12, 7.25) ACME 21.61 (−4.63, 54.95) 10.02 (−17.88, 43.57) 27.85 (−1.09, 66.81) 24.16 (−4.24, 62.87) 20.12 (−11.15, 55.55) Prop 0.25 53.75 −28.57 −11.55 7.11 Exposure-FEV1/FVC 1-OH-PYR ADE −0.07 (−1.19, 0.87) 7.85 (0.16, 22.24) 7.24 (0.48, 25.74) 2.3 (−2.55, 9.98) −4.37 (−11.78, 1.21) ACME −45.78 (−86.5, −14.94) −53.7 (−99.96, −24.19) −53.09 (−104.83, −22.12) −48.15 (−101.34, −19.82) −41.48 (−80.49, −6.95) Prop 0.16 −17.13 −15.79 −5.01 9.54 2-OH-PHE ADE −0.46 (−2.15, 1.03) 6.6 (0.24, 18.47) 3.8 (−0.19, 14.27) 1.49 (−2.04, 8.27) −2.81 (−8.64, 3.99) ACME −38.85 (−71.85, −12.55) −45.91 (−79.31, −20.92) −43.11 (−82.04, −17.22) −40.8 (−75.94, −14.06) −36.5 (−71.71, −10.4) Prop 1.17 −16.8 −9.66 −3.78 7.16 4-OH-PHE ADE −0.57 (−2.53, 1.67) 5.42 (0.39, 28.03) 2.76 (−1.01, 15.82) 0.77 (−2.01, 7.53) −5.35 (−14.07, 3.03) ACME −47.52 (−123.61, −19.92) −53.5 (−159.08, −31.69) −50.84 (−124.57, −27.55) −48.85 (−128.08, −26.42) −42.73 (−115.2, −15.2) Prop 1.18 −11.26 −5.73 −1.6 11.13 Exposure-FEF25-75% 2-OH-NAP ADE −0.39 (−6.86, 4.53) −5.28 (−58.35, 47.73) 7.47 (−19.69, 66.11) −6.48 (−50.08, 12.38) −28.67 (−57.63, −5.17) ACME −17.91 (−100.89, 194.67) −13.02 (−106.54, 218.93) −25.76 (−143.95, 181.83) −11.81 (−109.32, 225.76) 10.37 (−75.49, 265.41) Prop 2.12 28.84 −40.83 35.44 156.7 MBP ADE −0.54 (−9.42, 9.17) 0.62 (−25.73, 31.71) 19.47 (−7.2, 53.76) −1.98 (−31.46, 22.66) −18.05 (−66.04, −1.42) ACME −134.06 (−346.05, −39.06) −135.22 (−362.82, −39.24) −154.07 (−372.14, −45.94) −132.62 (−372.98, −25.52) −116.56 (−305.78, −17.94) Prop 0.4 −0.46 −14.46 1.47 13.41 Note.a estimated value of the direct effect (× 10−3); b estimated value of the indirect effect (× 10−3); c proportion of the mediation effect (%); d bold text represents a statistically significant value. SBP, systolic blood pressure; FEV1/FVC, the ratio of FEV1 to FVC; FEF25–75%, forced expiratory flow between 25% and 75% of vital capacity; 8-isoPGF2α, 8-iso-prostaglandin F2α; 8-OHdG, 8-hydroxy-2'-deoxyguanosine; EC-SOD, extracellular superoxide dismutase; GPx1, glutathione peroxidase 1; MDA, malondialdehyde. Other abbreviations are listed in Table S1. Table 4. Mediation effects of oxidative stress biomarkers on the association between exposures and cardiopulmonary health indicators
-
We conducted a randomized crossover experiment to investigate the effects of exposure to mixed environmental pollutants on cardiopulmonary health among a group of healthy college students in Beijing, China. Our study identified significant and positive associations between the urinary concentrations of 2-OH-PHE, 3-OH-PHE, and 4-OH-PHE and SBP as well as between the urinary concentrations of 2-OH-PHE, 4-OH-PHE, MBP, and DNP and DBP. Additionally, significant and negative associations were observed between FEV1/FVC and urinary levels of 1-OH-PYR, 2-OH-FLU, 2-OH-PHE, 3-OH-PHE, 4-OH-PHE, 9-OH-PHE, BuP, DEEP, and DEHP, as well as between FEF25–75% and 4-OH-PHE. These findings suggest that exposure to multiple pollutants may adversely affect cardiopulmonary health. Moreover, 8-OHdG and EC-SOD, commonly used biomarkers of oxidative stress, mediated the effects of various OH-PAHs on blood pressure and lung function.
Urinary 1-OH-PYR is a typical biomarker, and the measured concentrations in the present study were similar to those obtained in a cross-sectional study of community-dwelling people in China (median: 0.26 μg/g crea; IQR: 0.17, 0.37 μg/g crea)[27]. The concentrations of a variety of isomeric metabolites, including hydroxy-naphthalenes (OH-NAPs), OH-FLUs, and OH-PHEs, were similar to those reported in a previous study (median: OH-NAPs, 1.59 μg/g crea; OH-FLUs, 1.09 μg/g crea; OH-PHEs, 1.51 μg/g crea)[28], but much lower than the concentrations reported in a study of coke oven workers[23]. These comparisons indicate that the PAH exposure levels in our study were moderate or relatively low, which is consistent with the exposure of the general population. Additionally, BPA concentrations measured in this study were similar to those found in the urine of young residents in the community (mean: 1.43 μg/g crea)[29], and the PB concentrations were similar to those reported in a study of adult males in China (mean: BuP, 0.64 μg/g crea; ethyl paraben (EtP), 31.01 μg/g crea; methyl paraben (MeP), 68.98 μg/g crea; propyl paraben (PrP), 20.04 μg/g crea)[30]. Our study found that the PAE and MPAE concentrations were relatively higher than those reported in the National Health and Nutrition Examination Survey (NHANES)[31]. This discrepancy may be explained by the greater exposure of university students to personal care products and other plastic materials than the general population. Given that PAEs are among the most widely used plasticizers, their more frequent interactions with the study population likely contributed to the elevated levels that were observed. Based on a comparison with previous studies, the exposure levels of the pollutants investigated in this study were generally consistent with those found in the general population living in areas without severe pollution exposure.
Our findings highlight the adverse associations between exposure to various environmental pollutants and both blood pressure and pulmonary function, suggesting their potential adverse effects on cardiopulmonary health. As in previous studies, multiple PAH exposures were significantly associated with altered blood pressure in non-occupationally exposed populations[32]. Exposure to PAHs has been reported to increase the risk of CVDs[7], and OH-PAHs, which are biomarkers in urine, have been found to be significantly and positively correlated with CVDs[33]. A previous study conducted with the general population in China found significant and positive associations between urinary levels of 2-OH-FLU, 9-hydroxyfluorene (9-OH-FLU), and 1-hydroxyphenanthrene (1-OH-PHE), and the risk of CVDs[34]. The impact of PAHs on blood pressure has been studied predominantly in relation to PAHs with high molecular weights. However, previous studies have shown that PAHs with low molecular weights, particularly PHEs, have adverse cardiotoxic effects[35]. The negative association between MBP, a metabolite of PAEs with low molecular weights, and DBP is consistent with findings of previous studies[36]. A study conducted in the UK involving animal experiments demonstrated that MBP, acting as a typical EDC, inhibited steroid production in fetal testicular interstitial cells of primates and rodents[36]. Our study confirmed this finding. Our study also provides evidence of the adverse effects of environmental pollutants on blood pressure. However, although blood pressure is an established risk factor for CVDs, it does not comprehensively characterize cardiovascular health. Further investigations targeting other critical cardiovascular risk determinants, such as lipid profiles, inflammatory biomarkers, and vascular dysfunction indicators, are required to fully determine the hazardous effects of environmental pollutants on the cardiovascular system. In addition, we found significant associations between various urinary exposure biomarkers and pulmonary function indicators. This finding is consistent with the results of previous studies[37,38]. Exposure to PAHs and PAEs can lead to a decline in pulmonary function, suggesting that exposure to OPs in the environment has adverse effects on lung health. Few studies have investigated the association between individual PAH exposure and pulmonary function indicators, with most focusing mainly on occupational exposure in populations. Exposure to PAHs in the general population is lower than that in occupationally exposed populations. In studies on the association between PAH exposure and lung function in the general population, exploring the potential pathways and biomarkers of lung function reduction induced by PAH exposure could provide a theoretical basis for health protection strategies for non-occupationally exposed populations.
Our findings suggest that 8-OHdG, a classic and reliable biomarker of DNA oxidative damage, plays a critical role in oxidative stress induced by pollutant exposure[39]. Oxidative stress, through excessive ROS production, has been reported to lead to DNA oxidative damage, which may increase the risk of CVD or COPD[40]. This mechanism is likely a significant pathway through which pollutant exposure affects blood pressure and pulmonary function. Previous studies have shown that OH-PAHs, such as 2-OH-PHE and 4-OH-PHE, promote ROS generation through metabolic pathways, thereby exacerbating oxidative stress responses[41]. Our results are consistent with the findings of previous studies and further highlight the key mediating role of DNA oxidative damage. Additionally, our study identified a significant mediating role of EC-SOD on the effect of 1-OH-PYR on FEV1/FVC, suggesting that EC-SOD may influence lung function by modulating oxidative stress levels induced by exposure to 1-OH-PYR. Extracellular superoxide dismutase is a crucial antioxidant enzyme that operates predominantly in the extracellular matrix, scavenging superoxide anions to protect the alveoli and vascular endothelium from oxidative damage[42]. These findings suggest that 1-OH-PYR exposure may impair the protective function of EC-SOD, exacerbating oxidative damage to the airway and lung tissues, ultimately leading to a decline in lung function. Our study confirmed that intervention in oxidative stress is crucial for preventing the toxic effects of exposure to multiple OPs.
Our study had several limitations. First, the sample size was relatively small, which might have limited the generalizability of the findings. Second, this study was conducted over a relatively short period and focused on the effects of short-term exposure changes on cardiopulmonary health. The potential long-term effects warrant further investigation. Despite these limitations, this study has several strengths. First, the randomized crossover design provides greater internal validity than observational studies. Second, we simultaneously monitored multiple exposures and health indicators, offering a comprehensive assessment of their interrelationships. This approach enabled the construction of an association atlas by providing a more holistic evaluation than previous studies focused primarily on a single exposure or single exposure group. Finally, we conducted a mediation analysis from the perspective of oxidative stress, underscoring its critical role in mediating the toxic effects of exposure to multiple OPs. These findings highlight the importance of addressing oxidative stress in future studies and interventions aimed at mitigating the adverse health effects of environmental exposure.
-
Exposure to multiple environmental pollutants can adversely affect cardiopulmonary health. In addition, oxidative stress has been identified as a key mediator of the effects of OH-PAHs on blood pressure and lung function.
Associations of Exposure to Typical Environmental Organic Pollutants with Cardiopulmonary Health and the Mediating Role of Oxidative Stress: A Randomized Crossover Study
doi: 10.3967/bes2025.087
- Received Date: 2025-01-23
- Accepted Date: 2025-06-03
-
Key words:
- Cardiopulmonary health /
- Organic pollutants /
- Oxidative stress /
- Mediating effects /
- Risk assessment
Abstract:
The authors declare no competing interests.
| Citation: | Ning Gao, Bin Wang, Ran Zhao, Han Zhang, Xiaoqian Jia, Tianxiang Wu, Mengyuan Ren, Lu Zhao, Jiazhang Shi, Jing Huang, Shaowei Wu, Guofeng Shen, Bo Pan, Mingliang Fang. Associations of Exposure to Typical Environmental Organic Pollutants with Cardiopulmonary Health and the Mediating Role of Oxidative Stress: A Randomized Crossover Study[J]. Biomedical and Environmental Sciences. doi: 10.3967/bes2025.087 |








 Quick Links
Quick Links
 DownLoad:
DownLoad: