-
Human cytomegalovirus (HCMV) belongs to the β-Herpesvirinae subfamily within the Herpesviridae family and has a widespread global distribution. The seroprevalence of HCMV-IgG among women of reproductive age ranges from 40% to 60% in the middle-income brackets of developed countries, exceeds 80% in low-income women, and reaches 95% to 100% in developing countries[1-3]. After maternal infection, the virus can cause congenital infection of the fetus through the placenta, leading to microcephaly, sensorineural hearing loss, and cognitive impairment[4,5].
Even in developed countries, congenital HCMV infection, which is more prevalent than other congenital diseases (e.g., Down syndrome, congenital human immunodeficiency virus (HIV) infection, and spina bifida), remains the leading cause of congenital neurological disability in children. Currently, there are no safe and effective anti-HCMV drugs or vaccines that can be used to prevent HCMV infection.
Previous studies have reported that HCMV infection affects the Wnt signaling pathway, which is essential for developing the embryonic nervous system[6]. Investigating the Wnt pathway may aid in identifying new targets for the prevention and treatment of HCMV infection.
-
HCMV can be transmitted through bodily fluids, organ transplants, and the placenta during pregnancy. Multiple transmission routes contribute to the high prevalence of HCMV infections, making it a major global health concern. Women of childbearing age are the most commonly infected group, with CMV-IgG seroprevalence in women of childbearing age ranging from 45.6% to 95.7%, 45.6% to 65.9%, 60.2%, 58.3% to 94.5%, and 24.6% to 81.0% in Europe as a whole and in developed countries in Europe, Japan, Latin America, and North America, respectively[7]. Primary or recurrent HCMV infection during pregnancy can lead to vertical transmission to the fetus. Congenital HCMV infection accounts for approximately 0.67% of all births worldwide. In low-income countries, the prevalence is higher, at 1.42%[8].
HCMV-infected fetuses exhibit poor development, low survival rates, and high malformation rates. The proliferation and differentiation of neural stem cells (NSCs) are also affected[6]. Approximately 10% of babies affected by congenital CMV infection develop symptoms at birth, and up to 60% of these babies develop permanent neurological dysfunction[9]. A study in the United States suggested that the timing of congenital infection may be related to the severity of clinical symptoms. The risk of intrauterine infection increases when maternal infection occurs during the third trimester. However, fetal infection appears to pose a more significant threat to the fetus if it occurs early in pregnancy, with the potential for dissemination of the virus to multiple fetal tissues, including the brain[10].
The mechanisms underlying HCMV-induced adverse birth outcomes remain unclear. HCMV has been shown to infect a range of cells within the central nervous system, including oligodendrocytes, neural progenitor cells (NPCs), astrocytes[11], placental pericytes, cytotrophoblasts, and villous fibroblasts, with peri-placental cells being the most susceptible to HCMV infection[12]. Placental pericytes are essential for endothelial cell proliferation and placental microvasculature stability and integrity[13]. This information can aid in understanding HCMV-induced embryonic deformities.
Accumulating evidence suggests that congenital HCMV infection may be associated with subtle changes in human brain development (e.g., spatial learning, memory function, and language development)[14]. Congenital HCMV infection can lead to long-term effects in children, manifesting as mental retardation, sensory-transmitted otoacoustic and visual development impairments, convulsions, and seizures[15]. HCMV infection causes rapid downregulation of genes that maintain neural progenitor multipotency and establish neural identity, resulting in the premature and abnormal differentiation of NPCs and a reduction in their capacity to differentiate into astrocytes[16]. This may explain, at least in part, the abnormalities observed in the brain development of congenitally infected children[17]. Moreover, the abnormal calcium metabolism caused by HCMV infection, which results in abnormal expression of Ca/calmodulin-dependent protein kinase II (CaMKII) in the hippocampus, is also a contributing factor[18]. CaMKII plays a vital role in synaptic plasticity, an essential indicator of spatial learning and memory function[19].
Notably, HCMV has strict species specificity. It is difficult for the virus to replicate and produce complete progeny virus particles in animal tissues and cells, except in humans. The clinical symptoms of mouse cytomegalovirus (MCMV) infection in mice, which resemble those of HCMV infection, have been studied using the most widely used HCMV animal model. This model has been used in all aspects of CMV research[20-22]. However, considerable differences exist among these viruses. For instance, MCMV can inhibit human tumor growth, whereas HCMV cannot[23]. More importantly, MCMV cannot spread as much through the placenta as HCMV[24]. When interpreting findings based on mouse models, it is essential to exercise caution and to consider all information collectively.
-
The Wnt signaling pathway, a complex and diverse system, is critical for embryonic development and is highly conserved across both invertebrates and vertebrates. Its initial discovery in the study of the wingless gene in Drosophila revealed a fascinating world of autocrine and paracrine functions[25].
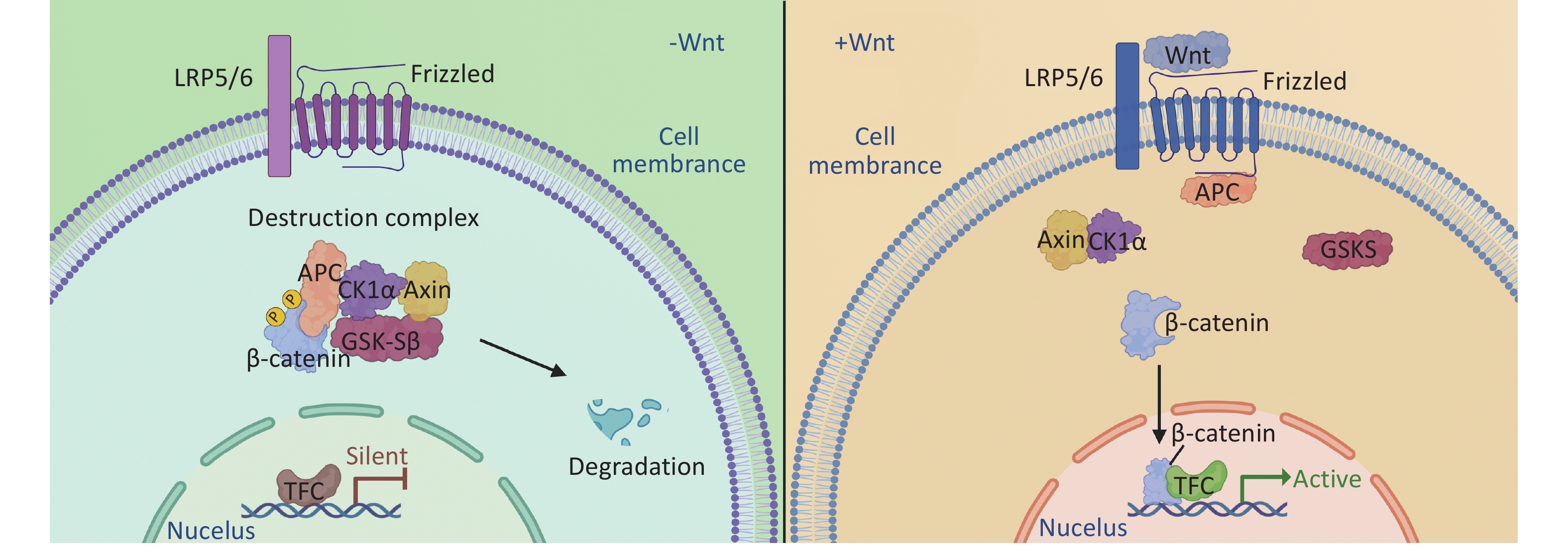
The canonical Wnt/β-catenin pathway, the most well-characterized branch, involves a series of signaling molecules. In the absence of Wnt ligands, β-catenin in the cytoplasm forms a degradation complex with Axin, adenomatous polyposis coli (APC), casein kinase 1α (CK1α), and glycogen synthase kinase-3β (GSK-3β), leading to its degradation via the ubiquitin-proteasome pathway[26-28]. However, in the presence of Wnt ligands, β-catenin phosphorylation is blocked, preventing its degradation and allowing its accumulation in the cells. β-catenin then translocates to the nucleus, where it binds to TCF/LEF transcription factors, ultimately activating the expression of downstream target genes[29] (Figure 1). β-catenin regulatory genes include cyclin D1 and c-myc, which are involved in cell cycle regulation and cell proliferation[30]; Dickkopf-1 (Dkk-1) is necessary for normal embryonic development through its negative feedback regulation of Wnt signal transduction[31]. Furthermore, matrix metalloproteinase-2 (MMP-2), MMP-9, and MMP-7 play important roles in cell proliferation, differentiation, migration, angiogenesis, and apoptosis[32].
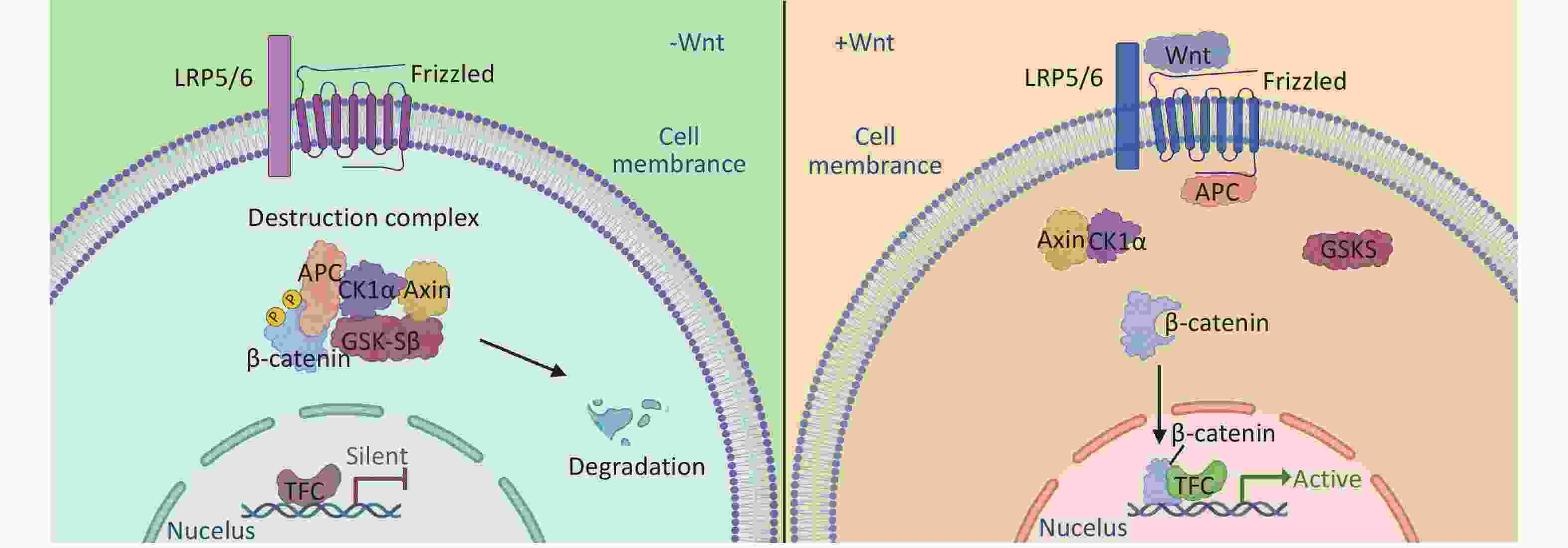
Figure 1. The accumulation and degradation of β-catenin in cells is a finely regulated process. In the absence of Wnt ligand stimulation, the β-catenin destruction complex, composed of Axin, CK1, GSK-3α/β, APC, and DVL, phosphorylates and subsequently degrades β-catenin. This process contributes to maintaining a stable level of intracellular β-catenin, thereby preventing cellular signaling disruptions resulting from its excessive accumulation. Upon the binding of Wnt ligands to receptors on the cell membrane, the activity of the β-catenin destruction complex is inhibited, allowing β-catenin to accumulate in the cytoplasm. The accumulated β-catenin translocates from the cytoplasm to the nucleus, where it interacts with nuclear transcription factors and initiates the transcription of target genes. This process is important for cellular differentiation, proliferation, and development.
The Wnt/β-catenin pathway, a pivotal player in embryogenesis, remains active throughout the lifespan of an organism. Its role in determining cell fate and establishing tissue polarity should not be underestimated[33,34]. Additionally, it is vital for the differentiation of various cell types, including neurons and mesenchymal stem cells[35-38]. Dysregulation of this pathway can lead to severe embryonic malformations and other grave consequences, underscoring the importance of our study[39].
Moreover, during embryonic development, Wnt signaling pathways control the differentiation of cell types in the mammalian cortex through dynamic changes[40]. Deletion of the Wnt7a gene results in the loss of a downstream target gene of the Wnt/β-catenin pathway, Hox A10/11, leading to difficulties in endometrial stromal differentiation and infertility in mice[41].
Recent evidence also underscores the essential role of this pathway in the differentiation of fetal cytotrophoblasts into an invasive phenotype during placentation[42]. Inhibition of β-catenin and TCF functions in cultured NSCs can decrease their proliferative ability, while in vivo inhibition causes extensive damage to neural hair in the cerebral cortex of embryonic mice[43]. This finding suggested that the canonical Wnt/β-catenin signaling pathway plays a crucial role in the early embryonic development of the nervous system (Table 1).
Wnt signaling pathways Functions in embryonic development Impact of abnormal expression on embryos References Wnt/β-catenin pathway Wnt1 Mid and hindbrain development; anterior-posterior patterning of the CNS Severe craniofacial skeletal defects; dramatic brain malformation [45-48] Wnt3a Presomitic mesoderm formation Embryonic defects; loss of presomitic mesoderm [49,50] Wnt3 Thalamus development, neural pattering, and vertebrate primary axis formation Medulloblastoma formation; tetra-amelia; defective brain development [51-60] Wnt7a Dendritic spine morphogenesis in hippocampal neurons Deficit in spatial memory [61] Wnt/PCP pathway Cell polarity establishment within an epithelial plane is involved in the early stages of mouse eye development Ocular defects; severe neural tube closure defects [62-64] Wnt/Ca2+ pathway Signal transduction for axon guidance and dendritic development; embryonic heart, bone, and lung development Axonal guidance defects; skeletal dysplasias [65-70] Table 1. The roles of Wnt in embryonic development
Furthermore, maternal ketamine anesthesia during pregnancy downregulates the Wnt/β-catenin signaling pathway in the hippocampus of offspring rats, leading to cognitive dysfunction[44]. This finding highlights the significance of this signaling pathway in early brain development.
-
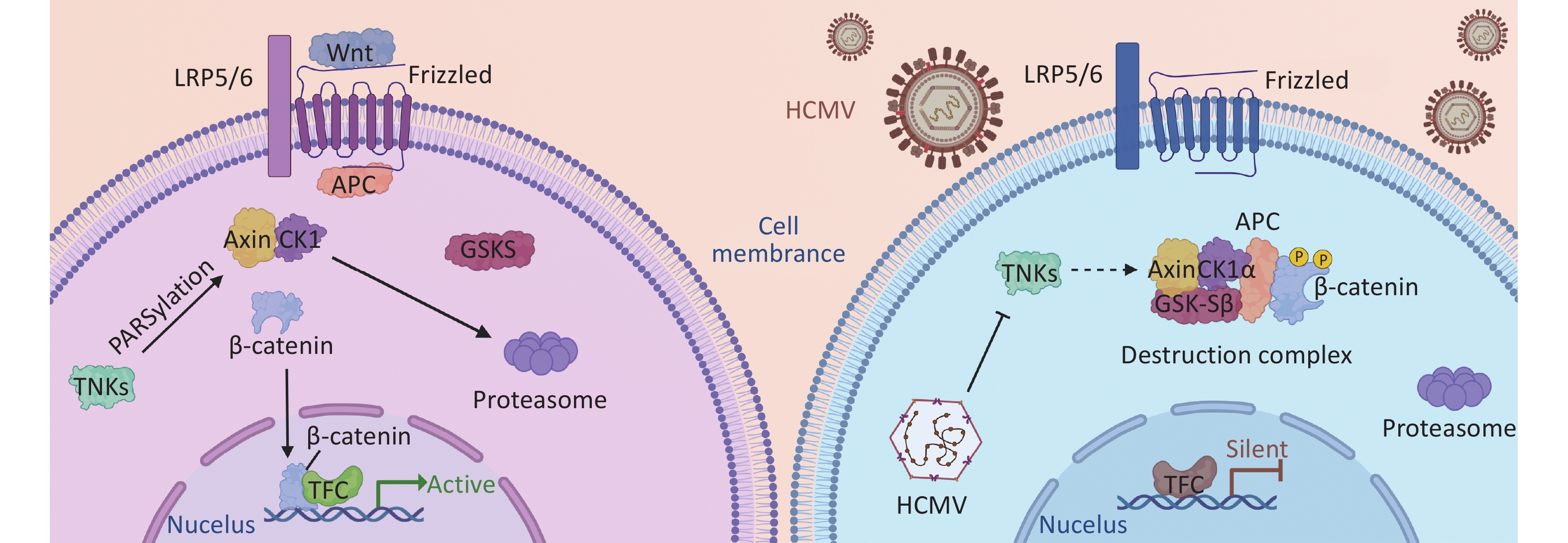
Studies have shown that HCMV inhibits the canonical Wnt/β-catenin pathway[71]. A key player in this pathway, β-catenin, is sequestered and degraded in infected cells. In addition, the transcriptional activity of β-catenin is inhibited in infected cells. It is well known that for β-catenin to drive the transcription of its target gene[72], it must first be translocated to the nucleus. HCMV infection leads to the accumulation and degradation of β-catenin in the perinuclear space. Thus, the transcriptional activity of Wnt/β-catenin and its downstream functions are blocked[32]. Axin1 is a critical protein in the β-catenin-destroying complex, and its concentration is involved in Wnt signal transduction. TNKS negatively regulates Axin1, and some studies have shown that during HCMV infection, HCMV stabilizes the expression of TNKS and reduces its PARylation activity, resulting in Axin1 accumulation and reduced PARylation, leading to β-catenin degradation during infection and affecting the expression of the Wnt signaling pathway[71] (Figure 2).
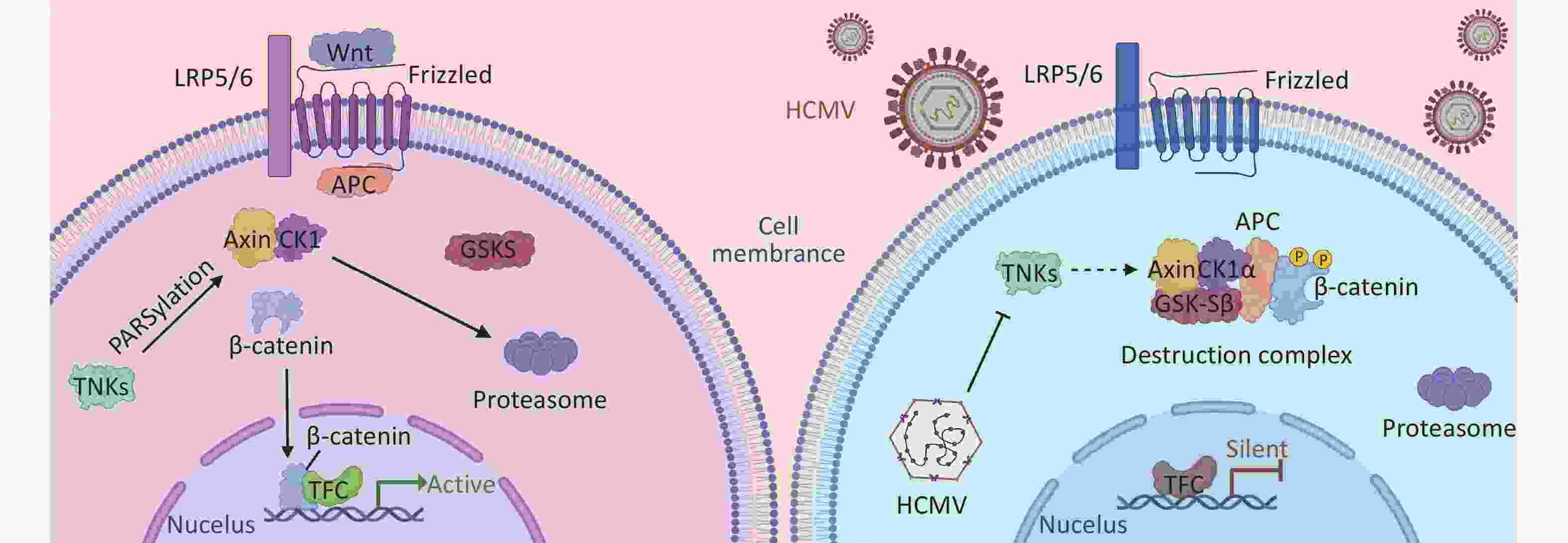
Figure 2. The NAD+-driven PARylation process plays a pivotal role in the proteasomal degradation of Axin, thereby preventing the formation of the β-linker disruption complex. The absence of this complex hinders the accumulation of β-linker proteins and their subsequent nuclear translocation for translation initiation, thereby influencing intracellular signaling. During HCMV infection, the PARylation activity of TNKS (PARP5a/b) is impeded, leading to the inhibition of Axin PARylation and consequently enhancing Axin stability. Increased Axin stability promotes the formation of the β-catenin destruction complex. This results in increased degradation of β-catenin, thereby preventing β-catenin-mediated transcription. Further investigation of this process will contribute to our understanding of intracellular signaling mechanisms.
This effect has been observed in SGHPL-4 cells, an extravillous trophoblast (EVT) cell line derived from the first-trimester placenta[73]. Researchers have proposed that HCMV infection impairs the ability of placental EVTs to adequately differentiate and invade the decidua. This could also hinder their ability to remodel the uterine spiral arteries during pregnancy, leading to shallow placentation[73-75]. HCMV acts on multiple steps of the TGF-β/Smad signaling pathway, which plays a vital role in trophoblast cell differentiation and EVT invasion to impede EVT proliferation and invasion, leading to narrow spiral arteries, high blood impedance, decreased placental blood perfusion, and substance exchange, resulting in poor pregnancy outcomes[76]. These results were also due to the interference of HCMV with the Wnt signaling pathway. We obtained the same findings for rat cytomegalovirus (RCMV). RCMV infection induced abnormalities in the rat embryonic nervous system, significantly inhibited NSC proliferation and differentiation, and suppressed the expression of key molecules in the Wnt/β-catenin signaling pathway, which in turn affected the differentiation of NSCs. This may be an important mechanism through which RCMV causes abnormalities in the embryonic nervous system[6].
Notably, noncanonical Wnt signaling pathways seem to play a role in regulating the canonical Wnt/β-catenin pathway during HCMV infection. Wnt5a interacts with the tyrosine-like orphan kinase ROR2, activating the Wnt/Planar cell polarity and Wnt/Ca2+ pathways[77]. However, during HCMV infection, infected cells become insensitive to normal Wnt5a ligand signaling while ROR2 expression significantly increases, inhibiting canonical signaling by repressing β-catenin TCF/LEF-1 transcriptional activity. Knockdown of over-expressed noncanonical ROR2 during infection can rescue some function of the canonical Wnt/β-catenin pathway in trophoblasts, suggesting that the canonical and noncanonical Wnt pathways are strongly intertwined, especially during HCMV infection[77].
These findings demonstrate the intricate relationship between HCMV and the Wnt pathway, and the delicate balance between them. Understanding how HCMV infection affects Wnt signaling may lead to the development of new ways of treating and preventing congenital HCMV infection-related disabilities in embryos.
-
To date, ganciclovir (GCV) is the drug of choice in China for the treatment of HCMV infection. Early sufficient dosage has shown remarkable clinical efficacy. However, the side effects of GCV on the human body should not be ignored, especially in newborns, with reversible neutropenia being the most common[78]. Recent studies have indicated that valganciclovir (VGCV) has better efficacy and fewer side effects[78]. Oral administration of this GCV prodrug eliminates the need for intravenous infusion and hospitalization during GCV treatment, thereby reducing the risk of phlebitis and hospital-acquired infections[79]. The US Food and Drug Administration (FDA) has approved letermovir. Unlike other traditional anti-HCMV drugs, this novel antiviral drug does not induce cross-resistance with existing anti-HCMV drugs[80]. It is primarily used to treat HCMV infection and disease prophylaxis in seropositive adult recipients[79].
In addition, new drugs are being developed. Maribavir is a promising anti-HCMV compound and has shown good safety[79]. At specific concentrations, BAI can also inhibit the proliferation of human NSCs infected with HCMV[81]. Given the critical role of the Wnt signaling pathway in HCMV infection, drugs targeting this pathway also show great promise. Previous studies have shown that some compounds that inhibit Wnt signaling are effective in inhibiting HCMV replication[82].
Additionally, a few neuroactive mood stabilizers have demonstrated anti-HCMV potential[83]. New treatment options and ideas have been proposed (e.g., RNAi-based therapeutics against HCMV and CRISPR/Cas9-based therapeutics)[84,85]. HCMV vaccines are also being developed. The research and application of these new insights may represent a leap forward in HCMV treatment.
Furthermore, even though tens of thousands of children suffer from permanent disabilities due to HCMV infection each year, women of childbearing age have limited knowledge about this virus, and authorities may have incomplete information on HCMV. Thus, it is essential to enhance public awareness of HCMV prevention. Future HCMV education campaigns should target the general public and focus on HCMV education and prevention among healthcare providers[86].
Human Cytomegalovirus Infection and Embryonic Malformations: The Role of the Wnt Signaling Pathway and Management Strategies
doi: 10.3967/bes2025.103
-
Key words:
- Human cytomegalovirus /
- Congenital cytomegalovirus infection /
- Wnt signaling pathway /
- β-catenin /
- Malformation of embryo /
- Embryonic development
Abstract: Human cytomegalovirus (HCMV) poses a significant risk of neural damage during pregnancy. As the most prevalent intrauterine infectious agent in low- and middle-income countries, HCMV disrupts the development of neural stem cells, leading to fetal malformations and abnormal structural and physiological functions in the fetal brain. This review summarizes the current understanding of how HCMV infection dysregulates the Wnt signaling pathway to induce fetal malformations and discusses current management strategies.
X.H., B.Z., L.X., and Z.L. organized the contents of the entire manuscript and wrote the sections. B.Z., J.C., and S.H. prepared the figures. D.W. and Z.L. contributed substantially to the conception and design of the study. All authors read and approved the final manuscript.The authors declare that they have no competing interests.
&These authors contributed equally to this work.
| Citation: | Xiaomei Han, Baoyi Zheng, Zhicui Liu, Junbing Chen, Shuting Huang, Lin Xiao, Dongfeng Wang, Zhijun Liu. Human Cytomegalovirus Infection and Embryonic Malformations: The Role of the Wnt Signaling Pathway and Management Strategies[J]. Biomedical and Environmental Sciences. doi: 10.3967/bes2025.103 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: