-
Ambient air pollution has raised global public health concerns worldwide. It includes the harmful pollutants emitted by industries, households, cars, and trucks. The disease burden of ambient environmental risk factors (air pollution, temperature extremes, etc.) is among the top ten health threats worldwide and accounts for nearly 20% of the global disease burden[1]. Moreover, the World Health Organization has determined that air pollution is a major environmental risk to health[2].
Currently, heart failure is also a critical public health problem. The condition is the terminal stage of many heart diseases. From cardiovascular risk factors to endothelial dysfunction, all heart diseases that progress to a severe or terminal stage eventually become heart failure[3]. Currently, heart failure is globally prevalent among an estimated 56 million or more individuals[4]. Driven by the aging population, the number of patients with heart failure has increased in recent years, and this trend is projected to persist in the future[4-6]. Despite improvements in treatment, the 5-year mortality rate remains at approximately 40%[7]. The places a substantial social and economic burden on society[8].
Many studies have investigated the link between air pollution and heart failure, and a large body of evidence suggests that exposure to air pollution may be an important risk factor for heart failure[9]. Air pollution contributes to the deterioration of heart failure and repeated hospitalizations[8]. Increased concentrations of most particulate matter (PM) and gaseous pollutants are positively correlated with an increased risk of on-site heart failure, hospitalization, and cardiovascular mortality[10]. However, other studies have reported contradictory results. A weight-of-evidence analysis revealed a lack of strong evidence to support the cause-and-effect relationship between short-term exposure to ambient ozone (O3) and adverse cardiovascular effects in humans[11]. Moreover, the specific mechanisms and pathways by which air pollution contributes to heart failure remain unclear, thereby emphasizing the need for further research to understand the potential biological processes and causal relationships. Biomarkers are measurable indicators of normal or pathological processes, or pharmacological responses, and play key roles in disease diagnosis and management[12].
In heart failure, specific biomarkers provide insights into key mechanisms such as myocyte stress, injury, inflammation, and fibrosis. For example, N-terminal pro-B-type natriuretic peptide (NT-proBNP) indicates myocyte stress, soluble suppression of tumorigenicity 2 (sST2) reflects fibrosis and remodeling activity, and troponin (troponin T or troponin I) signals myocardial injury. These biomarkers are associated with the risk of heart failure and its key pathological processes. Clinically relevant biomarkers play crucial roles in the diagnosis, management, and monitoring of heart failure. Table 1 summarizes the definitions, mechanistic insights, diagnostic thresholds, and prognostic associations of the key biomarkers commonly used in heart failure. These biomarkers have been demonstrated to be associated with the risk of heart failure as well as with specific pathological processes. They can be used to understand the underlying mechanisms and support new therapeutic strategies[12-16].
Biomarker Definition insights Cut point Sensitivity specificity RR for HF NT-proBNP The N-terminal fragment of proBNP, in response to myocyte stress. Myocyte stress < 300 pg/mL 99% 68% RR 1.35 per 100 pg/mL increase (death) sST2 Acts as a decoy receptor binding to IL-33, inhibiting antihypertrophic and antifibrotic response. Fibrosis and
myocyte stress35 ng/mL 95% 4% > 35 ng/mL, HR = 3.64 (adverse effects) Troponin T Myocardium-specific proteins, released in the early course of myocardial injury. Myocyte injury 0.014 ng/mL >95% > 95 % > 0.1 µg/L: HR 2.55 (in-hospital mortality) Note. NT-proBNP, N-terminal pro-B-type natriuretic peptide; sST2, soluble suppression of tumorigenicity 2; IL-33, interleukin-33; RR, relative risk; HR, hazard ratio; HF, heart failure. Table 1. Clinically relevant biomarkers in heart failure: definitions, mechanisms, and prognostic associations
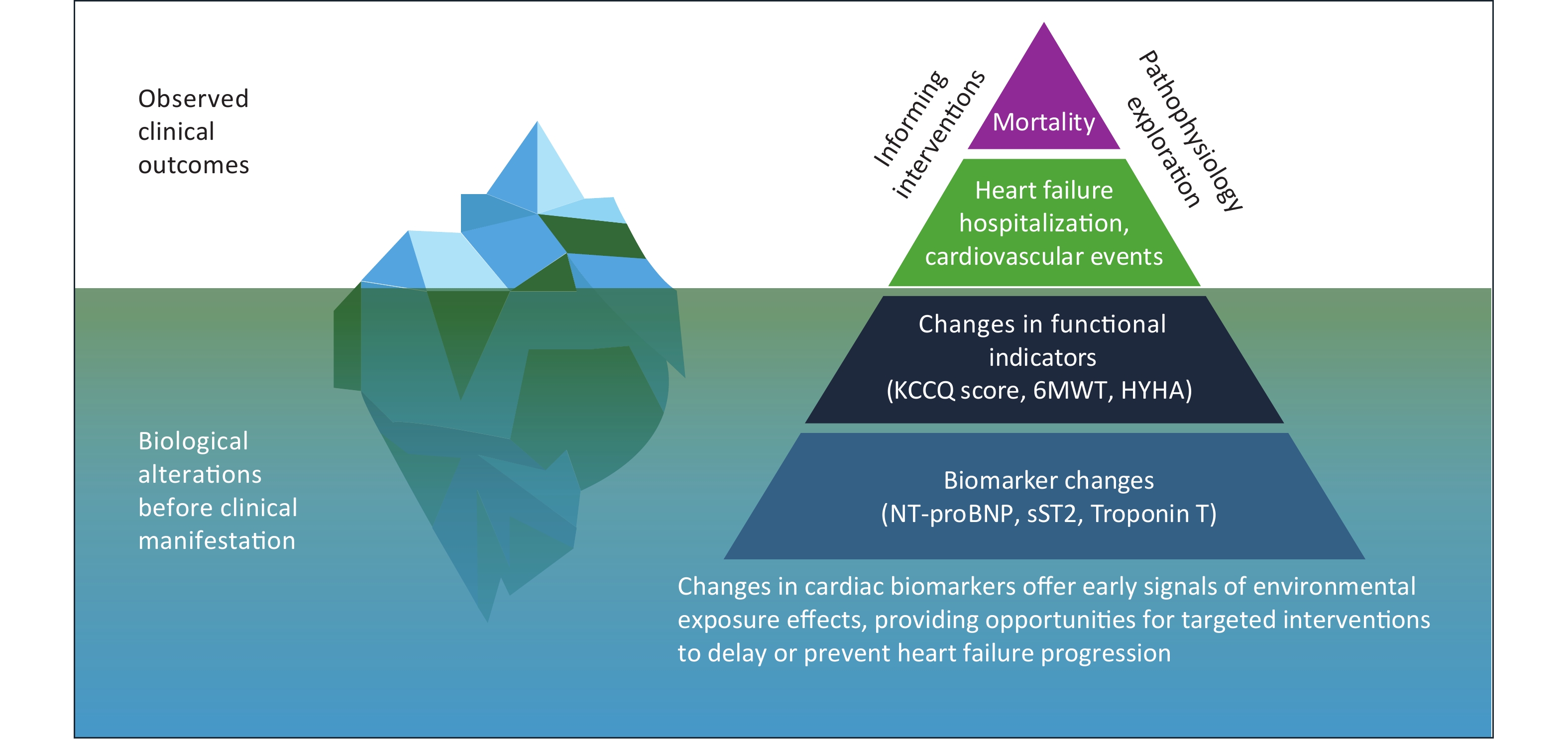
As shown in Figure 1, understanding the progression of heart failure requires going beyond traditional clinical outcomes such as hospitalization and death and focusing more on biomarkers as early indicators of disease progression. The pyramid framework illustrates that at the top are death and cardiovascular events, which are the outcomes most traditional studies have focused on. Below are changes in functional indicators, which reflect the heart’s adaptive adjustments before clinical symptoms appear. At the bottom are changes in biomarkers, which occur in the early stages of the disease and provide insights into underlying pathophysiological mechanisms. The iceberg model offers a complementary perspective: clinical outcomes are merely the visible tip of the iceberg, while the vast biological changes captured by biomarkers lie hidden beneath the surface. By studying biomarkers rather than just clinical events, researchers can gain a deeper understanding of the pathophysiological mechanisms underlying heart failure and identify earlier targets for prevention and intervention[13]. This conceptual framework forms the foundation of this study.
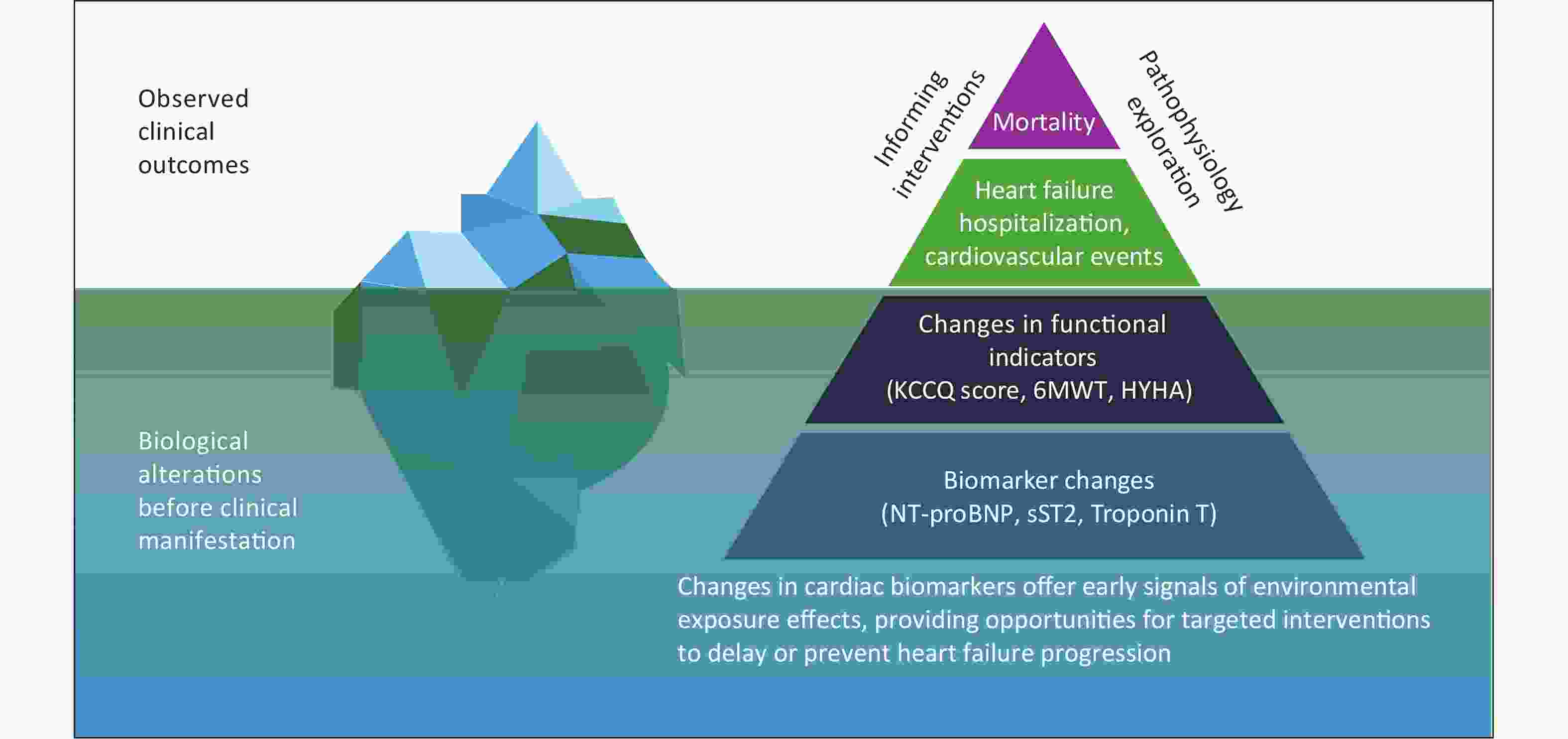
Figure 1. A biomarker-based approach to understanding air pollution-related pathophysiology in heart failure. The pyramid and iceberg model illustrate that mortality and major clinical events represent only the visible tip, while changes in functional indicators and early biomarker alterations occur beneath the surface. Studying biomarkers offers insights into underlying pathophysiology and enables earlier, targeted interventions to slow or prevent heart failure progression.
NT-proBNP, N-terminal pro-B-type natriuretic peptide; sST2, soluble suppression of tumorigenicity 2; KCCQ, Kansas City Cardiomyopathy Questionnaire; 6MWT, 6-minute walk test; NYHA, New York Heart Association functional classification.
-
Regarding the limited and highly heterogeneous existing relevant studies, we conducted a scoping review to systematically summarize how air pollutants impact heart failure biomarkers and contribute to heart failure, identified current research gaps based on available evidence. The results of this study will clarify the key concepts, map evidence, and inform further research supporting the environmental regulation and management of patients with heart failure. This study focused on clinically relevant biomarkers such as NT-proBNP, sST2, and troponin[14].
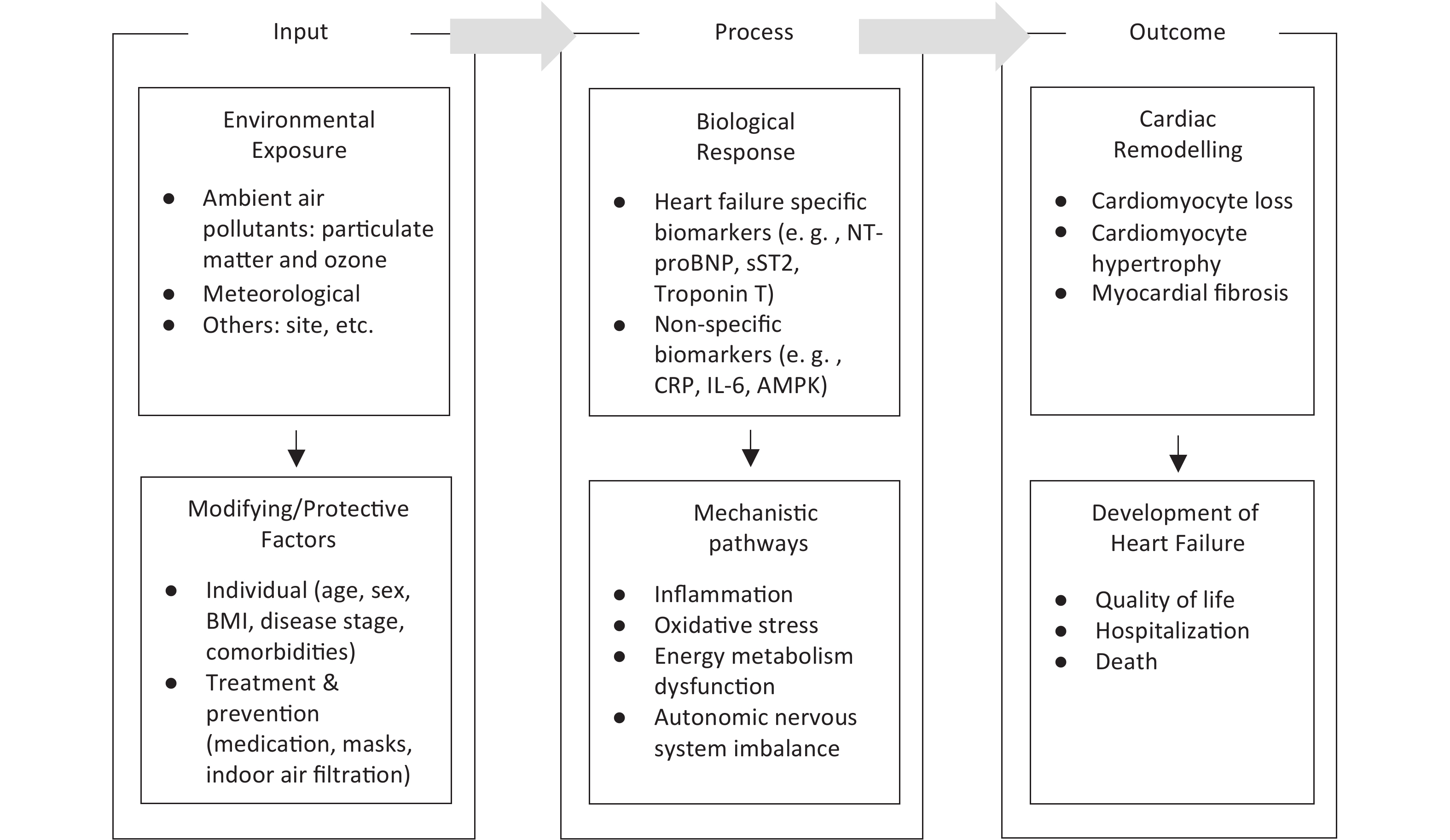
We used a conceptual framework to explore the specific research questions in this study. The conceptual framework is adapted from the Toxicological Paradigm[17]. The Paradigm is a classic model that illustrates the pathway from exposure of a susceptible population to toxins to biological response and disease, as shown in Figure 2.
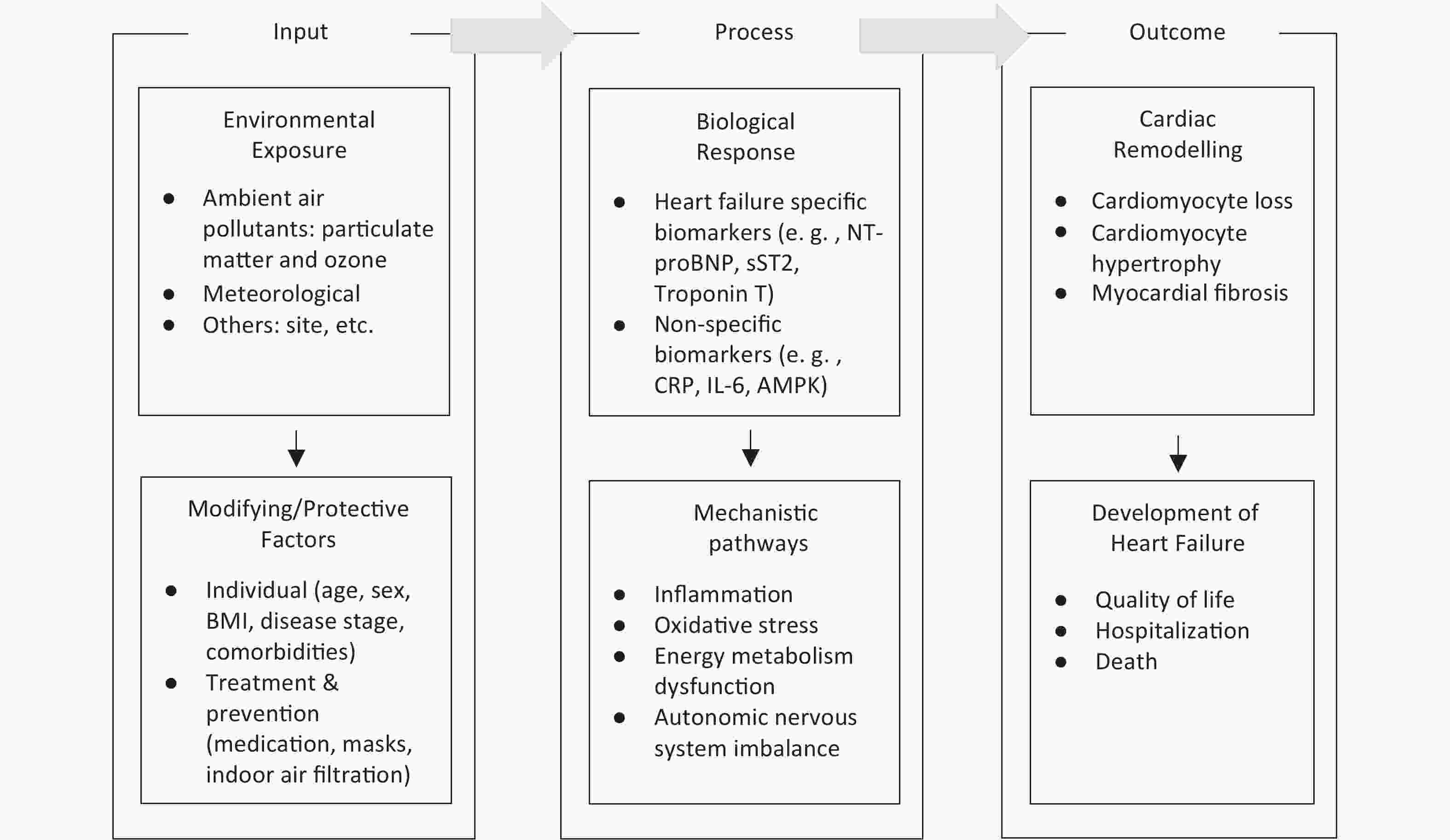
Figure 2. Conceptual framework linking environmental exposures, biological responses, and heart failure outcomes. Environmental exposures induce biological responses through pathways such as inflammation, oxidative stress, metabolic dysfunction, and autonomic imbalance. These responses can contribute to cardiac remodeling and the development of heart failure and can be modified by individual characteristics and treatment & preventive interventions. BMI, body mass index; NT-proBNP, N-terminal pro-B-type natriuretic peptide; sST2, soluble suppression of tumorigenicity 2; CRP, C-reactive protein; IL-6, interleukin-6; AMPK, adenosine monophosphate-activated protein kinase.
Based on the conceptual framework, we identified the primary research question of this study: What is the association between ambient air pollution and cardiac biomarkers in heart failure?
The specific research questions are:
• What evidence exists linking ambient air pollution to heart failure-specific biomarkers in patients with heart failure or individuals at elevated risk?
• What mechanistic pathways may underlie the effects of air pollution on these biomarkers?
• How might patient characteristics and therapeutic or preventive interventions modify these associations?
-
This literature review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR). The literature included in PubMed, Embase, and Web of Science databases was comprehensively researched. The association between air pollution and cardiac biomarkers in heart failure was considered the research question. The search was based on three key concepts: “air pollution”, “heart failure”, and “biomarkers”. The keywords in the “air pollution” category included air pollution, particulate matter, ozone, sulfur dioxide, nitrogen dioxide, and carbon monoxide. The keywords in the “heart failure” category included heart failure, systolic heart failure, and diastolic heart failure. The keywords in the “biomarker” category included biomarker, marker, natriuretic peptide, soluble suppression of tumorigenicity-2 or sST2, and troponin. Search techniques such as Boolean operators, nesting, truncation, and Medical Subject Headings (MeSH) terms were employed. The references of eligible studies and relevant review articles were also examined. We limited the publication dates to January 1, 1990 to August 31, 2024. The detailed search strategies are provided in the Supplementary Material.
-
An initial screening of all fields was performed to identify potentially relevant studies. The abstracts and full texts of all the potentially eligible articles were reviewed. In addition, a manual search of references from all selected articles was conducted to identify valuable studies. After removing duplicates and screening titles and abstracts, the full texts were assessed for eligibility.
Studies were included if they a) evaluated the relationship between exposure to criteria air pollutants such as particulate matter with an aerodynamic diameter ≤ 2.5 µm (PM2.5), particulate matter with an aerodynamic diameter ≤ 10 μm (PM10), O3, nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon monoxide (CO), and cardiac biomarkers in heart failure populations; b) included human or animal models with specific focus on heart failure or pre-heart failure conditions, assessed biomarkers such as NT-proBNP, sST2, Troponin T/I, or other cardiovascular biomarkers; c) reported quantitative outcomes using appropriate statistical descriptions; d) were peer-reviewed original research articles.
According to the definition of a biomarker, the ideal biomarker should be able to measure biological processes and predict the incidence or outcome of a disease. However, most biomarkers do not measure biological processes or have predictive value[18]. Moreover, clinically relevant biomarkers can be used as surrogate endpoints, providing more timely and sensitive information than event endpoints, such as death and hospitalization, reducing sample size and shortening study duration. Therefore, this study focused on clinically relevant biomarkers, including NT-proBNP, sST2, and troponin.
Studies were excluded if biomarkers were not evaluated, heart failure or pre-heart failure was not included as a key variable, or if relevant air pollutants were not measured. Reviews and editorial were excluded from the analysis.
-
The quality of evidence ratings for this study followed the Navigation Guide[19,20], with an initial rating of “moderate” for human evidence and “high” for non-human evidence. The overall quality was then adjusted for five predefined downgrading factors, including cross-study risk of bias, indirectness, inconsistency, imprecision, and publication bias, each reducing the rating by one or two levels depending on severity. For human evidence, three upgrade factors were also considered: large magnitude of effect, dose-response relationship, and presence of confounding factors that minimized the observed effect, which increased the rating by one to two levels. The detailed assessment criteria followed the principles of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE)[21]. Two researchers (GL and YJ) independently assessed the domains in each study. In cases of disagreement, a third researcher (HX) was involved. If a consensus could not be reached, the most conservative option was selected (avoidance of upgrades or downgrades).
-
For the selected articles, data extraction focused on study characteristics across human and nonhuman studies, including population, study design, exposure metrics, outcome measures, and main findings related to the association between air pollution and heart failure biomarkers. In this review, short-term exposure was defined as several hours to days, and long-term exposure was defined as several weeks to months. Key comparisons highlighted the differences in exposure duration, pollutants studied, and biomarkers analyzed. Due to the heterogeneity of the study designs and methodologies, a narrative synthesis approach was adopted to summarize and interpret the findings.
-
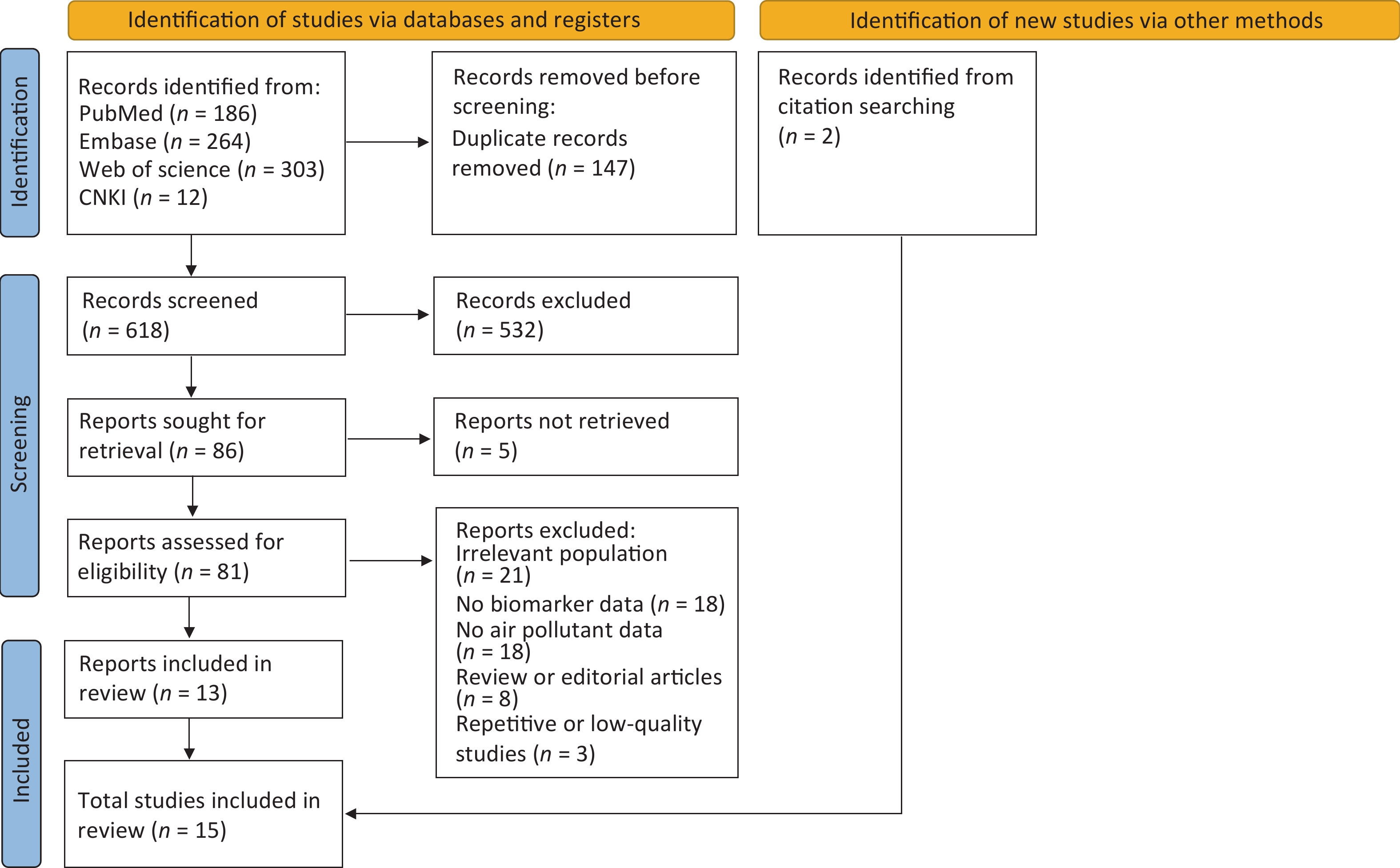
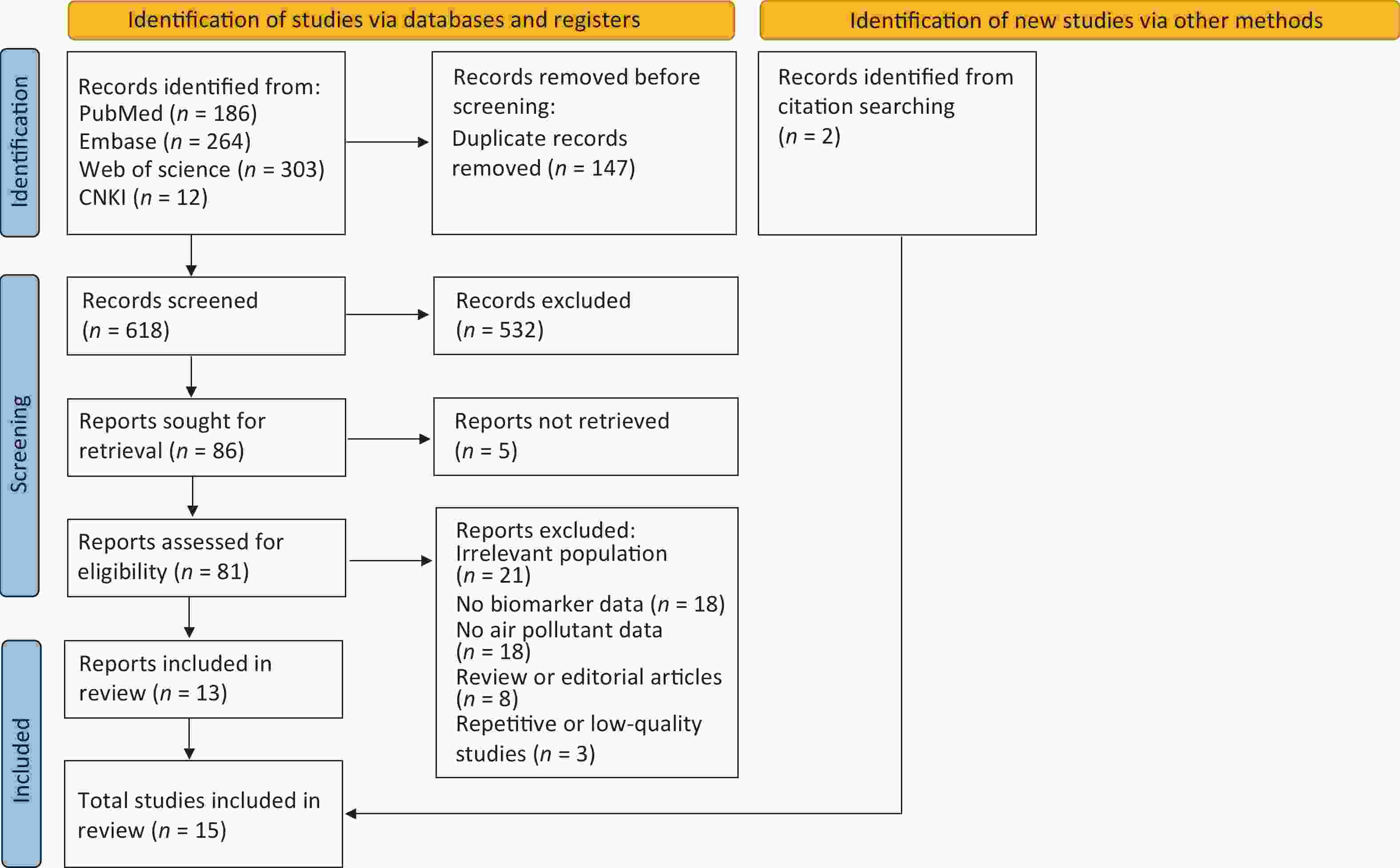
A total of 765 studies were identified from the following databases: 186 from PubMed, 264 from Embase, 303 from Web of Science, and 12 from CNKI. After removing 147 duplicate studies, 618 studies were screened by title and abstract, of which 532 were excluded because they were irrelevant. A total of 81 studies with full text were assessed for eligibility, of which 68 were excluded for the following reasons: irrelevant population (n = 21), lack of biomarker data (n = 18), lack of air pollutant data (n = 18), review or editorial articles (n = 8), and repetitive or low-quality studies (n = 3). This narrowed the selection to 13 studies that were included in the review. Fifteen studies were included, with 2 identified via citation searching. Figure 3 illustrates the study selection process.
Table S1 presents the general characteristics of the included studies (Reference, Country, Publication, Year, Study Year, Data source, Methods, Outcome, Population, Pollutants, Lag Model, and Exposure Assessment). Of the 15 studies included, 5 examined cardiac biomarkers such as NT-proBNP/ B-type natriuretic peptide (BNP), troponin, and sST2. These studies focused on heart failure patients or at least pre-heart failure populations using analytical methods including case-crossover analysis and repeated-measures panel designs. The primary pollutants studied included PM2.5 and NO2, with lags modeled from short-term (days) through long-term (months or years). The remaining 10 studies focused on other cardiovascular or inflammation-related biomarkers (e.g., interleukin-6 (IL-6), C-reactive protein (CRP), low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol). Human studies were primarily conducted in Europe and the United States, whereas non-human studies were predominantly performed in China.
-
Long-term exposure to ambient air pollution has been associated with an increased risk of heart failure onset. A prospective study of long-term combined exposures reported that various air pollutants, including PM and NO2, were associated with an increased risk of incident heart failure. The study also found that genetic susceptibility, evaluated by the heart failure genetic risk score (calculated using a formula including 12 single-nucleotide polymorphisms [SNPs]), increased the risk of heart failure[22].
Similarly, a long-term exposure study of 5.1 million adults in Canada concluded that PM, NO2, and O3 were correlated with an increased incidence of heart failure[23]. This study also found that the concentration-response curves for heart failure incidence were supra-linear with PM2.5 and NO2 and sub-linear with O3, suggesting that even slight increases in PM2.5, significantly increased the risk of heart failure, with an earlier trend toward saturation at higher concentrations. O3, on the other hand, did not significantly increase the risk of heart failure in the low concentration range, and it was only at higher concentrations (greater than 40 ppb) that the risk of heart failure increased dramatically with increasing O3 concentration[23].
In addition to its role in heart failure onset, long-term exposure to air pollutants is associated with worse clinical outcomes, including hospital readmission and mortality[10]. A retrospective study included a data analysis reported on 12,857 patients with acute coronary syndromes and showed that long-term exposure to higher levels of NO2 and PM10 was correlated with an elevated hospital readmission risk for heart failure in patients with ST-segment elevation myocardial infarction, particularly when these pollutant levels rose beyond certain thresholds[24]. A systematic review and meta-analysis found that each 10 μg/m3 increase in PM and NO2 significantly increased the risk of heart failure for long-term exposures, with the effects being more pronounced for long-term exposure, while no significant associations were observed for SO2 and CO at any exposure duration[25]. In support of these observational findings, a Mendelian randomization study provided genetic evidence for a causal relationship between long-term PM exposure and increased risks of myocardial infarction, heart failure, and adverse lipid profiles[26].
In addition, one study showed that increasing adenosine monophosphate-activated protein kinase (AMPK) activity may be promising in reducing the adverse effects of long-term PM2.5 exposure on cardiovascular health[27]. This study suggests that targeting energy metabolism pathways may offer therapeutic potential for mitigating air pollution-related cardiovascular risks.
-
Several studies have demonstrated that short-term exposure to ambient air pollutants is associated with cardiovascular events. A short-term time series-study conducted by Yang et al. showed that exposure to air pollution was associated with an increase in the number of emergency ambulance dispatches[28]. Another study, which included the health insurance data of nearly one million people, showed that short-term exposure to PM in areas of moderate or high air pollution was associated with an increased incidence of acute heart failure[29].
A short-term repeated measurement study including 28 heart failure patients reported that each 10 μg/m3 increase in PM2.5 was associated with a 0.8% increase in BNP on the same day, but the finding was not statistically significant due to the small sample size and large within-person variability in BNP[30].
In contrast, reducing exposure to air pollutants can lower cardiac biomarker levels. A short-term intervention trial demonstrated that a respiratory filter significantly reduced particulate matter concentrations and BNP levels[31]. A large-scale, short-term study involving 2,732 patients with pre-heart failure showed that acute exposure to PM2.5 was significantly associated with increased cardiac troponin T, fibrinogen, and NT-proBNP concentrations. Notably, the associations were more pronounced among patients aged < 60 years and those living in rural areas, suggesting a heightened susceptibility in these subpopulations[32]. Troponin is a highly sensitive and specific marker of cardiomyocyte damage[14]. Higher high-sensitivity troponin (hs-troponin) levels are linked to an elevated incidence of heart failure and increased risk of cardiovascular mortality[33,34], making it a key indicator of pollution-induced myocardial injury.
Similarly, a short-term prospective study that included 1,003 patients with post–myocardial infarction (post-MI) indicated that PM exposure was associated with elevated concentrations of IL-6 and fibrinogen[35]. However, a short-term repeated-measures panel study with a cohort of 132 patients with heart failure and chronic stable conditions showed no correlation between pollutant exposure and the levels of IL-6 or CRP, suggesting that modern cardioprotective therapies may mitigate the harmful health effects of air pollutants[36]. Moreover, a meta-analysis found that O3 exposure was significantly associated with heart failure risk under short-term, but not long-term, exposure conditions[25]. Subgroup analyses from various studies revealed greater pollutant-induced increases in inflammatory and vascular biomarkers, such as IL-6, fibrinogen, and lipoprotein-associated phospholipase A2 (Lp-PLA2). Notably, more pronounced pollutant effects on biomarker concentrations were observed among individuals with a lower body mass index (BMI ≤ 25 kg/m2), elevated glycated hemoglobin A1c (HbA1c) levels (> 6.5%), and during winter[37]. In addition, a panel study reported greater fibrinogen increases in response to particulate exposure among non-smokers in certain cities[35].
-
The quality assessment detailed in Table 2 indicates that the overall quality of the evidence was moderate or low. The downgrading of human evidence was mainly attributed to potential misclassification of exposures, limited sample size, large confidence intervals, and unexplained heterogeneity. As for nonhuman evidence, methodological limitations, such as problems with randomization and blinding, as well as the limited extrapolation of animal models to human health outcomes, have contributed to the downgrading of quality. Additional considerations of upgrade factors for human evidence were mainly when confounding factors weakened the observed associations, suggesting that the actual effect may be more robust than previously reported. The detailed reasons for each downgrade and upgrade decision are provided in Supplementary Table S2.
Study Initial rating Downgrade factors (human and non-human) Upgrade factors (human only) Overall grade Resulting rating Risk of Bias across studies Indirectness Inconsistency Imprecision Publication bias Large magnitude of effect Dose response Confounding minimizes effect Barclay, J. L., et al. Moderate −1 0 0 −1 0 0 0 1 −1 Low Wellenius, G. A., et al. Moderate −1 0 0 −1 0 0 0 1 −1 Low Vieira, J. L., et al. Moderate 0 0 0 0 0 0 0 0 0 Moderate Allaouat, S., et al. Moderate 0 0 0 −1 0 0 0 0 −1 Low Zhang, S. Q., et al. Moderate 0 0 0 0 0 0 0 0 0 Moderate Brüske et al. Moderate −1 0 −1 0 0 0 0 1 −1 Low Wang, Q., et al. Moderate 0 0 −1 0 0 0 0 0 −1 Low Rückerl, R., et al. Moderate −1 0 0 −1 0 0 0 1 −1 Low Zhang, Z., et al. High 0 −2 0 0 0 NA NA NA −2 Low Wu, F., et al. High −1 −1 0 0 0 NA NA NA −1 Moderate Wang, H., et al. High −1 0 0 −1 0 NA NA NA −2 Low Ramot, Y., et al. High −1 0 0 −1 0 NA NA NA −2 Low Wold et al. High −1 −1 0 −1 0 NA NA NA −3 Low Wang, Z., et al. High −1 −1 0 −1 0 NA 1 NA −2 Low Xue, B., et al. High −1 −2 0 0 0 NA NA NA −3 Low Table 2. Quality assessment of human and non-human evidence
-
Although many studies have investigated the cardiovascular effects of air pollution, relatively few have focused specifically on populations with heart failure and clinically relevant biomarkers such as NT-proBNP, sST2, and troponin[38]. Most available studies have focused on general inflammatory or metabolic biomarkers, such as IL-6, high-density lipoprotein cholesterol, and CRP, often in populations with pre-heart failure or with small sample sizes[26,35,36]. Moreover, different types of cardiovascular diseases have underlying genetic and physiological differences, resulting in varied susceptibilities to air pollution[39].
A comparative analysis of human and nonhuman studies highlighted both valuable contributions and critical gaps in understanding the association between air pollution and heart failure biomarkers. Table 3 shows that non-human studies have primarily focused on specific disease models, such as heart failure, hypertension, and obesity, under controlled experimental conditions. These studies mainly investigated pollutants such as PM2.5 and O3, with exposure durations ranging from short- to long-term. Non-human studies have also offered detailed insights into the underlying mechanisms through which air pollution affects cardiac health, including inflammation, oxidative stress, apoptosis, and metabolic disruption, which collectively contribute to cardiac remodeling and the progression of heart failure. In contrast, human studies have included a wider range of populations, such as patients with heart failure, pre-heart failure, and post-myocardial infarction. The pollutants examined in human research are more diverse, including PM2.5, PM10, O3, NO2, and CO, with exposure durations ranging from daily or weekly short-term exposure to longer-term exposure over months or years.
Non-human Studies [n = 7] Human Studies [n = 8] Population Characteristics Specific disease models (heart failure, hypertensive, obese-diabetic, stroke-prone rats) [n = 2]
AMPKα2-deficient/C57BL/6J mice [n = 3]
H9c2 rate myocardial cells [n = 2]Heart failure [n = 3]
Pre-heart failure (post-myocardial infarction) [n = 5]Methods Controlled exposure [n = 5]
Cell culture experiments [n = 2]Observational study (Cross-sectional analyses, Repeated-measures panel study, Prospective longitudinal study) [n = 6]
Experimental study (Randomized controlled trial) [n = 1]
Instrumental/Quasi-Experimental Method (Mendelian randomization) [n = 1]Pollutants PM2.5 [n = 7]
O3 [n = 1]PM2.5 [n = 8], PM10 [n = 4], O3 [n = 2], NO2 [n = 4], SO2 [n = 2], CO [n = 2], NOX [n = 1], Black carbon [n = 1],ultrafine(< 0.1µm) [n = 1], coarse particles(2.5–10µm) [n = 1] Exposure Duration a Short-term (hours to days) [n = 5]
Long-term (weeks to months) [n = 2]Short-term (daily or weekly exposure) [n = 6]
Long-term (years to decades) [n = 2]Biomarkers and Outcomes Inflammatory& Cardiovascular Risk markers (TNF-α, IL-6, IL-1β,fibrinogen,TGF-β,Collagen,SERCA2a, MHC,ANP,α-tubulin) [n = 6]
Metabolic markers (TC, HDL, LDL, AMPK activity, ATP, CPT1/CPT2, MDH, Lactate, Pyruvate, PPARα, etc.) [n = 3]
Oxidative stress markers (MDA、SOD、NOX4) [n = 2]
Heart failure-specific biomarker (BNP, ANP) [n = 1]
Hematological markers (RBC, WBC, lymphocytes ) [n = 1]
Apoptosis markers (p53、Bax、Bcl-2、Caspase-3) [n = 1]Heart failure-specific biomarker (BNP, NT-proBNP, Troponin T, Troponin I) [n = 4]
Inflammatory& Cardiovascular risk markers (CRP, IL-6, fibrinogen, D-Dimer, von Willebrand Factor, E-selectin) [n = 3]
Hematological markers (RBC, WBC, Platelet Count) [n = 2]
Metabolic markers (TG,HDL-C,LDL-C, Lp-PLA2, Fasting insulin,Fasting glucose) [n = 2]Potential Future Directions • Strengthen Heart Failure-Specific Research
• Prioritize Gaseous Pollutants in Research (O3, NO2, SO2, CO)
• Integrate Mechanistic and Translational Biomarkers
• Advance Longitudinal and Preventive Studies
• Improve Exposure AssessmentNote. [n = X] indicates the number of studies for each category. AMPK, adenosine monophosphate-activated protein kinase; PM2.5, particulate matter with an aerodynamic diameter ≤ 2.5 μm; PM10, particulate matter with an aerodynamic diameter ≤ 10 μm; NO2, nitrogen dioxide; SO2, sulfur dioxide; CO, carbon monoxide; O3, ozone; NOX, nitrogen oxides; BNP, B-type natriuretic peptide; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; ANP, atrial natriuretic peptide; RBC, red blood cells; WBC, white blood cells; CRP, C-reactive protein; IL-6, interleukin-6; IL-1β, interleukin-1 beta; TGF-β, transforming growth factor beta; SERCA2a, sarcoplasmic reticulum Ca2+ ATPase 2a; MHC, myosin heavy chain; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Lp-PLA2, lipoprotein-associated phospholipase A2; ATP, adenosine triphosphate; CPT1/CPT2, carnitine palmitoyltransferase 1/2; MDH, malate dehydrogenase; MDA, malondialdehyde; SOD, superoxide dismutase; NOX4, nicotinamide adenine dinucleotide phosphate oxidase 4.
a. Short-term and long-term exposures are defined differently in human and non-human studies due to differences in lifespan, metabolic rates, and research objectives.Table 3. Comparative summary of key dimensions in human and non-human studies with potential research directions
However, studies exploring the relationship between air pollution and biomarkers in well-defined heart failure populations are limited, and even fewer studies have focused on clinically useful biomarkers of heart failure (e.g., NT-proBNP, sST2, and troponin). The animal models are typically genetically homogeneous. They may not fully capture the genetic, lifestyle, and environmental diversity observed in human populations, thereby reducing the applicability of the findings to broader human settings. The potential synergistic effects of multiple pollutants, such as the combined impact of PM2.5 and O3, have also received limited attention.
Integrating mechanistic insights from non-human studies with epidemiological evidence from human research is essential to understand better the causal pathways linking air pollution to heart failure-specific biomarkers. Addressing these gaps could further our understanding of how air pollution affects heart failure-specific biomarkers and contribute to the development of targeted therapies or interventions for at-risk populations.
-
The associations between air pollutants and heart failure biomarkers varied across pollutant types, exposure durations, and study populations. PM2.5, the most extensively studied pollutant, showed the strongest associations. Short-term exposure to PM is significantly linked to an elevated risk of heart failure, and long-term exposure is associated with a notable magnitude (a 74.8% increase in risk per 10 μg/m3 of PM2.5). Consistent with these findings, nonhuman studies reveal that PM2.5 exposure disrupts myocardial energy metabolism by reducing adenosine triphosphate (ATP) production, decreasing peroxisome proliferator-activated receptor alpha (PPARα) expression, impairing fatty acid β-oxidation and the tricarboxylic acid (TCA) cycle, and increasing glycolysis, ultimately leading to cardiac dysfunction. Additionally, PM2.5 exposure intensifies oxidative stress by increasing malondialdehyde (MDA) and inducible nitric oxide synthase (iNOS) levels, reducing superoxide dismutase (SOD) activity, and upregulating nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX4), contributing to inflammation, with elevated tumor necrosis factor-alpha (TNF-α) and Interleukin-1 beta (IL-1β), and to cardiomyocyte apoptosis[40,41]. Long-term exposure induces adverse cardiac remodeling, including left ventricular hypertrophy, fibrosis, and dysfunction, consistent with incipient heart failure. AMP-activated protein kinase catalytic subunit alpha 2 (AMPKα2) deficiency exacerbates these effects, whereas enhancing AMP-activated protein kinase (AMPK) activity shows the potential to mitigate air pollution-related health impacts. Intervention studies have demonstrated that reducing particulate exposure through respiratory filters can significantly lower BNP levels.
In contrast, O3 generally exhibits a weak or no association with cardiovascular biomarkers in human studies, and nonhuman studies have similarly shown minimal direct cardiac toxicity from acute O3 exposure. However, strain-specific responses suggest that underlying cardiovascular or metabolic disorders may modulate susceptibility to O3. Several studies have reported links between PM and other air pollutants and increased cardiac biomarkers; however, the findings remain inconsistent, which may be due to differences in study design, population characteristics, and methods of exposure assessment.
-
Several studies have shown that air pollution results in the development of heart failure through various mechanisms. These mechanisms include inflammation, oxidative stress, autonomic nervous system dysfunction, and energy metabolism[9,42,43].
-
Air pollution can lead to heart failure by activating the inflammatory response, as supported by many non-human experimental studies. Mechanistic non-human studies have discovered three sequential phases: pro-inflammatory, healing (fibrosis), and remodeling. The pro-inflammatory stage is dominated by cytokines such as tumor necrosis factor (TNF), IL-1β, IL-6, and IL-8. The healing stage involves tissue death and scar formation caused by macrophage infiltration. The third stage is remodeling, which results in chronic inflammation and fibrosis owing to an ongoing immune response. The three successive stages of the inflammatory response play crucial roles in the development of cardiac dysfunction and heart failure[44,45]. Chronic inflammation also promotes the upregulation of adhesion molecules, coagulation factors, and pro-inflammatory mediators such as CRP and IL-6, further accelerating heart failure progression[46]. Biomarkers such as sST2, which are elevated during the inflammatory and fibrotic phases, are independently associated with higher rates of hospitalization for heart failure and mortality, highlighting their clinical relevance in both disease monitoring and outcome prediction[47].
-
Various air pollutants, including PM and chemicals, trigger oxidative stress, which is an important pathological mechanism that results in heart failure[48]. Oxidative stress leads to cardiac remodeling and dysfunction by activating metal matrix proteases and degrading extracellular matrix proteins[49,50]. In addition, oxidative stress is known to trigger subcellular remodeling, abnormal Ca2+ processing, and cardiomyocyte loss due to apoptosis, necrosis, and fibrosis[49]. An experimental study of acute exposure showed that exposure to PM2.5 resulted in increased oxidative stress, leading to myocardial apoptosis through the activation of NOX4[51]. PM2.5 exacerbates these processes by generating reactive oxygen species (ROS), with NOX4 playing a pivotal role in driving myocardial apoptosis and fibrosis[51,52]. An experimental study further demonstrated that chronic exposure to PM2.5 induces adverse cardiac remodeling, including left ventricular hypertrophy, fibrosis, and dysfunction[53]. The study also identified specific markers, such as increased expression of atrial natriuretic peptide (ANP), myosin heavy chain (MHC), and collagen, and decreased expression of Sarcoplasmic Reticulum Ca2+ ATPase 2a (SERCA2a), which reflects a pro-fibrotic phenotype and early heart failure[53]. Collectively, these results demonstrate the involvement of oxidative stress in the pathologic impact of PM2.5 on the development of heart failure. Myocardial injury caused by oxidative stress may also contribute to elevated troponin levels. Elevated troponin levels have been linked to an increased risk of incident heart failure and cardiovascular death[12,33].
-
The autonomic nervous system is critical in the pathophysiology of heart failure and is a key target for its treatment of heart failure[54]. Many epidemiological studies have revealed that pollutants can affect blood pressure, resting heart rate, and heart rate variability, which are mainly controlled by the autonomic nervous system[55]. Human and non-human biological studies have also found that pollutants can stimulate defensive sensory nerves, contributing to pollutant-induced autonomic dysfunction[56]. Disruption of the balance between the sympathetic and parasympathetic nervous systems can result in undesirable consequences, which can worsen heart failure[57]. Sympathetic activation increases the myocardial oxygen demand and workload, leading to cardiomyocyte injury, cardiac remodeling, and hypertrophy. The inhibition of parasympathetic activation in heart failure results in arrhythmias and sudden cardiac death[57]. Autonomic nervous system dysfunction, particularly sympathetic overactivation, may contribute to elevated NT-proBNP levels by increasing cardiac wall stress[58]. Elevated NT-proBNP levels can predict the incidence of heart failure, disease progression, and cardiovascular deaths[12,15].
-
Energy metabolism plays a crucial role in heart failure, which is characterized by a notable decrease in high-energy phosphate levels, mitochondrial dysfunction, and increased reliance on glucose as the primary energy source[59]. Fine PM contributes to myocardial injuries through myocardial energy metabolism by regulating PPARα expression and translation[60]. This process is critical for regulating cardiac lipids, glucose metabolism, and beta-oxidation. The level of PPARα expression directly affects the ability of cardiac tissues to utilize fatty acids for energy. Metabolic dysfunction impairs contractile function and is associated with poor outcomes in patients with heart failure.
-
These findings have important implications for clinical practice and public health policy. The clear link between PM exposure and cardiac biomarkers in patients with heart failure emphasizes the need to intervene in at-risk populations to mitigate the adverse cardiovascular effects of air pollution.
Physicians should incorporate environmental risk factors into comprehensive heart failure care, including clinical management and personalized guidance on pollution avoidance; raise awareness among cardiovascular physicians and patients about the cardiovascular risks of air pollution; and promote routine risk assessment to support more effective management of pollution-related cardiovascular threats.
Public health interventions that minimize individual exposure to air pollution are also critical. Encouraging personal protective equipment, such as masks, and promoting indoor air filtration systems can help vulnerable individuals, particularly those with pre-existing cardiovascular conditions, reduce their exposure during periods of high pollution. However, because these measures are less effective for gaseous pollutants such as O3, additional protective strategies should be considered. These may include real-time O3 alerts, policy-driven reduction of O3 precursors, and the promotion of healthier indoor environments with proper ventilation rather than recirculation.
To mitigate these detrimental effects at the population level, stricter air quality regulations targeting the reduction of key pollutants should be implemented. These measures can be implemented through legislation and regulations to reduce emissions from industrial activities, transportation, and residential sources, thereby mitigating the overall cardiovascular burden in populations exposed to high pollutant levels[61]. This knowledge could inform future pharmacological innovations to prevent or alleviate pollution-induced cardiovascular damage, thereby offering new avenues for reducing hospitalizations and mortality associated with heart failure. Biomarker monitoring may help guide personalized prevention strategies and optimize heart failure management in vulnerable individuals.
-
More studies on heart failure-specific populations and clinically relevant biomarkers are needed to advance research on the relationship between ambient air pollution and heart failure. Particularly, research on high-risk populations with established heart failure should be emphasized, as individual characteristics may modify vulnerability to pollutant-related adverse effects. Additionally, prioritizing research on under-studied gaseous pollutants, including O3, NO2, SO2, and CO, is essential to uncover their cardiovascular impact. Regarding the complexity of real-world exposure, future studies should examine the cumulative and interactive effects of multiple pollutants as well as their potential combined effects with climate change and extreme weather conditions. Improved exposure assessments through personal monitoring and modeling advancements can provide precise and individualized pollution exposure data and improve comparability across studies.
Furthermore, integrating mechanistic and translational biomarkers, including those linked to oxidative stress, inflammation, and cardiac remodeling, can provide insights from animal models to human studies and elucidate key pathways. Further studies on biomarkers associated with the mechanisms of air pollution in heart failure development (e.g., inflammation and oxidative stress) are critical for the development of targeted therapies. Finally, longitudinal studies incorporating biomarkers along with therapeutic interventions can offer valuable insights into preventive strategies against pollution-induced heart failure progression.
-
This scoping review has several limitations. First, the number of eligible studies was limited, and many did not focus specifically on heart failure populations or clinically relevant biomarkers, making it difficult to conduct a quantitative synthesis. Second, most studies were conducted in high-income countries, where differences in pollutant exposure levels and population characteristics may limit the generalizability of the findings to low- and middle-income settings. Third, relatively few recent studies have been identified, which may reduce the relevance of the findings to current air pollution profiles and clinical practices.
-
This review summarizes the association between air pollutants and heart failure biomarkers. Compared to studies focusing on clinical outcomes, such as mortality and hospitalization, research on the association between air pollutants and heart failure biomarkers, such as NT-proBNP, sST2, and troponin, is limited.
PM2.5, the most extensively studied pollutant, showed the strongest associations. In contrast, O3 generally exhibited a weak or no association with cardiovascular biomarkers in human studies.Similarly, non-human studies showed minimal direct cardiac toxicity from acute O3 exposure.
Future research should address these gaps by prioritizing studies on heart failure-specific populations and under-researched gaseous pollutants to facilitate the development of targeted preventive and therapeutic strategies. Stricter air quality regulations and individual-level interventions are critical for reducing cardiovascular risks related to exposure to air pollutants.
Air Pollution and Cardiac Biomarkers in Heart Failure: A Scoping Review
doi: 10.3967/bes2025.120
- Received Date: 2025-02-12
- Accepted Date: 2025-09-04
-
Key words:
- Air pollution /
- Heart failure /
- Biomarkers /
- Natriuretic peptides /
- Troponin /
- sST2
Abstract: Ambient air pollution is increasingly being recognized as a risk factor for heart failure; however, its effects on cardiac biomarkers remain unclear. This scoping review assessed the existing evidence on the association between air pollution and cardiac biomarkers in heart failure, described the key concepts, synthesized data, and identified research gaps. Following the PRISMA-ScR guidelines, PubMed, Embase, Web of Science, and CNKI databases were searched for studies on air pollution, heart failure, and biomarkers. A total of 765 records were screened, and 81 full texts were assessed for eligibility, resulting in 15 studies. The results showed that the exposure to particulate matter was associated with elevated N-terminal pro-B-type natriuretic peptide and troponin levels. Several studies have linked particulate matter exposure to a higher cardiovascular risk and heart failure biomarkers. Inflammatory and oxidative stress markers were consistently elevated across studies, supporting the biological relevance of these associations. However, few studies have focused specifically on populations with heart failure or clinically relevant biomarkers, and the evidence for gaseous pollutants remains inconclusive. These findings highlight the need to integrate environmental risk assessment into heart failure care and inform policy efforts to reduce the pollution-related cardiovascular burden. Further research should address these gaps through improved exposure assessments and the integration of mechanistic evidence.
Conception or design of the study was contributed by Gang Li, Yanhui Jia, Yunshang Cui, and Mary A. Fox; acquisition, analysis, or interpretation of data by Gang Li, Yanhui Jia, Yunshang Cui, Hongbing Xu, Yuhui Zhang, Shaowei Wu, Yunxing Jiang, Tongyu Ma, and Mary A. Fox; drafting of the manuscript by Gang Li, Yanhui Jia, and Yunshang Cui; and critical review and revision of the manuscript by Gang Li, Yanhui Jia, Yunshang Cui, Hongbing Xu, Yuhui Zhang, Shaowei Wu, Yunxing Jiang, Tongyu Ma, and Mary A. Fox. All authors have approved the manuscript and ensured its accuracy or integrity.None.
&These authors contributed equally to this work.
| Citation: | Gang Li, Yanhui Jia, Yunshang Cui, Shaowei Wu, Tongyu Ma, Yunxing Jiang, Hongbing Xu, Yuhui Zhang, Mary A Fox. Air Pollution and Cardiac Biomarkers in Heart Failure: A Scoping Review[J]. Biomedical and Environmental Sciences. doi: 10.3967/bes2025.120 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: