-
The Beijing-Tianjin-Hebei (BTH) region has the most severe atmospheric compound pollution in China[1]. The effectiveness of single-city governance is limited, given the cross-regional transmission characteristics of air pollution[2]. In 2017, the Chinese government formally defined the “2+26” cities and implemented unified standards and joint law enforcement to enhance regional environmental quality. Handan City is not only one of the “2+26” cities but also a typical city in the BTH region[3]. We have been conducting systematic testing of refined pollutant components from various typical emission sources in Handan City to obtain their spectra and construct a high-resolution emission inventory of pollution source emissions for the BTH region, thereby improving the scientific basis for environmental decision-making[4-6]. However, systematic research studies on the characteristics of fine particulate matter (PM)2.5-bound components and their impact on environmental pollution remain limited, as are those on the population health risks of such particles emitted by cooking fumes in the catering industry in the BTH region.
Numerous studies have demonstrated that PM2.5 serves as a significant carrier of toxic and harmful substances that pose a significant threat to human health[7-10], being able to permeate the human body after binding to polycyclic aromatic hydrocarbons (PAHs)[11-12]. The propensity of inhaled PM2.5-bound PAHs to accumulate in vital organs can precipitate inflammatory responses, lung cancer, and other severe ailments in the pulmonary and respiratory systems[13-15]. Studies have shown that the concentration of gaseous-phase PAHs in the atmosphere is typically higher than that of particulate-phase PAHs[16-17]. However, particulate-phase PAHs have attracted more widespread attention as nearly 90% of these molecules are absorbed by PM2.5, which renders them more likely to remain in the environment for a longer time than gaseous-phase PAHs do, thus posing a high health risk to the exposed population[18-20]. Particulate-phase PAHs are pervasive in human undertakings, such as industrial production, biomass combustion, motor vehicle exhaust, and cooking activities[21-23]. Owing to their unique complexity and prolonged exposure profiles compared with other emission sources, those from cooking sources have attracted substantial scholarly attention regarding their associated health hazards[24-26].
Studies have already established that PAHs generated during cooking pose health risks to humans[27-29]. The extent of these risks is primarily influenced by the cooking method, type and quality of cooking oil, and fuel used[30] as well as the ventilation conditions in the kitchen. Notably, PM2.5-bound PAH concentrations can vary considerably among different cooking styles, with those generated via deep-frying, pan-frying, and roasting (34.6–609.0 ng/m3) typically being significantly higher than the concentrations emitted during steaming and boiling (10.5–29.5 ng/m3)[31-33]. Some studies have found that the choice of cooking oil significantly influences PAH concentrations, with higher-quality oils yielding lower levels[34]. Factors such as cooking temperature, food lipid content, and ingredient composition also influence PAH emissions[35]. In one study[36], the exposure risks of women to PAH compounds were investigated under various kitchen configurations; namely, indoor kitchens without partitions, indoor kitchens with partitions, separate enclosed kitchens outside the home, open kitchens, and open kitchens under stairways. The highest levels of PM2.5-bound PAHs occurred in open kitchens under stairways owing to the small space size and poor ventilation, resulting in the highest lifetime exposure risk per million population (400) among all types of kitchens[36]. Therefore, studying the characteristics of PM2.5-bound PAHs emitted from various cooking styles will enhance our understanding of the primary origins of these potentially harmful molecules stemming from cooking activities.
Even under identical cooking conditions, PAHs may affect various populations differently owing to age- and sex-related disparities in metabolic rates[16,37]. For instance, children and adolescents exhibit considerable fluctuations in their respiratory rates and body weight parameters during growth, whereas older adults generally exhibit lower respiratory rates and reduced body weights compared with their younger and middle-aged counterparts as a result of metabolic slowdown and other related factors[38-41]. To date, the population-differentiated health risks associated with PAHs emitted from different cooking styles have not been extensively studied. Furthermore, the precise relationships among body weight (BW), exposure duration (ED), and cancer risk remain unclear. Hence, a comprehensive understanding of PM2.5-bound PAH emissions from cooking sources and their health implications in different populations is imperative for effectively managing cooking-related emissions and safeguarding public health. Given the heightened cancer potency of particulate-bound PAHs relative to that of the gaseous forms, the inhalation, ingestion, and dermal contact of PAH-laden cooking emissions carry substantial cancer risks for humans[42], can cause diseases of the respiratory system in children and adults, and may damage DNA[43-44]. Consequently, cooking methods that pose elevated cancer risks require increased vigilance, particularly in vulnerable demographics.
To enhance our understanding of the health risks associated with PM2.5-bound PAHs originating from diverse cooking sources, we sampled PM2.5 emissions from 11 cooking styles and used gas chromatography-triple quadrupole mass spectrometry (GC-MS/MS) to quantitatively analyze 16 PAHs specified by the US Environmental Protection Agency (USEPA). Moreover, the human health risks of PM2.5-bound PAHs from three exposure routes (ingestion, inhalation, and dermal contact) and the respiratory deposition doses (RDDs) of the compounds were assessed. This study aimed to (a) analyze the concentration and composition of PM2.5-bound PAHs emitted from different cooking styles; (b) assess the health risks of PM2.5-bound PAH exposure through inhalation, ingestion, and dermal contact routes to individuals in different age and sex groups; and (c) explore the effects of PM2.5-bound PAHs from different cooking styles on respiratory health in children and adults.
-
Handan City, which is located at the southernmost end of the BTH region, serves as a key urban center for the coordinated development of the region and the Central Plains Economic Zone. It holds a crucial demographic position in the BTH region owing to its sizable population, which ranks among the highest in Hebei Province, with a total of 9.3669 million people in 2021. Moreover, Handan has a comprehensive economic foundation with a diverse structure that encompasses manufacturing, agricultural, and service industries. The regional gross domestic profit of 411.48 billion yuan in 2021 is a result of the leveraging of the strategic geographical location, well-balanced population demographics, and robust economic framework of this pivotal city. Thus, cooking fumes were collected on a commercial street in Handan City from July 8 to August 3, 2022. To cover almost all cooking methods, 11 cooking styles were selected for comprehensive testing and analysis: barbecue (BB), Sichuan and Hunan cuisine (SHC), Zhejiang cuisine (ZC), Northeast cuisine (NC), Western fast food (WFF), Chinese fast food (CFF), Noodle (ND), Canteen (CT), Chinese cuisine (CC), Beijing cuisine (BC), and Guangdong cuisine (GC). Detailed descriptions are provided in Supplementary Tables S1 and S2.
Sampling was performed during standard lunch (11:30–13:30) and dinner (18:30–20:30) hours, aligning with the operational schedules of catering establishments. The end of the oil smoke purifier was chosen as the sampling point[45]. PM2.5 samples were collected onto 47 mm Teflon membranes (Whatman Inc., Maidstone, UK) using a portable atmospheric particulate matter sampler (Beijing Municipal Bureau of Labor and Social Security, China) at an isokinetic sampling flow rate of 5 L/min, ensuring that the concentration of PM2.5 sampled was consistent with the concentration in the chimney. At least four samples were collected from each restaurant over a continuous hour-long sampling period. To minimize errors, the on-site blanks and samples were sealed, transported under identical conditions, and stored at –20 °C until analysis. The principles of sample collection can be found in a previously reported study[46]. Additionally, rigorous quality assurance and control measures for samples collected from Teflon membranes were implemented[47-48] to ensure the effectiveness of the sampling method.
-
All the PM2.5 sample-containing filter membranes, including the seven blank filters, were cut into small pieces and weighed. Each piece was then individually placed into prenumbered 66 mL extraction cells, and the matter components were extracted in a 1:1 (v/v) mixture of hexane and methylene chloride in an ASE 350 accelerated solvent extraction system (Thermo Fisher Scientific, Waltham, MA, USA). The extract was concentrated to 1 mL and purified on a composite silica gel column (10 g of activated silica gel and 6 g of anhydrous sodium sulfate) that had been prewashed with 40 mL of n-hexane followed by a 40 mL mixture of n-hexane and dichloromethane[49]. The purified extract was then evaporated to near dryness. Subsequently, 5 µL of an internal standard solution (L429-RS Recovery Standard Stock Solution) was added and the mixture was diluted to 50 µL with n-hexane. The analysis was conducted at the Research Center for Eco-Environmental Sciences of the Chinese Academy of Sciences.
-
To more accurately quantify the PAH contents in all extracts, a GC-MS/MS method that has been widely used for the quantitative testing of PAHs was established[50]. The following 16 priority PAHs listed by the USEPA were measured: naphthalene (Nap), acenaphthylene (Acy), acenaphthene (Ace), fluorene (Fle), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flu), pyrene (Pyr), benzo[a]anthracene (BaA), chrysene (Chr), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), indeno[1,2,3-cd]pyrene (IcdP), dibenzo[a,h]anthracene (DBahA), and benzo[ghi]perylene (BghiP).
The extracts were analyzed using both GC-MS/MS (7010; Agilent Technologies, Santa Clara, CA, USA) and GC (7890B; Agilent). The inlet temperature was set to 280 °C, and non-split injection was performed with an injection volume of 1.0 µL and a flow rate of 1.2 mL/min. Separation was achieved using a DB-5MS column (30 m × 250 μm × 0.25 μm), with helium (99.9999%) as the carrier gas at a flow rate of 1.2 mL/min. The following temperature program was used: an initial 3 min hold at 80 °C; increase to 230 °C at a rate of 30 °C/min; an additional 2 min hold; increase to 300 °C at a rate of 3 °C/min; and a final 5 min hold[51]. All other parameters are detailed in Supplementary Table S3.
-
Rigorous quality control and assurance measures were implemented during the sample preparation and analysis processes. A program blank was run for every five PAH samples to ensure instrument stability. A mixed solution containing Nap-d8, Acy-d8, Phe-d10, Flu-d10, BaA-d12, Chr-d12, BaP-d12, DBahA-d14, BghiP-d12, Ace-d10, Pyr-d10, and BeP-d12 standards was used as the internal standard and added to all samples to monitor the extraction and purification procedures. The recovery rates of all deuterated PAHs ranged from 83.9% to 109.0%, which were within the acceptable range. To ensure method reliability, all samples were tested in replicates, and the standard deviation of the results was within 10%. Three or more blank samples underwent the same preprocessing and testing steps, and all target PAH concentrations in the blank samples were below the detection limit. For a single PAH quantification method, the detection limit was determined by adding the lowest concentration of the PAH standard, which was defined as three times the standard deviation obtained from seven repeated measurements (Supplementary Table S4).
-
To evaluate the toxicity of the 16 PAHs present in cooking fumes from the 11 cooking styles, we introduced toxic equivalents for the health risk analysis. The benzo[a]pyrene toxic equivalent (TEQBaP) was used to assess the toxicity of other PAHs relative to benzo[a]pyrene according to the methodology outlined by Ssepuya et al.[52]. TEQBaP was calculated using Equation (1), and the percentage contribution of each PAH to total carcinogenicity was calculated using Equation (2)[53]:
$$ {TEQ}_{B\mathrm{a}P}=\sum _{i=1}^{16}({C}_{i,j}\times {TEF}_{i}) $$ (1) $$ {({\text{%} Carc.{\%} Potential})}_{i}=\frac{{(RC\times TEF)}_{i}}{\sum _{i=1}^{16}{(RC\times TEF)}_{i}}\times 100 $$ (2) where $ {TEQ}_{B\mathrm{a}P} $ represents the toxic equivalent quantity (ng/m3), $ {C}_{i,j} $represents the average concentration of the ith PAH species in the jth cooking style (ng/m3), $ {TEF}_{i} $represents the toxicity equivalent factor of the PAH compound (TEF values for the 16 PAHs are presented in Supplementary Table S4[54]), and $ RC $ represents the relative abundance marker of an individual PAH ($ RC={\mathrm{P}\mathrm{A}\mathrm{H}}_{i}/\mathrm{B}\left[\mathrm{a}\right]\mathrm{P}) $.
The incremental lifetime cancer risks (ILCRs) associated with ingestion, inhalation, or dermal contact exposure to cooking fumes emitted from the different cooking styles were evaluated in four different groups stratified by age and sex[41,55,56]: male and female children (≤ 5 a), adolescent males and females (6–17 a), adult males and females (18–59 a), and older adult males and females (≥ 60 a). The ILCRs for the three exposure routes were calculated as follows[57]:
$$ {ILCR}_{ing}^{i,j}=\frac{{C}_{i,j}\times {TEF}_{i}\times \sqrt[1/3]{\dfrac{BW}{70}}\;{\times\; CSF}_{ing}\times {IR}_{ing}\times ED\times EF}{BW\times AT}\times CF $$ (3) $$ {ILCR}_{inh}^{i,j}=\frac{{C}_{i,j}\times {TEF}_{i}\times {\sqrt[1/3]{\dfrac{BW}{70}}\times CSF}_{inh}\times {IR}_{inh}\times ED\times EF}{BW\times AT\times PEF} $$ (4) $$ \begin{aligned} & {ILCR}_{der}^{i,j}=\\ & \dfrac{{C}_{i,j} \times {TEF}_{i}\times \sqrt[1/3]{\dfrac{BW}{70}} \times {CSF}_{der}\times SA\times AF\times ABS\times ED\times EF}{BW\times AT}\times CF \end{aligned} $$ (5) $$ {ILCR}_{total}^{i,j} = {ILCR}_{ing}^{i,j}+{ILCR}_{inh}^{i,j}+{ILCR}_{der}^{i,j} $$ (6) where $ {C}_{i,j} $represents the average concentration of the ith PAH species in the jth cooking style (ng/m3); $ {CSF}_{ing} $, $ {CSF}_{inh} $, and $ {CSF}_{der} $ represent the carcinogenic slope factors of the ingestion, inhalation, and dermal contact routes (7.3, 3.85, and 25 mg·kg−1·d−1, respectively[58]); $ {IR}_{ing} $ (mg/d) and $ {IR}_{inh} $ (m3/d) represent the ingestion and inhalation rates, respectively; $ ED $ represents the exposure duration (years); $ CF $ represents the conversion factor (10–6); $ EF $ represents the exposure frequency (d/year); $ BW $ represents the body weight (kg); $ AT $ represents the average contact time (25,550 d)[59]; $ PEF $ represents the particulate emission factor (1.36 × 109 m3/µg)[60]; $ SA $ represents the dermal surface area (m2/d); $ AF $ represents the dermal adherence factor (mg/cm2); and $ ABS $ represents the dermal adsorption fraction (unitless, 0.13)[61]. The detailed parameters are listed in Supplementary Table S5.
-
Owing to the uncertainty involved in calculating cancer risks, the influencing factors mainly include weight, duration and mode of exposure, and other related parameters. To conduct an uncertainty analysis of the cancer risk of PAHs emitted from different cooking styles, Monte Carlo simulations were performed using Crystal Ball 11.1. The simulations involved 20,000 random iterations, with a confidence level of 95%. The detailed parameters used in the simulation are listed in Supplementary Table S6.
-
The inhalation of PAHs emitted from cooking fumes poses a significant threat to respiratory health[44]. In this study, the RDD was estimated on the basis of the PM2.5 deposition (Mdep) rate[62]. The inhalable fraction (IF) was first determined for calculating the respiratory deposition fraction (DF) of PM2.5 in the upper airway, tracheobronchial, and alveolar regions, and the corresponding RDD was then obtained. The following equations were used for these calculations[63-64]:
$$ IF=1-0.5 \;(1-\frac{1}{1+0.0007{d}_{p}^{2.8}}) $$ (7) $$ \begin{aligned} {DF}_{UA}= & IF\times (\frac{1}{1 + \text{exp} \left(6.84 + 1.183ln{d}_{p}\right)}\;+\\ & \frac{1}{1 + \text{exp} \;(0.924 - 1.885ln{d}_{p})}) \end{aligned} $$ (8) $$\begin{aligned} {DF}_{TB}=& \left(\frac{0.00352}{{d}_{p}}\right)[ \text{exp} \left(-0.234{\;(\text{ln}{d}_{p}+3.40)}^{2}\right) +\\ & 63.9 \text{exp}\; (-0.819{\;(\text{ln}{d}_{p}-1.61)}^{2}) ] \end{aligned} $$ (9) $$ \begin{aligned} {DF}_{AL}=& \;\left(\frac{0.0155}{{d}_{p}}\right)[\text{exp}\left(-0.416\;{(\text{ln}{d}_{p}+2.84)}^{2}\right) +\\ & 19.11\text{exp}\;(-0.482{\;(\text{ln}{d}_{p}-1.362)}^{2}) ] \end{aligned} $$ (10) $$ {M}_{dep}=PM\times {V}_{m}\times \left(DF\right) $$ (11) where $ {d}_{p} $ represents the diameter of the particle; $ {DF}_{UA} $, $ {DF}_{TB} $, and $ {DF}_{AL} $ represent the deposition fractions in the upper airway, tracheobronchial, and alveolar regions, respectively; $ {M}_{dep} $represents the PM2.5 deposition rate in parts of the body (ng/h); $ PM $ represents the mass concentration of the 16 PAHs in different cooking styles (ng/m3); $ {V}_{m} $ represents the inhalation rate (0.33 and 0.83 m3/h for children and adults, respectively[65]); and $ DF $ represents the total deposition fraction (sum of $ {DF}_{UA} $, $ {DF}_{TB} $, and $ {DF}_{AL} $).
-
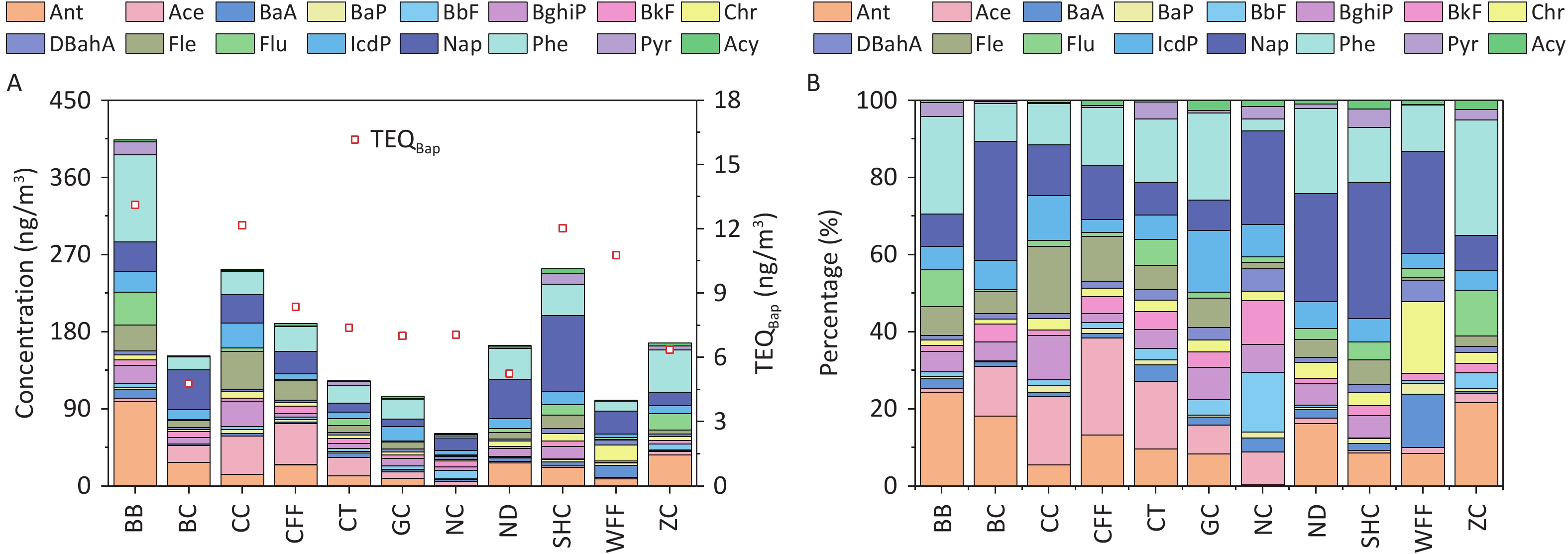
The concentrations of PAHs emitted from different cooking styles are shown in Figure 1A. The total concentrations of the 16 PAHs (ΣPAHs) ranged from 61.10 to 403.80 ng/m3. Notably, the BB sample had the highest concentration of ΣPAHs, exceeding those of the other cooking styles by 1.6–6.6 times. Consistent with another study, Chinese cooking styles such as CC, SHC, and CFF, which involve stir-frying and deep-frying, exhibited higher ΣPAH levels[66].
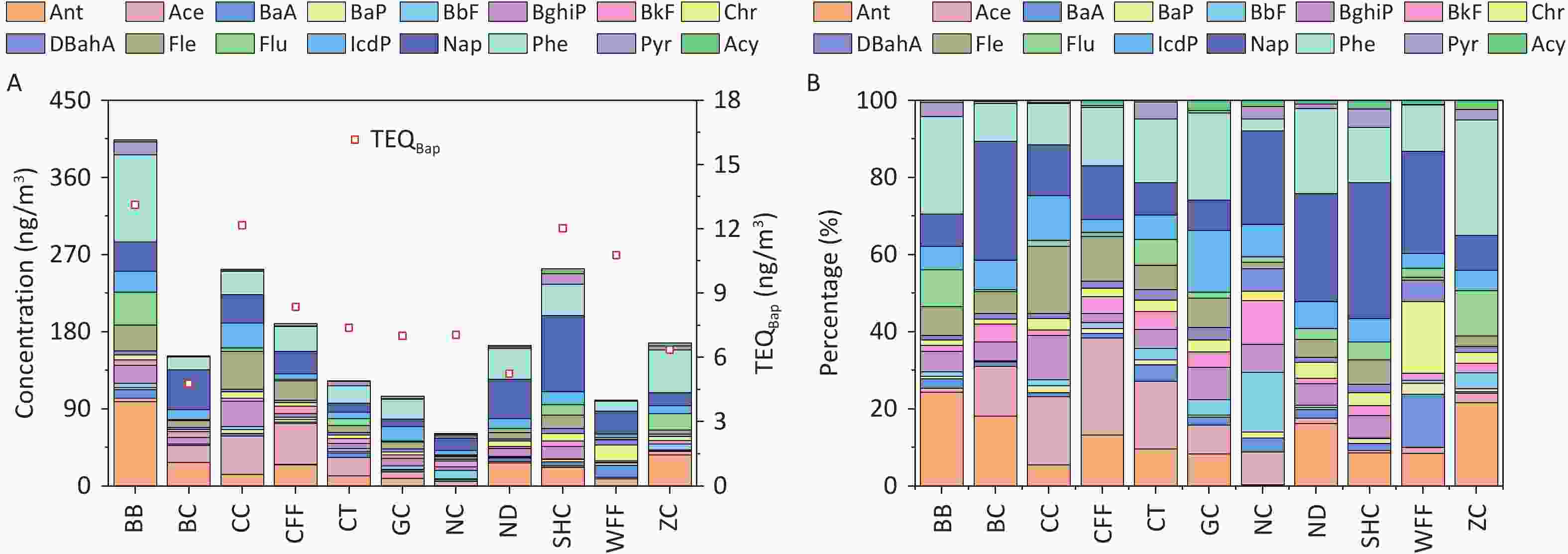
Figure 1. (A) PAH concentrations and TEQ values, and (B) percentages of PAHs emitted from different cooking styles.
The composition of ΣPAHs varied among the Bdifferent cooking styles (Figure 1B). The Nap concentrations in the SHC, BC, ND, WFF, and NC samples accounted for 35.2%, 30.8%, 28.0%, 26.4%, and 24.3% of ΣPAHs, respectively, whereas they ranged from 7.9% to 14.0% in samples from the other cooking styles. Phe and Ant were the dominant PAHs in most cooking styles, accounting for 3.0%–30.0% and 0.3%–24.4% of ΣPAHs, respectively. In particular, high levels of Phe and Ant were noted in the BB sample, accounting for 25.2% and 24.4%, respectively, owing to their presence in particulate-phase PAHs and their increase with higher cooking temperatures[67-68]. BaP was most prominent in the WFF sample, comprising 2.9% of ΣPAHs, whereas it ranged from 0.5% to 1.8% in the samples from the other cooking styles and was absent in the BC sample.
-
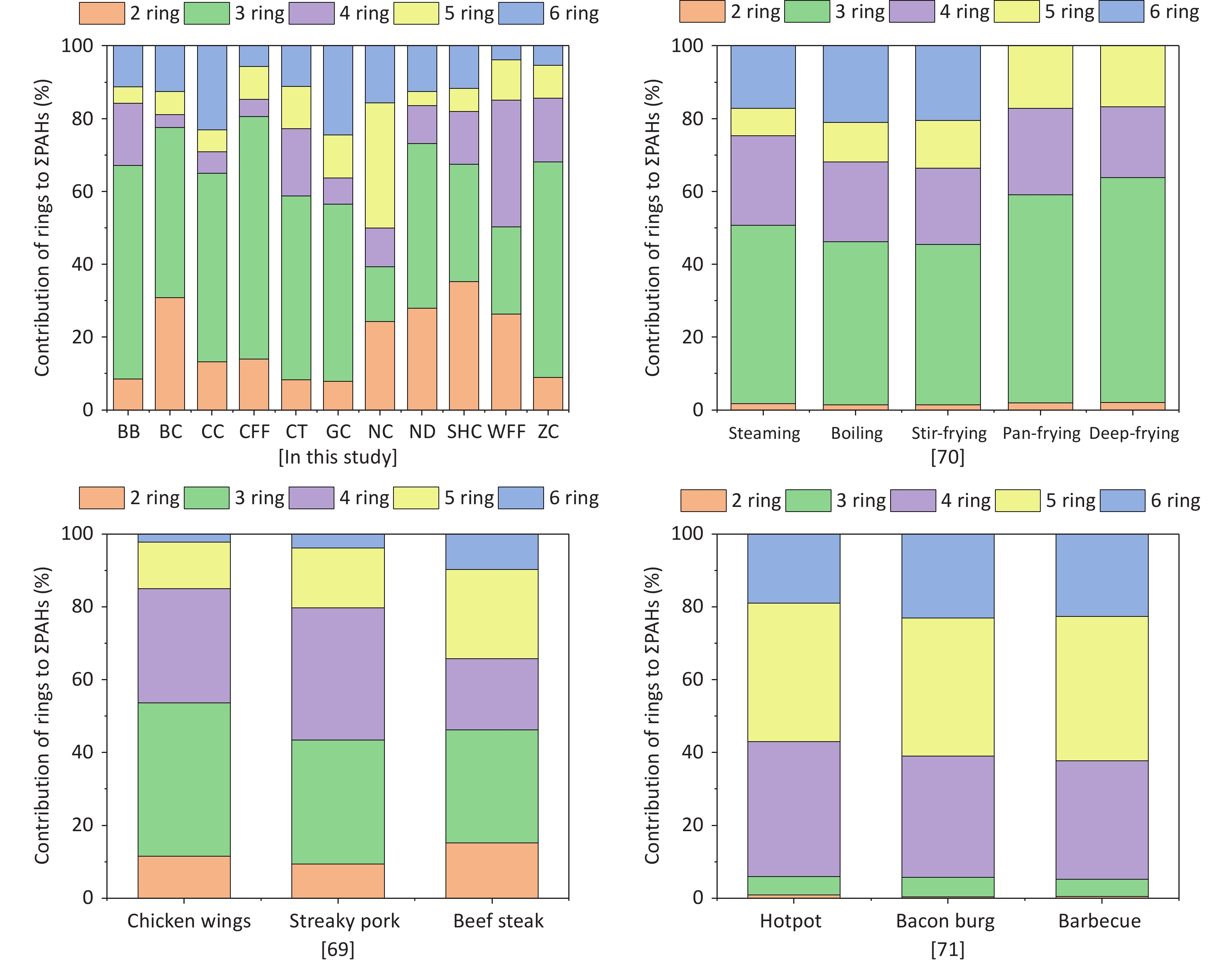
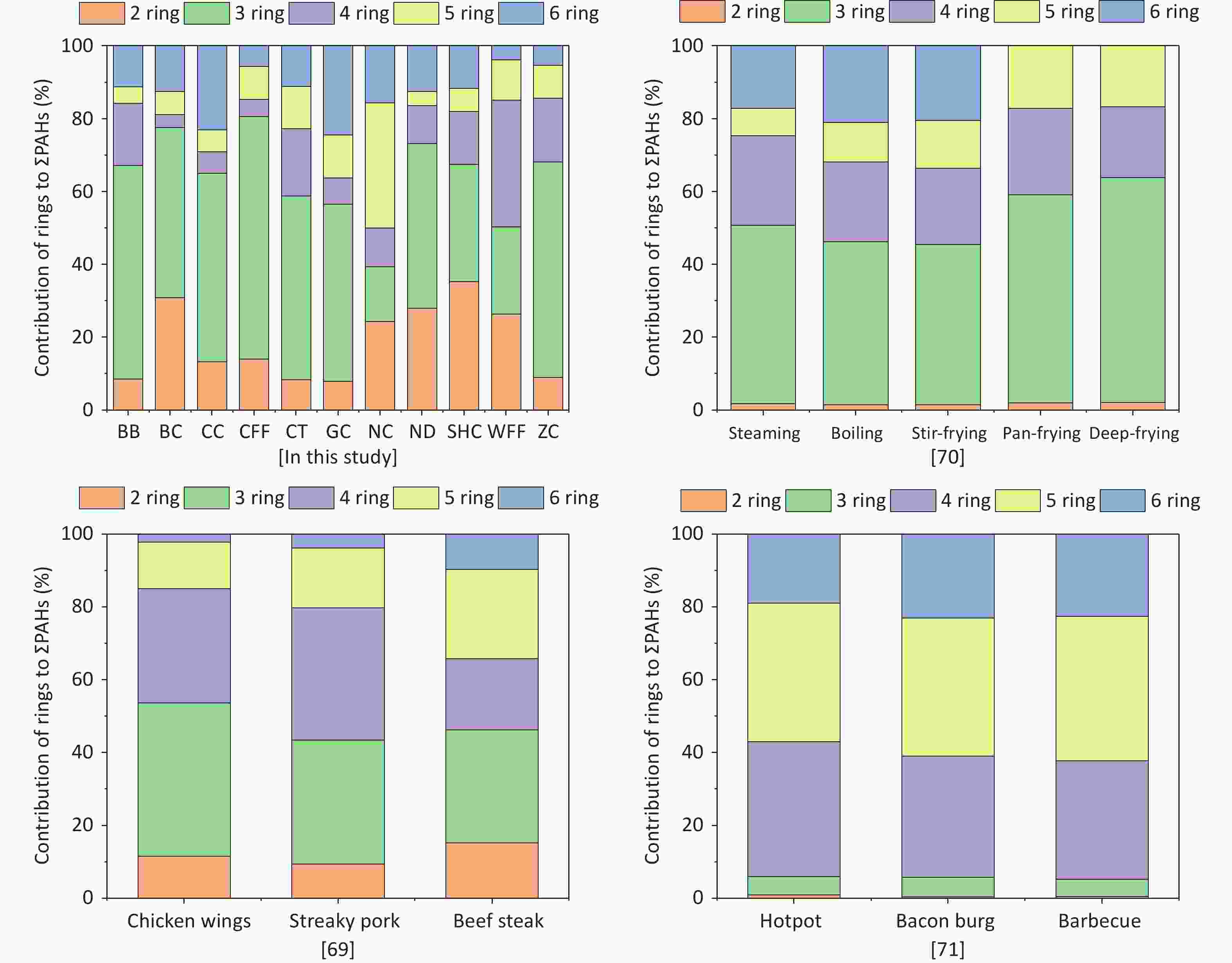
For all cooking methods, 2- and 3-ring PAHs constituted 39.4%–80.6% of ΣPAHs (Figure 2). Additionally, the contribution of 4-ring PAHs to ΣPAHs in the WFF sample reached 34.8%, and that of 5-ring PAHs in the NC sample was 34.4%, which was significantly higher than those of the other cooking types. In this study, low-molecular-weight PAHs were the main contributors to ΣPAHs for the majority of the cooking styles investigated. In another study, 3- and 4-ring PAHs were the main contributors to ΣPAHs during meat cooking [69], which was consistent with the results of some of the cooking styles in this study.
-
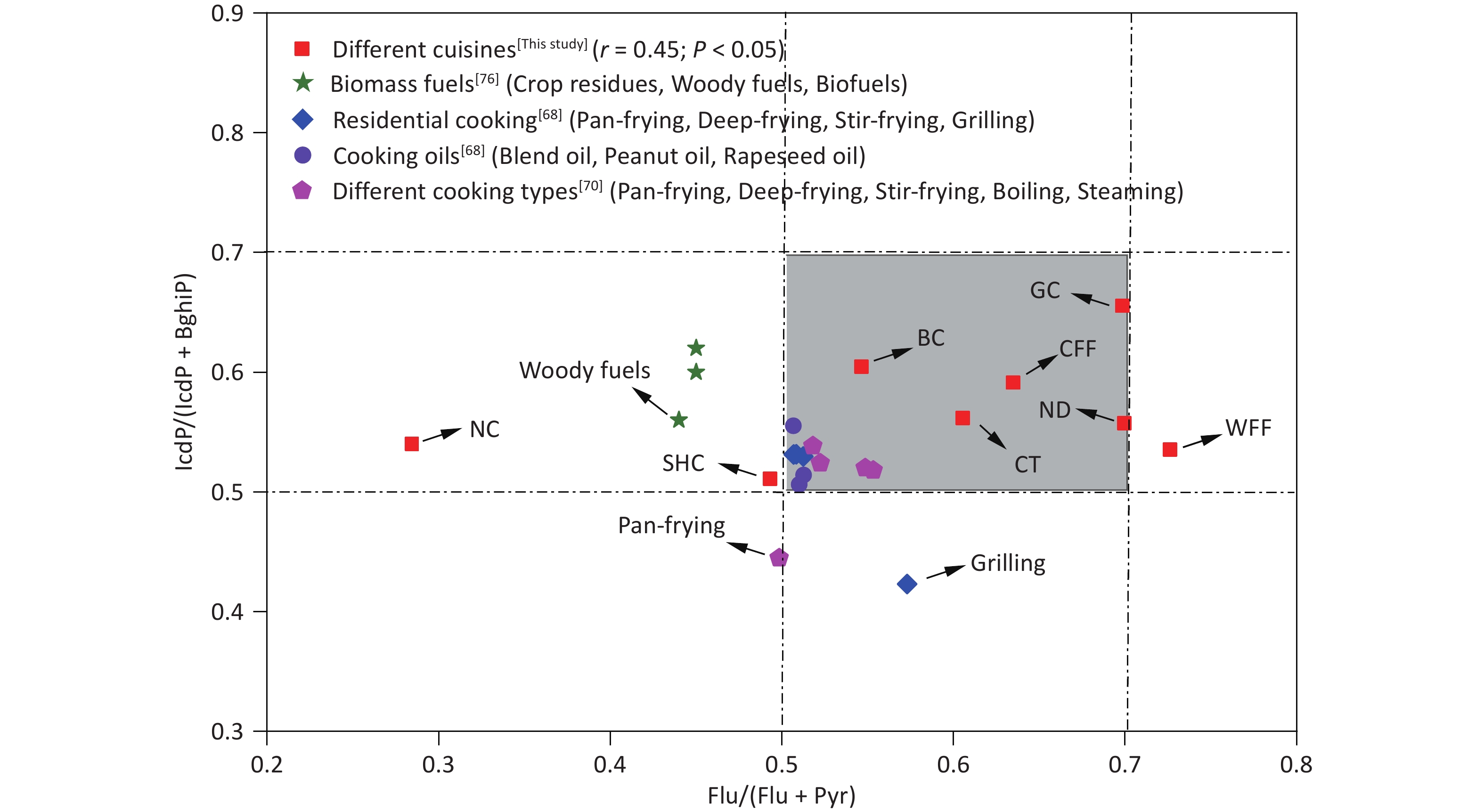
Specific diagnostic ratios of PAH isomers, including Flu/(Flu+Pyr), Ant/(Ant+Phe), and IcdP/(IcdP+BghiP), have been commonly used as indicators to identify potential sources of PAHs in the environment[70-72]. Notably, however, not all such diagnostic ratios are universally applicable. For instance, the Ant/(Phe+Ant) ratio is susceptible to photocatalytic effects and is more suitable for analyzing gaseous-phase PAHs, whereas Flu/(Flu+Pyr) and Icdp/(Icdp+BghiP) are typically used to assess particulate-phase PAHs[73]. Ratios such as Flu/(Flu+Pyr) and IcdP/(Icdp+BghiP) have been used to identify potential sources of PAHs in fossil fuel combustion, biomass fuel, coal combustion, and industrial processes[74-76]. As shown in Figure 3, the Flu/(Flu+Pyr) and IcdP/(IcdP+BghiP) ratios for the various cooking styles were predominantly in the ranges of 0.29–0.71 and 0.50–0.66, respectively, aligning with the diagnostic ranges observed in residential cooking in a previous study[68]. Some variability in the diagnostic ratios of Flu/(Flu+Pyr) and IcdP/(IcdP+BghiP) existed among the different cooking styles, which was mainly due to the differences in cooking methods, oil temperature, and ingredients. This discovery of the stable nature of these diagnostic ratios in most cooking styles underscores their usefulness as critical benchmarks for identifying PAH sources in various culinary environments.
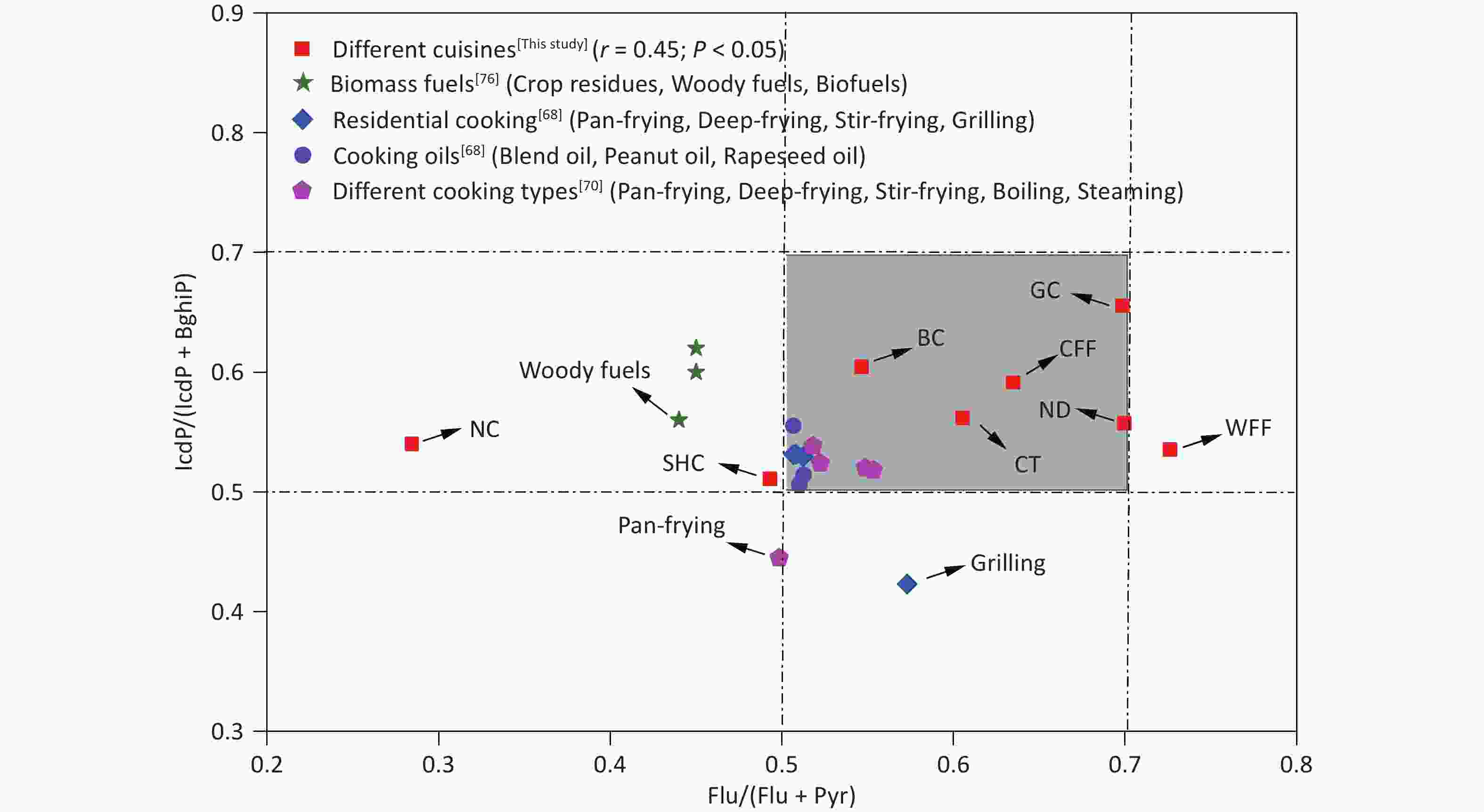
Figure 3. Range of Flu/(Flu + Pyr) and IcdP/(IcdP + BghiP) ratios in PAHs from this study and in other studies. BC: Beijing cuisine; CFF: Chinese fast food; CT: Canteen; GC: Guangdong cuisine; NC: Northeast cuisine; ND: Noodle; SHC: Sichuan and Hunan cuisine; WFF: Western fast food; BghiP, benzo[ghi]perylene; Flu: fluoranthene; IcdP: indeno[1,2,3-cd]pyrene; Pyr: pyrene.
-
The TEQBaP values for all the cooking styles ranged from 4.78 to 13.12 ng/m3 (Figure 1A). In general, BB (13.12 ng/m3), CC (12.17 ng/m3), SHC (12.03 ng/m3), and WFF (10.78 ng/m3) exhibited significantly higher toxicity than did the other cooking styles, with TEQBaP values ranging 1.3–1.5 times higher than the average value. Notably, the TEQBaP values from these cooking styles exceeded the World Health Organization non-mandatory guide value of 1 ng/m3[77]. To facilitate a comprehensive analysis of the toxicity contributions of individual PAHs to the TEQBaP of different cooking styles, the percentage contribution of each PAH to total carcinogenicity was calculated (Supplementary Table S7). For all cooking styles, the high-ring PAHs DBahA, BaP, and IcdP contributed the most to TEQBaP, with average contribution rates of 42.4%, 20.1%, and 15.3%, respectively. The individual PAHs of the other cooking styles contributed less than 6.8% on average. Generally, the toxicity of TEQBaP depends largely on the concentration of high-ring PAH monomers with high TEFs, which are typically produced by high-temperature pyrolysis during cooking.
-
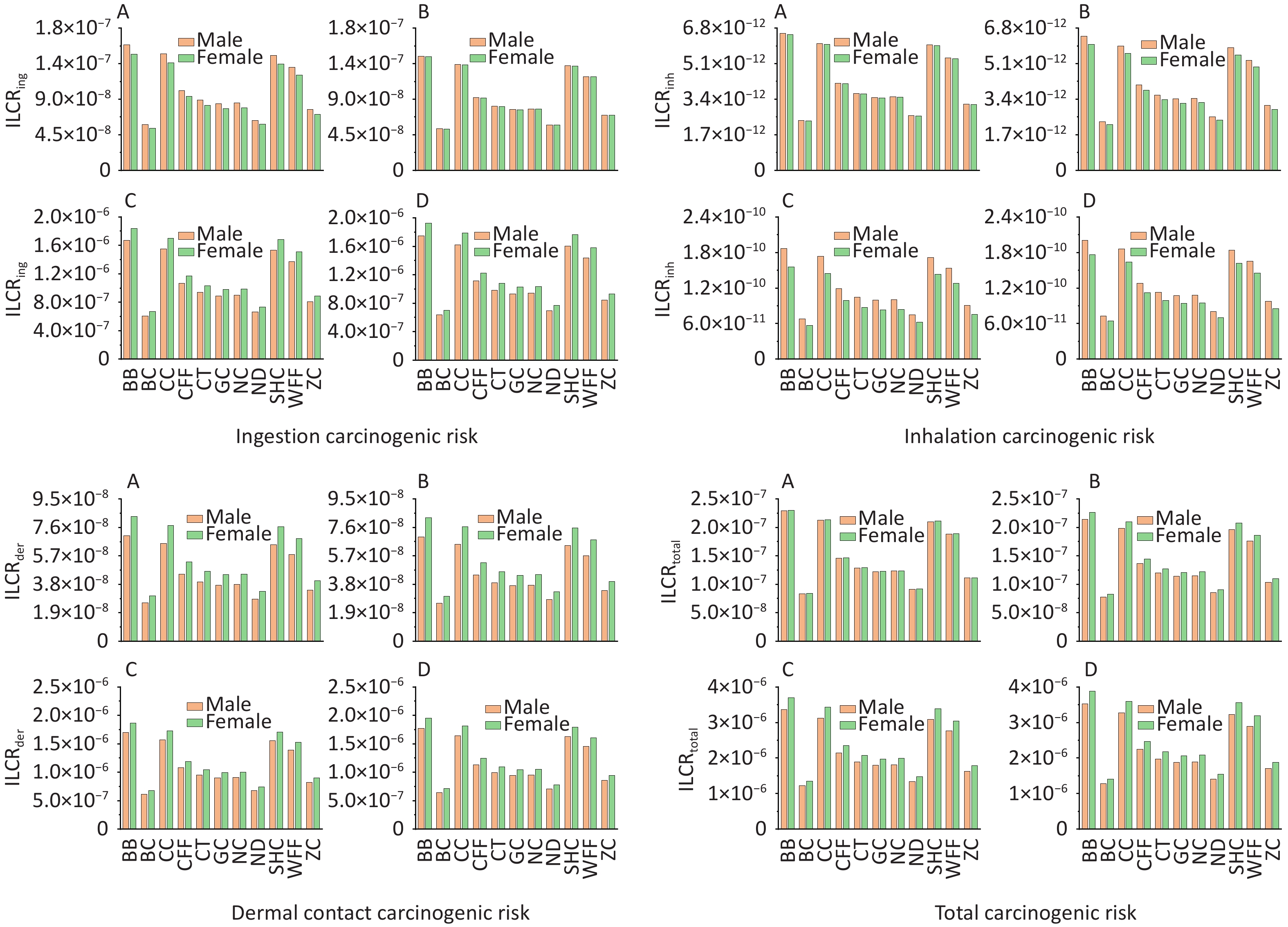
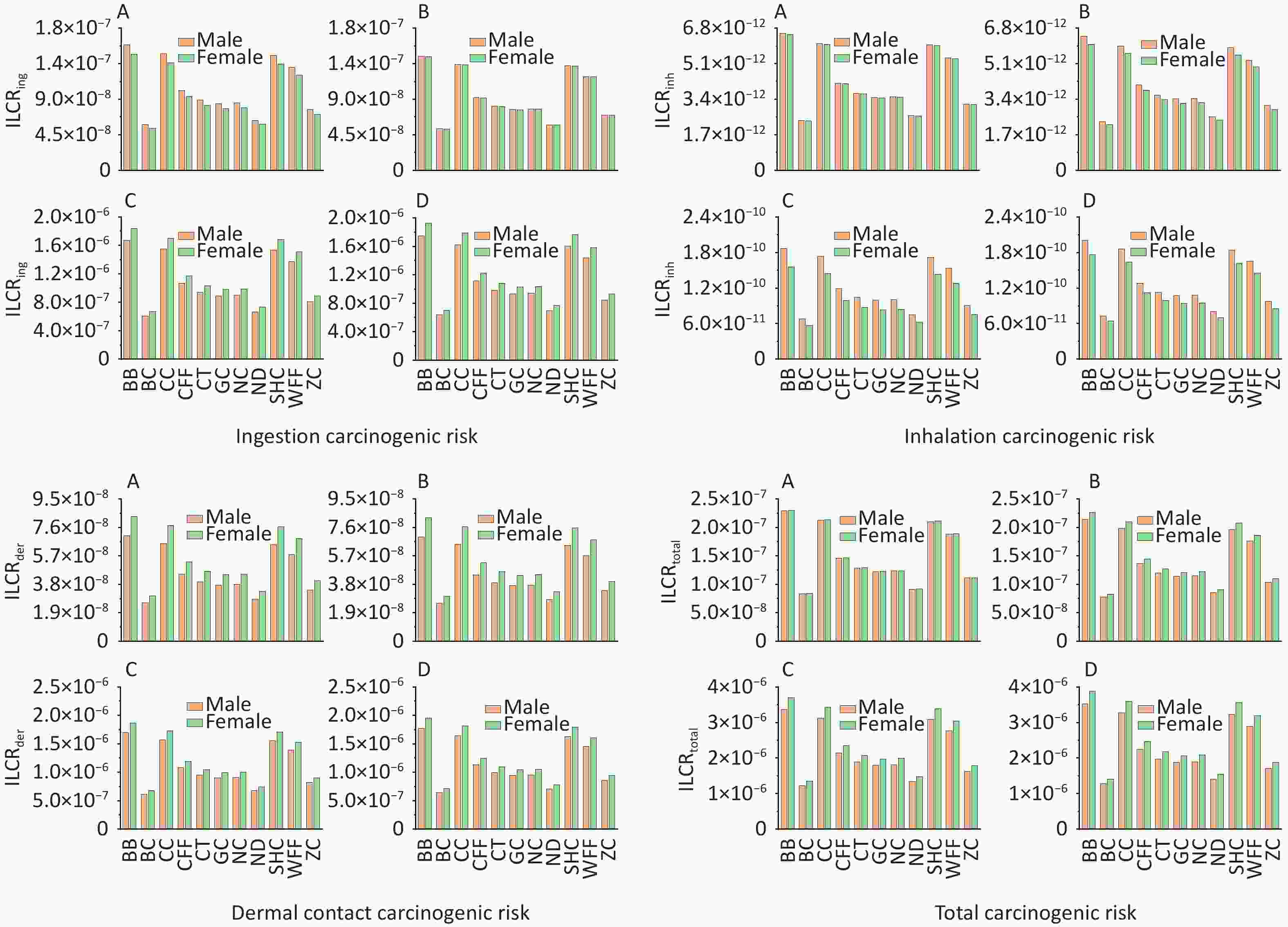
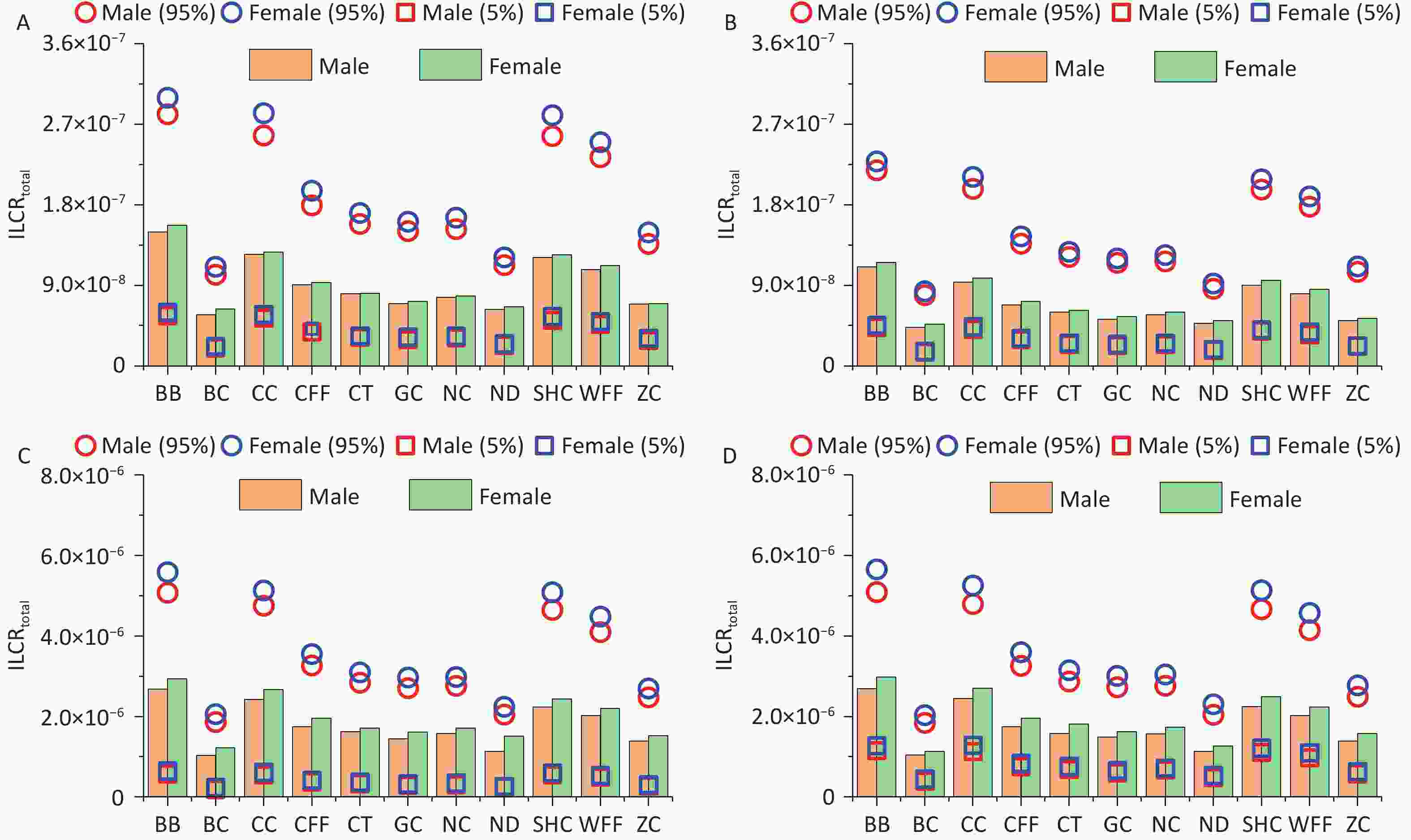
According to USEPA recommendations[78], ILCR < 10–6 indicates that the potential health risk is negligible; 10–6 < ILCR < 10–4 indicates that the potential health risk is within the acceptable range; and ILCR > 10–4 indicates a serious potential health risk. The ILCRs associated with the three exposure routes to PAHs from different cooking styles were assessed in four different groups stratified by age and sex and prioritized as ingestion > dermal contact > inhalation (Supplementary Table S8). For all cooking styles, the average ILCRtotal values ranged from 8.26 × 10−8 to 3.88 × 10−6 for females and from 7.81 × 10−8 to 3.53 × 10−6 for males. The ILCRtotal values for adults (1.23 × 10−6 to 3.70 × 10−6) and older adults (1.28 × 10−6 to 3.88 × 10−6) exceeded the acceptable limit of 1.00 × 10–6, and females showing a higher cancer risk (Figure 4). The ILCRtotal values were higher in the older adult group than in the other age groups. Generally, the ILCRtotal values were lower for children than for older adults, exhibiting a trend of first decreasing and then increasing with increase in age. Notably, the ILCRtotal values were significantly higher for BB, CC, SHC, and WFF than for the other cooking styles, which were mainly influenced by BaA, BaP, DBahA. In addition to the higher TEF values of these PAH monomers, the higher concentrations of some PAH monomers were also influencing factors.
-
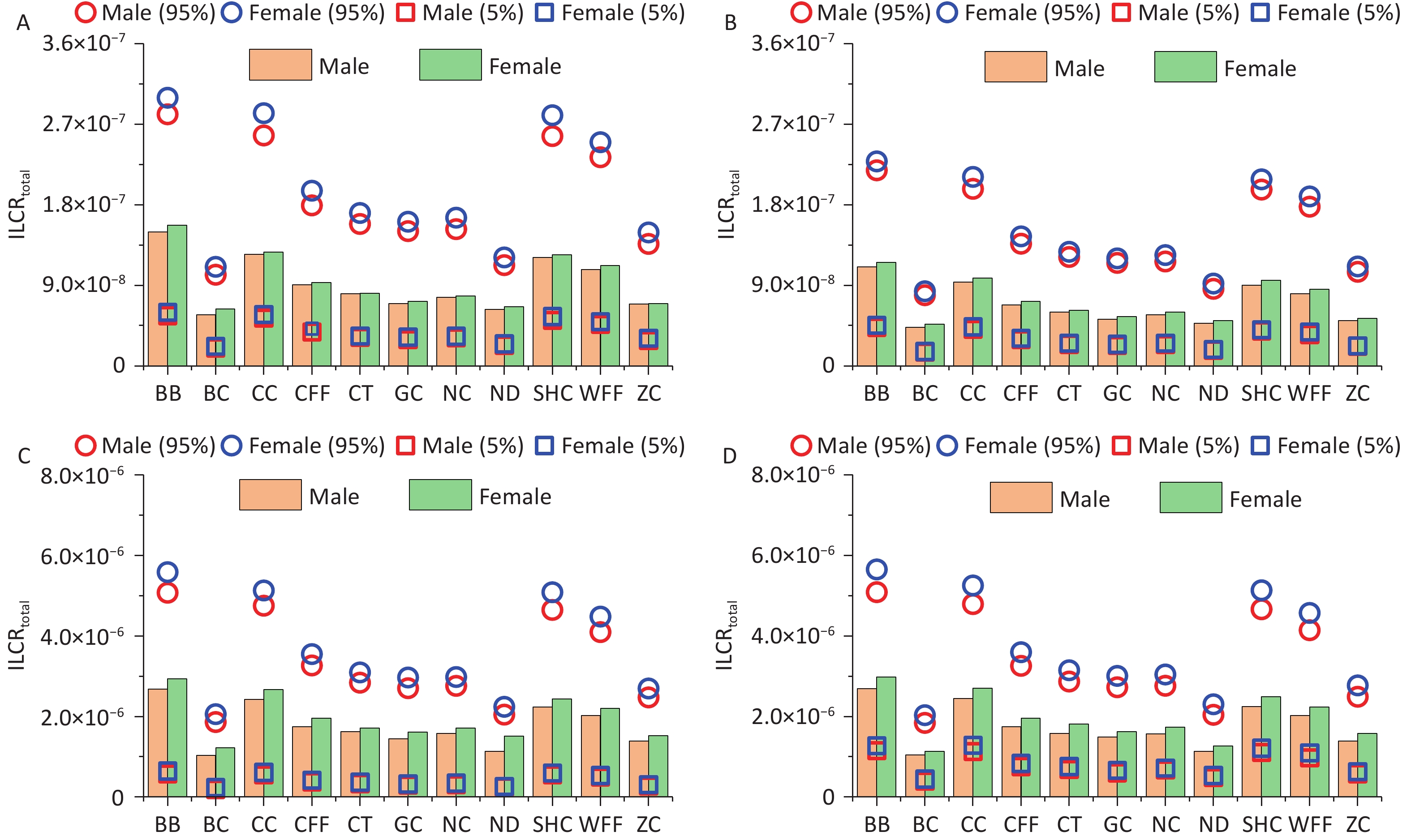
Monte Carlo simulation was used to calculate the ILCRtotal values for different age groups and sexes exposed to PM2.5-bound PAHs emitted from different cooking styles. The mean ILCRtotal values for all cooking styles and different groups ranged from 4.29 × 10-8 to 2.98 × 10-6, with the values of the 5th percentile spanning from 1.53 × 10-8 to 6.45 × 10-7 and those of the 95th percentile ranging from 7.89 × 10-8 to 5.65 × 10-6 (Figure 5). Notably, the ILCRtotal values for adults and older adults exceeded 1 × 10-6 regardless of cooking style, and the cancer risks were generally higher for females than for males. Consistent with previous research findings, the ILCRtotal values often first decreased and then increased, indicating that health risks may be higher in children and older adults than in adolescents, which are similar to the conclusions drawn from other studies[65].
-
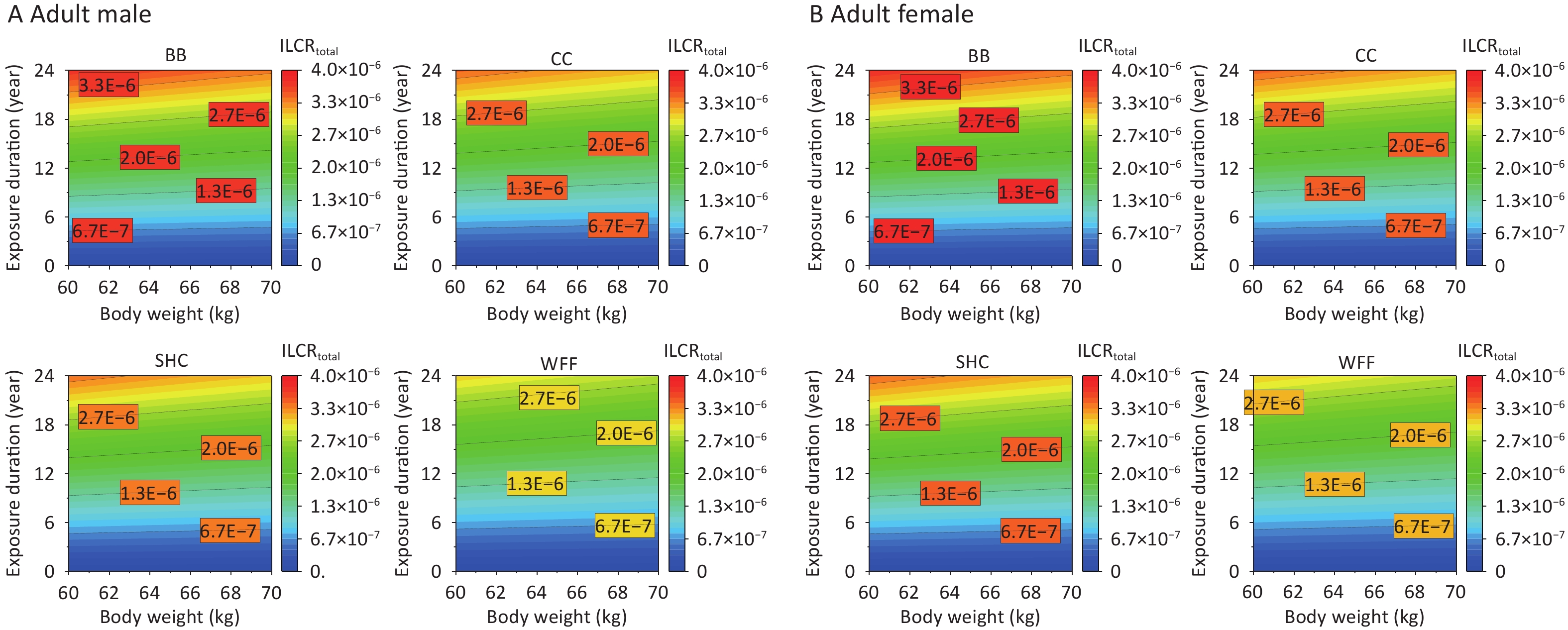
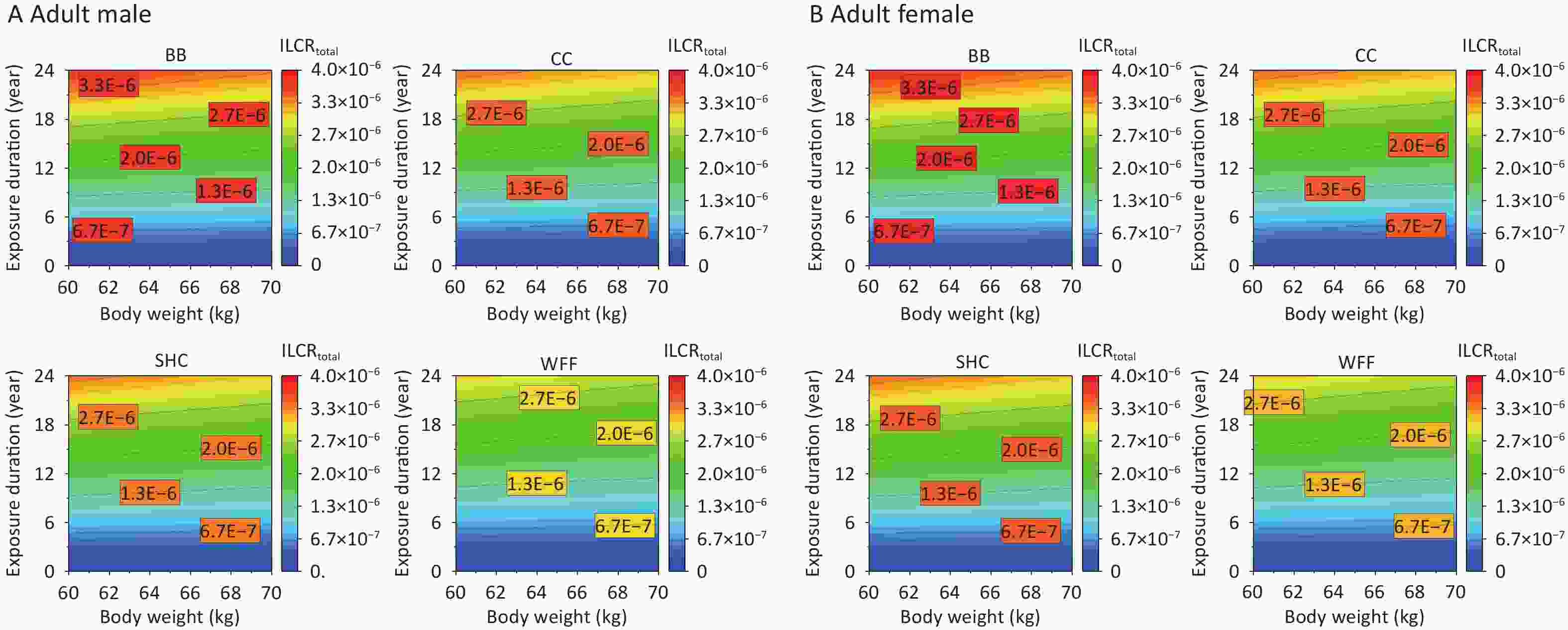
As the exposed population ages, their ED and BW change more pronouncedly, which consequently alters their cancer risks from PAHs emitted from various cooking environments[5]. The impacts of ED and BW on the ILCRtotal of adult males and females were further studied on the basis of recommended values for other parameters (Supplementary Table S5). Given that the cancer risk among individuals of different age groups is primarily affected by BW, the average weight of the adults was initially set to 60 kg[58,79] and varied between 60 and 70 kg. Meanwhile, the ED was set to span from 0 to 24 years for adults. The various cooking styles exhibited increased cancer risks under specific ED and BW conditions, with BB particularly affecting the ILCRtotal and requiring a shorter ED to pose a cancer risk to males at an equivalent BW (Figure 6A). By contrast, females showed a relatively higher ILCRtotal response, indicating that females in different age groups may face a greater risk of cancer as their ED and BW increase.
-
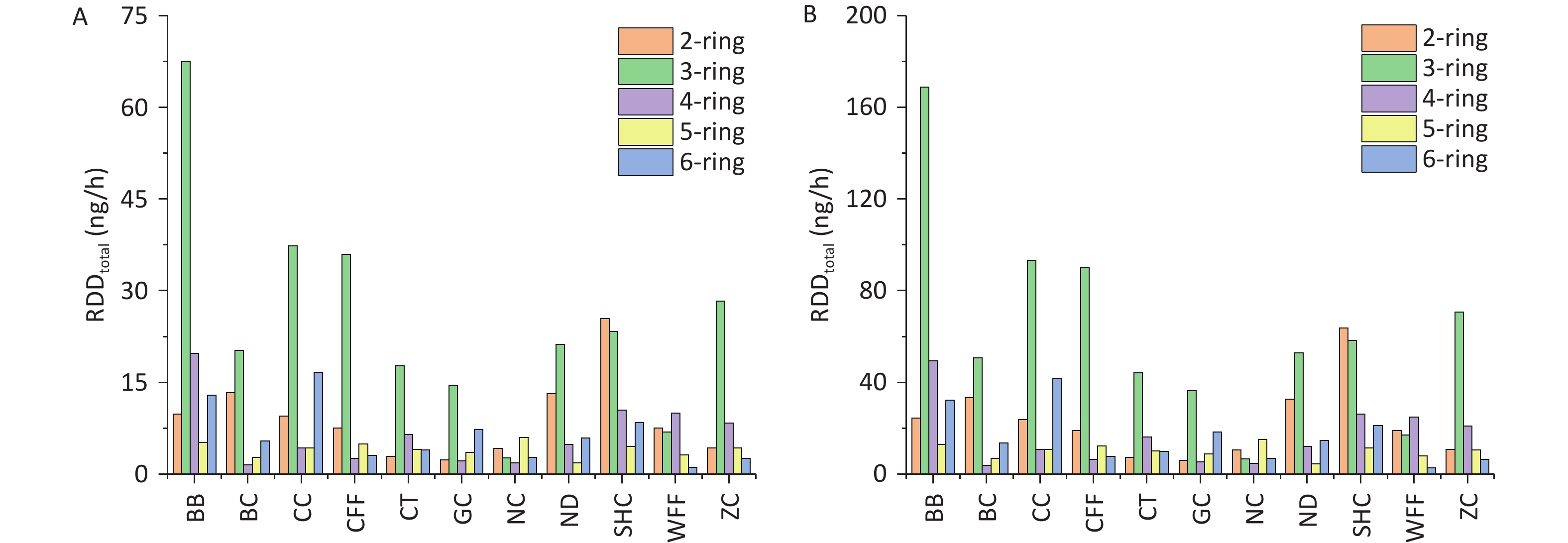
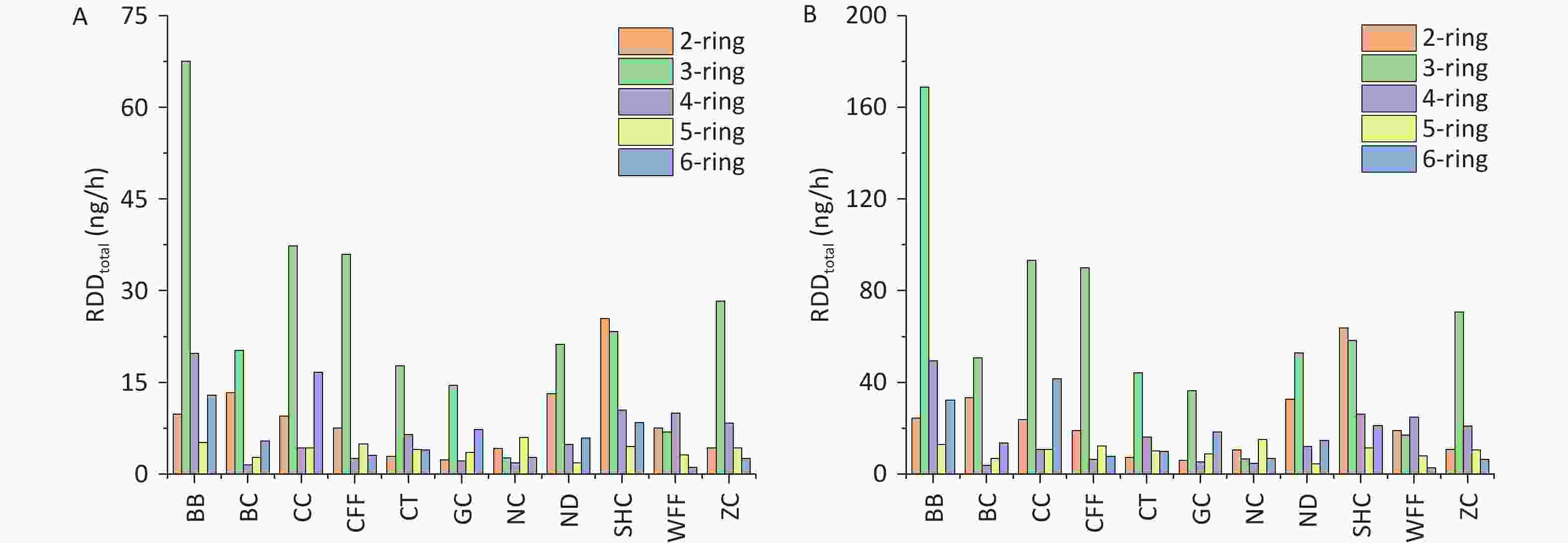
The RDD values of PM2.5-bound PAHs in the three regions of the respiratory system are shown in Figure 7. For all cooking styles, the total RDD (RDDtotal) values for children ranged from 17.44 to 115.30 ng/h, whereas those for adults ranged from 43.61 to 288.24 ng/h. Notably, the values in the upper airway (RDDUA), alveolar (RDDAL), and tracheobronchial (RDDTB) regions followed the hierarchical order RDDUA > RDDAL > RDDTB (Supplementary Table S9). Significant variations in RDD were observed among the different cooking styles, with the average RDDUA, RDDAL, and RDDTB values being 41.10, 6.45, and 3.63 ng/h, respectively, for children and 102.74, 16.13, and 9.06 ng/h, respectively, for adults. This disparity can be attributed to inhalation rates being higher in adults than in children, resulting in increased deposition doses. For the BB, SHC, CC, and CFF cooking styles, the RDD values in the three respiratory regions were respectively 2.2, 1.41, 1.40, and 1.05 times higher than the average RDD values for all cooking styles. Notably, for all cooking styles, the average contributions of BaP to RDDUA, RDDAL, and RDDTB in children and adults were 0.44 and 1.09 ng/h, 0.07 and 0.17 ng/h, and 0.04 and 0.10 ng/h, respectively.
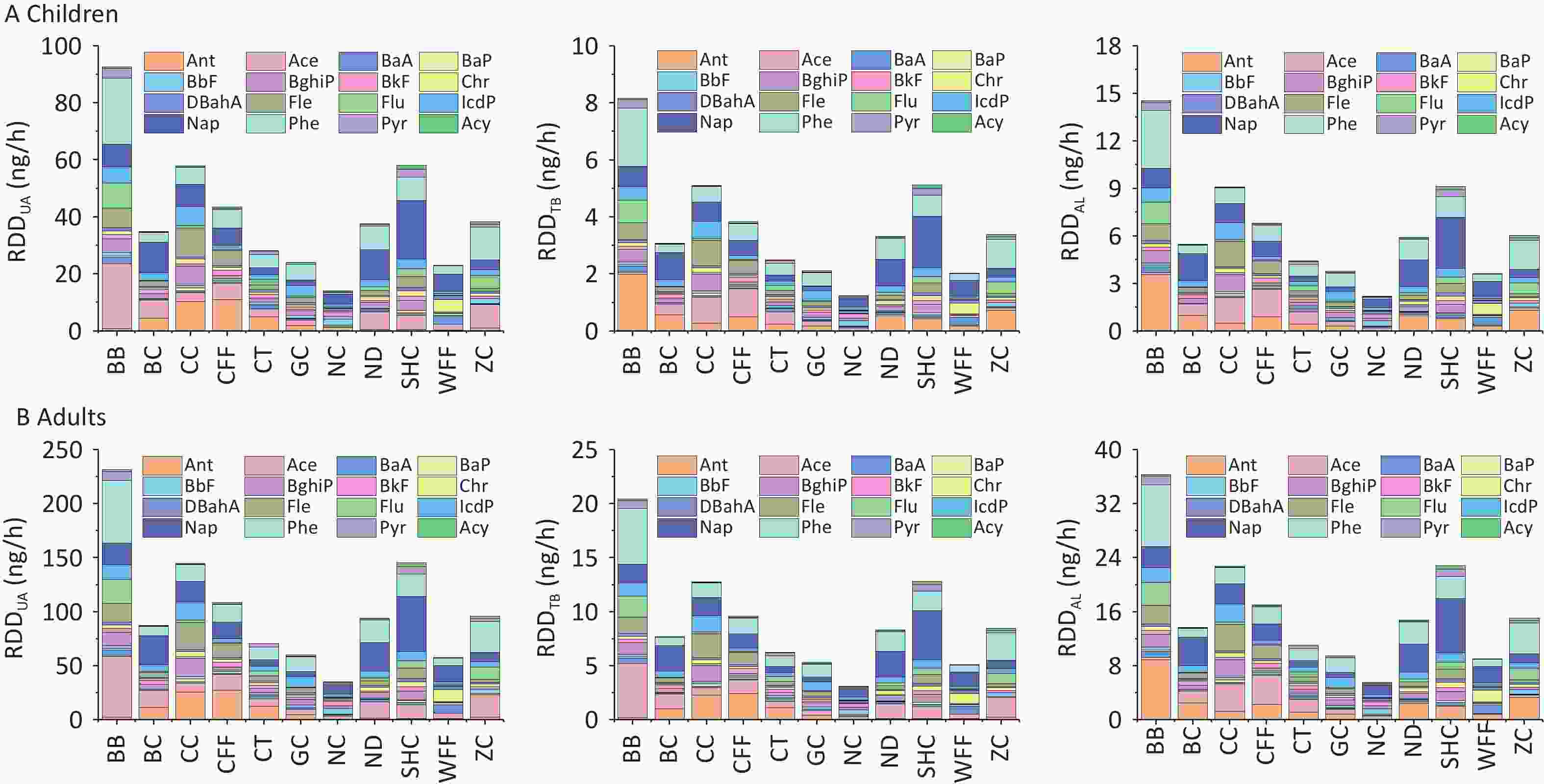
Figure 7. RDDUA, RDDTB, and RDDAL of PM2.5-bound PAHs emitted from different cooking styles in (A) children and (B) adults.
With regard to the contribution of PAHs of different ring numbers to the RDDtotal values, the cumulative impact of 2- and 3-ring PAHs on RDDtotal exceeded that of PAHs with other ring numbers. For the different cooking styles, the mean RDDtotal values of the 2- and 3-ring PAHs were respectively 9.11 and 25.07 ng/h for children and 22.79 and 62.67 ng/h for adults. The contribution of 3-ring PAHs from BB to the RDDtotal was markedly greater than that from similar PAHs from the other cooking styles. By contrast, the contribution of 2-ring PAHs from SHC to the RDDtotal was higher than that from similar PAHs from the other cooking styles, which was due to the high Phe and Ant contents. Similarly, the 2- and 3-ring PAHs contributed the most to the RDD values for the three respiratory system regions. However, 5- and 6-ring PAHs are more toxic[20]. The combined contributions of 5- and 6-ring PAHs from BB and CC to the RDDtotal were respectively 18.16 and 20.95 ng/h for children and 45.41 and 52.38 ng/h for adults.
-
Generally, a higher fat content correlates with increased PAH production during cooking, resulting in significantly elevated levels of PAHs from fat pyrolysis in BBs compared with other cooking styles[27,35,80]. Additionally, higher cooking temperatures are associated with increased PAH formation[67]. The NC, CT, and GC styles generally require lower cooking temperatures, resulting in lower PAH concentrations[70]. In another study, high levels of PAHs were detected in BB, CFF, and Sichuan cuisine from commercial kitchens in the northwestern region[67]. However, the PAHs produced by the different cooking methods in this study were relatively high, which may have been influenced by various factors, such as differences in the ingredients, edible oil consumption, and food composition. In a previous study, Nap concentrations were observed to be higher in Chinese- and Western-style restaurants[81]. Notably, however, not all cooking styles result in high Nap concentrations, which may be due to the cooking method itself and the efficiency of fume-cleaning devices[82-83]. BaP, classified as a Group 1 carcinogen by the International Agency for Research on Cancer, was detected in nearly all cooking fume samples, posing substantial health risks[30,80]. The high presence of BaP in WFF can be attributed to deep-frying, which produced levels of this PAH second only to those produced by BB[84]. In previous studies, the contribution of rings to ΣPAHs has commonly served as an indicator of emission sources, with 2- and 3-ring PAHs shown to be primarily associated with wood combustion[85-86]. However, higher molecular weight PAHs, including 5- and 6-ring members, are predominantly derived from coal, organic matter, and higher plants[53,74,87]. Studies have established that high-molecular-weight PAHs are the principal components of particulate-phase emissions from traffic exhaust[88-89]. The PAHs present in liquefied petroleum gas, which is used as a cooking fuel, are predominantly high-ring compounds, whereas those containing 2 and 3 rings are more predominant in natural gas[90]. See and Balasubramanian[70] showed that different cooking methods affect the ring composition, with the 3-ring PAHs accounting for a much higher proportion of ΣPAHs than the other ring members. According to another study, the contribution of 4-ring PAHs to ΣPAHs is more prominent[71]. Lee et al.[91] showed that the proportion of various ring members in ΣPAHs is independent of the amount of oil used. However, the proportion of 4-ring PAHs may be influenced by other factors, such as the fat content of the meat, added seasonings, and cooking temperature[48,92]. The results of this study showed that cooking methods, types of ingredients, and other factors had significant impacts on the composition of PAHs. Moreover, we found that some cooking styles resulted in relatively high levels of low-ring PAHs, suggesting that cooking sources may be significant contributors of low-molecular-weight PAHs in the atmosphere.
Notably, PM2.5-bound PAHs generated during cooking not only cause environmental pollution but also pose significant health risks to individuals of different ages and sexes. In this study, higher ILCRtotal values were found for females, which could be due to their generally lower BWs and longer EDs to cooking environments compared with males. Notably, the ILCRtotal values were lower for children than for older adults, which may be due to the shorter exposure time of infants and having a lower risk of developing cancer[65] and because of the prolonged exposure of older adults to cooking environments despite their heavier body mass. The risk of carcinogenicity could be reduced by having as little exposure as possible to a cooking environment, especially for cooking styles with higher cancer risks. Regarding the cancer risks associated with the three exposure routes, the ILCRing values were higher for male children and male adolescents, indicating that males may have a greater cancer risk than that of females in these age groups. This difference primarily arises because at similar BWs, males generally exhibit higher respiratory rates and intake parameters than females do. Although the intake parameters of both sexes were similar in adults and older adults, females tend to have a lower overall BW, resulting in them exhibiting a higher ILCRing value and suggesting that they may face a greater cancer risk than that faced by males[93]. The ILCRinh value is influenced by the disparity in respiratory rates between males and females, with males exhibiting a higher respiratory rate, which potentially increases their cancer risk[44]. The ILCRder values indicated that the older adults had the highest cancer risk, which may be due to the BW and ED parameters, with older adults being exposed longer to emissions compared with infants and weighing the least between the two adult groups. Overall, given the multifaceted influences on health risks associated with PM2.5-bound PAHs in diverse cooking environments, prolonged exposure to these molecules should be minimized, particularly among vulnerable groups such as children and older adults. The establishment of effective ventilation measures can mitigate these health risks[94]. The results indicated that minimizing the ED in cooking environments is crucial for reducing cancer risks, particularly for cooking styles associated with higher ILCR values. Notably, lower initial weight values, with consistent ED and BW gain, generally correlated with increased cancer risk, explaining why infants are more susceptible, as observed in a previous study[81]. Over the long term, adults endure prolonged exposure to cooking environments, amplifying their cumulative cancer risk relative to that for children.
Among the three respiratory system regions, the upper airway serves as the primary barrier for preventing particulate matter from entering the body, whereas smaller amounts of finer particulate matter can penetrate the alveolar region and potentially reach the tracheobronchial area. The RDD values of the upper airway, alveolar, and tracheobronchial regions were significantly higher for PAHs from BB, SHC, CC, and CFF than for those from the other cooking styles. This may be due to the substantial quantity of oil used in these culinary techniques in concert with the emission of numerous particulate fumes, and thus should be highly noted. Several studies have shown that long-term accumulation of PAHs in the alveolar region can pose a significant health threat to the respiratory system and other organs of the human body[63,95]. The annual deposition of PM2.5 in the upper respiratory tract, alveoli, and bronchi of chefs working in various kitchen environments was found to gradually decrease, with significant variation depending on the cooking methods[44], indicating that certain cooking techniques present greater long-term health risks to exposed populations than previously estimated. Furthermore, the combined contributions of 5- and 6-ring PAHs from BB and CC to RDDtotal were significantly higher than those of similar PAHs from the other cooking styles. Thus, the deposition doses of these high-ring PAHs in the respiratory system pose an increased risk to human health, warranting their significant attention.
-
In this study, the concentrations and fractions of PM2.5-bound PAHs emitted from 11 cooking styles were analyzed, and the associated health risks of cooking source emissions to different age and sex groups were evaluated. The study enhances our understanding of the cumulative impact of PM2.5-bound PAHs emitted from different cooking styles on human health risks and points out the direction for reducing the risks associated with cooking sources. Despite these discoveries, certain limitations remain. These were primarily reflected in the variations in behavioral characteristics among different populations that were influenced by factors such as age, occupation, and lifestyle habits. Existing models are unable to fully capture these subtle differences, resulting in discrepancies between the actual exposure levels of sensitive populations and the estimated values. Additionally, smoke dispersion during cooking should not be underestimated. Even when individuals are not in the kitchen, cooking fumes can spread to non-kitchen areas, posing potential health risks. Future research could be conducted in two key directions. First, adopting diverse data collection methods, such as questionnaire-based surveys and smart device monitoring, can facilitate deeper exploration of individual exposure behavior patterns, refine exposure assessment models, and improve the accuracy of health risk assessments. Second, more samples from different cooking methods and advanced monitoring technologies could be used to comprehensively evaluate the concentrations of PAHs dispersed by cooking fumes in both kitchen and non-kitchen areas as well as their associated health risks to various populations from different cities (areas). In conclusion, this study provides a scientific basis for developing targeted protective measures to mitigate the harmful effects of cooking fumes on human health.
Health Risks from Exposure to PM2.5-bound Polycyclic Aromatic Hydrocarbons in Fumes Emitted from Various Cooking Styles and Their Respiratory Deposition in a City Population Stratified by Age and Sex
doi: 10.3967/bes2025.129
- Received Date: 2024-12-20
- Accepted Date: 2025-06-03
-
Key words:
- Cooking fumes /
- PM2.5-bound PAHs /
- Respiratory deposition dose /
- Health risk /
- Older adults
Abstract:
The authors have no conflicts of interest to declare.
| Citation: | Junfeng Zhang, Xi Chen, Ke Gao, Shuiyuan Cheng, Wenjiao Duan, Liying Fu, Jianjia Li, Shushu Lan, Cuilan Fang. Health Risks from Exposure to PM2.5-bound Polycyclic Aromatic Hydrocarbons in Fumes Emitted from Various Cooking Styles and Their Respiratory Deposition in a City Population Stratified by Age and Sex[J]. Biomedical and Environmental Sciences. doi: 10.3967/bes2025.129 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: