-
Ischemic stroke is the leading cause of death in the Chinese population. The incidence of cerebral infarction is higher in high-altitude regions, particularly those above 3,500 m, than in populations residing at lower altitudes[1]. There are various speculations regarding the mechanisms behind this phenomenon, one of which is that the low oxygen content and cold climate at high altitudes may increase the occurrence of vascular diseases[2]. The multifactorial effect of high-altitude environments on residential populations makes it challenging for researchers to determine the specific pathways through which these diseases occur. Given that vascular blockage is the direct cause of cerebral infarction, alterations in the vascular structure and blood flow components may be the most direct factors influencing the human body at high altitudes.
Physiologically elevated red blood cell (RBC) count and hemoglobin (HGB) level are considered direct risk factors for cerebral infarction[3]. Previous studies have also found a significant increase in erythrocyte levels in individuals with high-altitude strokes[4]. Nonetheless, limited research has confirmed the role of erythrocyte compensatory elevation at high altitudes in the onset of cerebral infarction. Plateau migrants could experience compensatory acceleration of cerebral blood flow velocity in the early stage of reaching the plateau, which may be related to the partial pressure of oxygen, carbon dioxide, and PH levels. This increase is related to an increase in hematocrit (HCT)[5]. Some reports have suggested that native Andean populations have a lower blood flow in the brain, whereas Tibetan populations have increased internal carotid artery blood flow[6]. RBC indices continued to increase with increasing altitude. However, the effect of erythrocytes in long-term high-altitude environments on brain blood supply is not clear. In this study, we aimed to investigate the association between red cell indices and cerebral blood flow velocity in migrants and natives at high altitudes and to provide clinical evidence for the mechanisms of high-altitude cerebrovascular diseases.
To analyze changes in cerebral blood flow at high altitudes, 30 high-altitude natives (HAN) and 96 high-altitude migrants (HAM) were recruited from the Shigatse Branch, Xinqiao Hospital (altitude: 3,800 m) from May 2021 to July 2024. The high-altitude participants were from altitudes between 3,500 and 4,800 m in Tibet. Moreover, HAN have been living on the plateau for generations. While, the HAM were those who migrated to the plateau for > one year and had no experience at higher altitudes for at least three months. During the same period, 101 age- and sex-matched low-altitude controls were recruited from the Health Examination Center of Daping Hospital (altitude: 300 m). Participants were excluded if they had: (1) a family history of cerebropathy; (2) any neurological disorders or other disorders that may potentially affect cerebral hemodynamics; (3) severe cardiac, pulmonary, hepatic, renal diseases or any kinds of tumor; (4) mental illness (e.g., schizophrenia); (5) recent transfusion history; (6) any acute illnesses (e.g., infections, diarrhea) (7) any drugs affecting blood velocity or viscosity (e.g., pentoxifylline). Our study was approved, without any reservations, by the Institutional Review Board of Shigatse Branch, Xinqiao Hospital and Daping Hospital. This study was conducted in accordance with the principles of “Measures for the Ethical Review of Biomedical Research Involving Human Subjects”, “Measures for the Ethical Review of Life Sciences and Medical Research Involving Human Subjects”.
We collected the demographic characteristics and medical history data of all participants. The levels of SpO2 (Saturation of Peripheral Oxygen) were assessed using a fingertip oximeter after the patients refrained from breathing pure oxygen for at least 30 minutes upon admission. Following informed consent from each participant, fasting blood was collected between 06:00 am and 07:00 am to avoid the potential circadian rhythm effects. The RBC, HGB and HCT levels were measured using an automatic blood analyzer in the clinical laboratory of two hospitals.
Owing to the limited medical resources available at high altitudes, we opted to use transcranial Doppler (TCD) instead of high-resolution imaging examinations or other direct observation methods to observe cerebral vascular structures in the population. Additionally, all participants underwent physical examinations and auxiliary tests, including electrocardiography, echocardiography, and brain CT or MRI resonance imaging to rule out severe cardiovascular and cerebrovascular diseases. The left ventricle ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) were determined based on the measurements from echocardiography. Furthermore, the maximum velocity (Vmax), mean velocity (Vmean), and pulsatility index (PI) of the basilar artery, left terminal internal carotid artery (LTICA), and right terminal internal carotid artery (RTICA) were determined using TCD measurements. The TCD procedure was performed by an experienced technician and verified by a senior doctor.
Statistical analyses were performed using the GraphPad Prism software (version 8.0; GraphPad Software, USA). Unless otherwise stated, the mean ± standard deviation was used to present the data. Differences between groups were compared using appropriate tests, such as Mann-Whitney U tests, two-tailed independent t-tests, or chi-squared tests. All correlations were analyzed using partial correlation analyses adjusted for age, sex, comorbidities, SpO2, LVEF, and LVFS to exclude the contributions of potential confounding factors. We considered two-sided P values < 0.05 as statistically significant.
As shown in Supplementary Table S1, there were no significant differences in the comorbidities (hypertension, diabetes, and hyperlipidemia) among the three groups. As expected, the red cell indices in HAM were significantly higher than those observed in low altitude group. Additionally, the levels of RBC and HGB were higher in HAM than in HAN. As altitude increased, blood oxygen levels decreased significantly. Therefore, the SpO2 level of the HAM group was significantly lower than that of the low altitude group, whereas there was no difference between the two high-altitude groups. In terms of cardiac indicators, none of the participants had organic heart disease or severe cardiac structural changes as shown by echocardiography. Additionally, there were no differences in LVEF or LVFS between the HAM and low altitude groups.
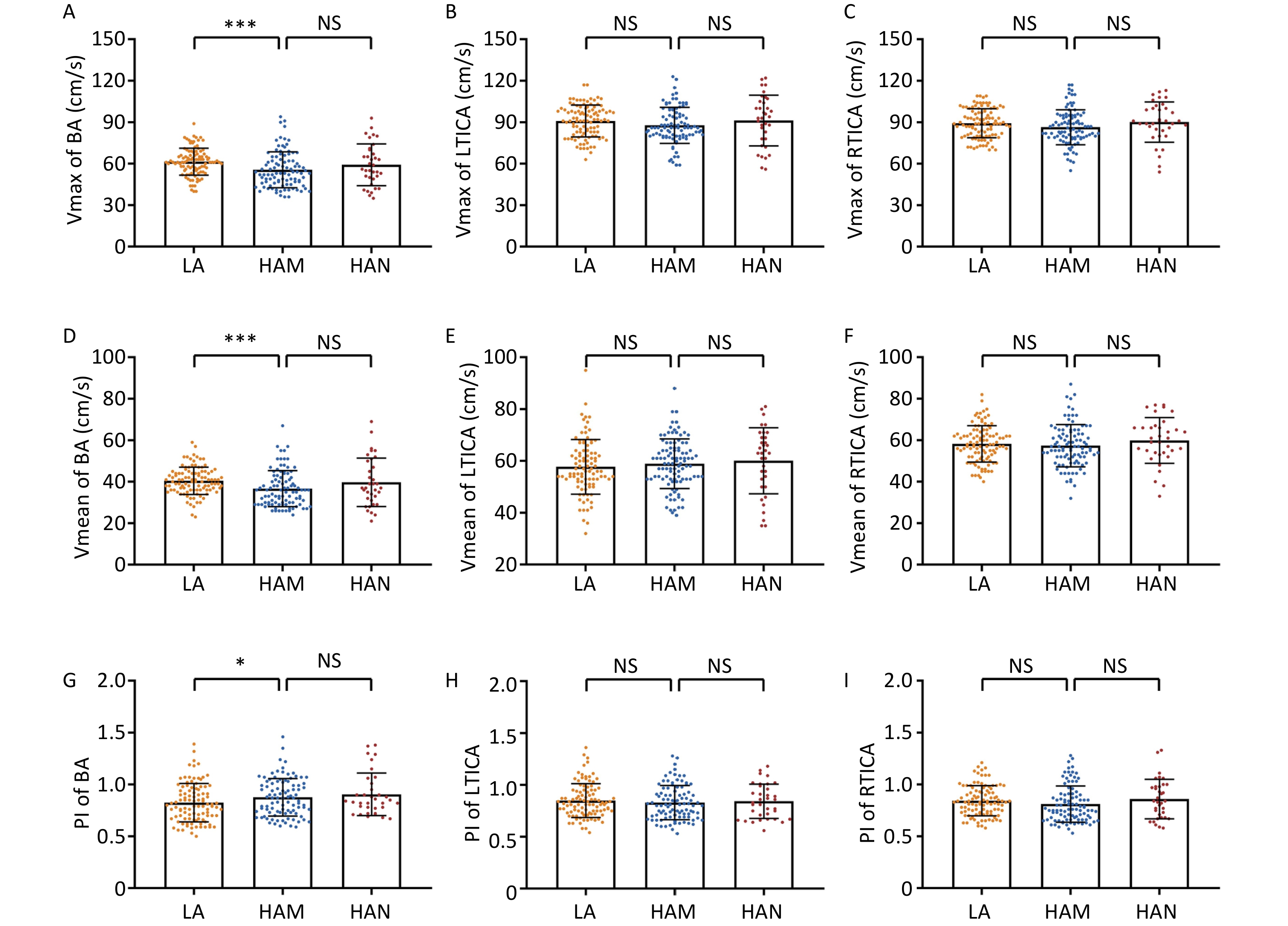
We then analyzed the data from the TCD measurements. The participants of HAM had significantly lower levels of Vmax (55.60 ± 12.95 m/s vs. 61.45 ± 9.77 m/s, P < 0.001) and Vmean (36.65 ± 8.67 m/s vs. 40.46 ± 6.61 m/s, P < 0.001) in basilar artery than low altitude group, while significant higher levels of PI (0.88 ± 0.18 vs. 0.82 ± 0.19, P = 0.041) (Figure 1A, D, G). Moreover, we found no significant differences in Vmax, Vmean, and PI of the basilar artery between HAM and HAN. As for LTICA and RTICA, although the Vmax decreased trendily in HAM, no statistical significances were observed compared to low altitude group (LTICA: 87.77 ± 13.01 m/s vs. 90.84 ± 11.52 m/s, P = 0.080; RTICA: 86.41 ± 12.67 m/s vs. 89.27 ± 10.47 m/s, P = 0.070) and HAN (Figure 1B–C). Furthermore, no significant differences were found in the Vmean and PI of the TICA among the three groups (Figure 1E–F, H–I).
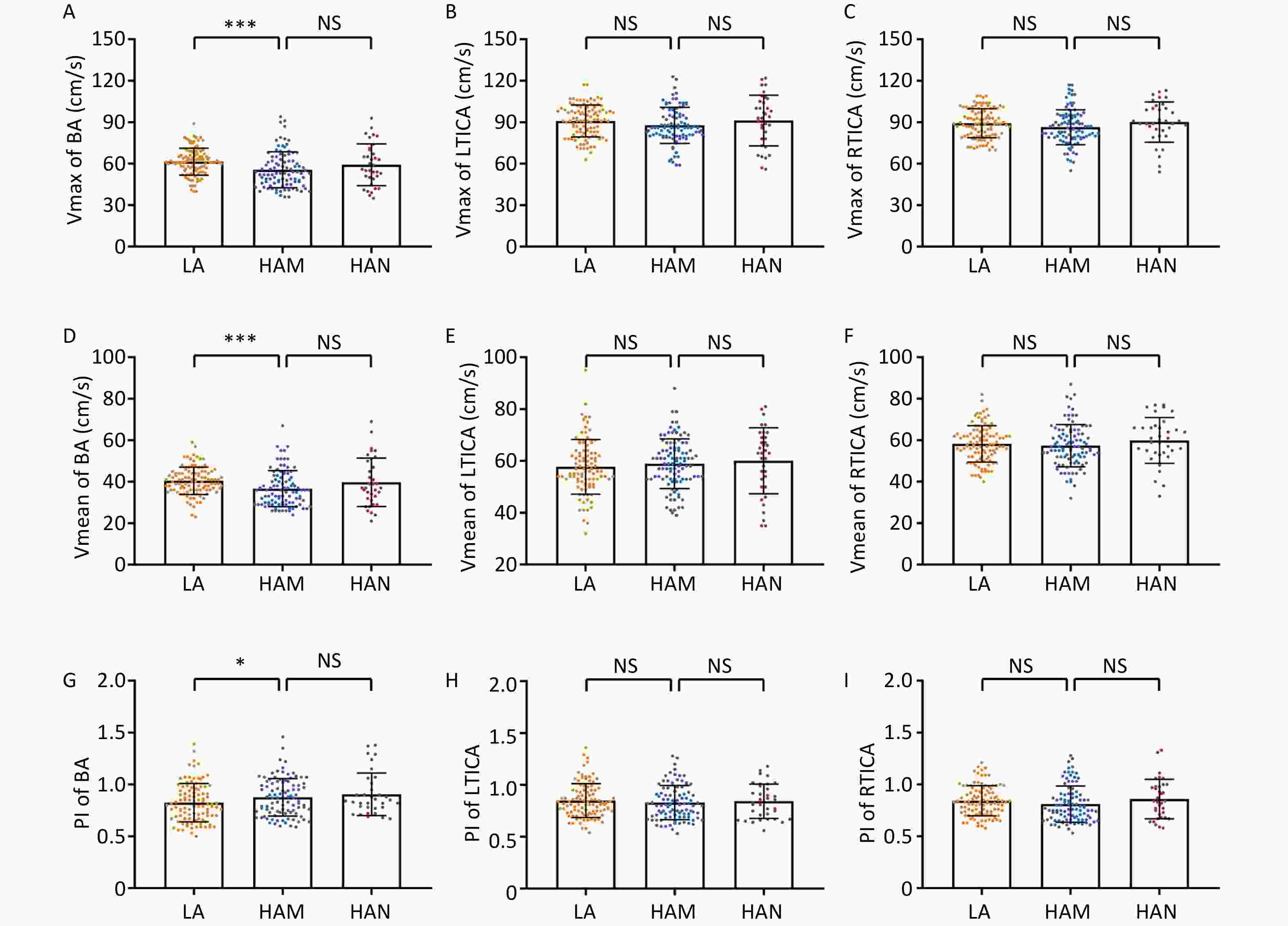
Figure 1. Comparison of Vmax, Vmean and PI of BA, LTICA and RTICA between LA, HAM and HAN. A–C Comparison of Vmax of BA (A), LTICA (B) and RTICA (C) between groups of LA, HAM and HAN. D–F Comparison of Vmean of BA (D), LTICA (E) and RTICA (F) between groups of LA, HAM and HAN. G–I Comparison of PI of BA (G), LTICA (H) and RTICA (I) between groups of LA, HAM and HAN. Vmax, max velocity; Vmean, mean velocity; PI, pulsatility index; BA, basilar artery; LTICA, left terminal internal carotid artery; RTICA, right terminal internal carotid artery; LA, low-altitude participants; HAM, high-altitude migrants; HAN, high-altitude natives. ***P < 0.001. *P < 0.05. NS denotes no statistical significance.
High-altitude erythrocytosis is an important risk factor for cerebral infarction[1]. This study demonstrated that migrants to high-altitude areas exhibit a decrease in Vmax and Vmean and an increase in the PI of the basilar artery. However, there were no significant differences in cerebrovascular parameters between migrants and natives. This trend may be associated with various mechanisms, one potential reason could be that the decrease of cerebral blood flow velocity is due to the adaptive elevation of hemoglobin in patients[7]. The increase in PI may be related to factors such as ischemia and hypoxia-induced small vessel contraction as well as a relative rise in intracranial pressure[8]. We conducted a more thorough analysis of the relationship between the RBC indices and TCD-related parameters.
We found a more significant decrease in the blood flow velocity in the basilar artery, whereas the flow velocity in the internal carotid artery exhibited a downward trend. We speculate that this outcome might be related to the path of the blood vessels because the internal carotid artery detected through the eye tends to be more tortuous and exhibits more anatomical variations, which can affect blood flow velocity monitoring. Conversely, the basilar artery remained relatively straight and consistent during monitoring.
We conducted further analyses to examine the correlations of red cell indices with the velocity and index of cerebral vessels while adjusting for age, sex, comorbidities, SpO2, LVEF, and LVFS. As illustrated in Figure 2A–C, despite there were no correlations between RBC and the Vmax of basilar artery. However, RBC showed negative correlations with the Vmax of LTICA (r = –0.220, P = 0.014) and RTICA (r = –0.225, P = 0.013) in all high-altitude groups. Additionally, HGB was negatively correlated with the Vmax of the basilar artery in HAM (r = –0.224, P = 0.036) and all high-altitude participants (r = –0.248, P = 0.006) (Figure 2D). Moreover, HGB was negatively correlated with the Vmax of TICA in HAM (LTICA: r = –0.286, P = 0.007; RTICA: r = –0.274, P = 0.010), HAN (LTICA: r = –0.450, P = 0.018; RTICA: r = –0.493, P = 0.009), and all high-altitude participants (LTICA: r = –0.322, P < 0.001; RTICA: r = –0.310, P < 0.001) (Figure 2E–F). Regarding Vmean, the red cell indices were negatively correlated with basilar artery in all high-altitude participants (RBC: r = –0.208, P = 0.021; HGB: r = –0.311, P < 0.001) (Figure 2G, J). The Vmean of basilar artery had a negative correlation with HGB in the HAM group (r = –0.289, P = 0.006). In addition, the Vmean of both the LTICA and RTICA had significant negative correlations with the red cell indices in HAM, HAN, and all high-altitude individuals (Figure 2H–I, K–L). Notably, although the HAN group had poorer correlations than the HAM group (Figure 2), the r values of HAN were relatively larger. This could be attributed to the small sample size. However, no correlation between the erythrocyte count and velocity in the low altitude group was observed.
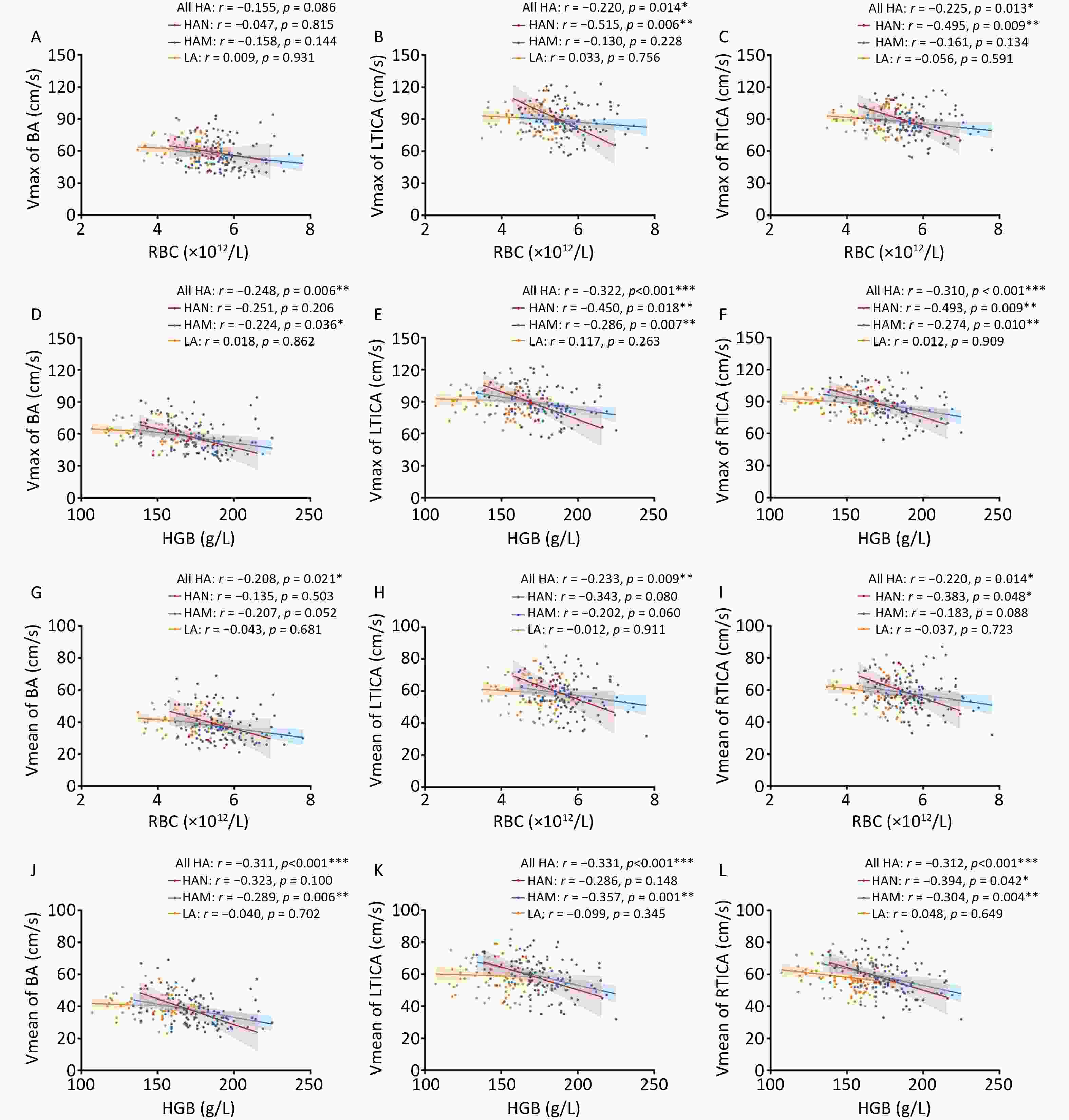
Figure 2. Correlations of red cell indices with velocity of BA, LTICA and RTICA in LA, HAM, HAN and all HA. A–C Correlations of RBC with Vmax of BA (A), LTICA (B) and RTICA (C) in LA, HAM, HAN and all HA groups. D–F Correlations of HGB with Vmax of BA (D), LTICA (E) and RTICA (F) in LA, HAM, HAN and all HA groups. G–I Correlations of RBC with Vmean of BA (G), LTICA (H) and RTICA (I) in LA, HAM, HAN and all HA groups. J–L Correlations of HGB with Vmean of BA (J), LTICA (K) and RTICA (L) in LA, HAM, HAN and all HA groups. Vmax, max velocity; Vmean, mean velocity; BA, basilar artery; LTICA, left terminal internal carotid artery; RTICA, right terminal internal carotid artery; LA, low-altitude participants; HAM, high-altitude migrants; HAN, high-altitude natives; RBC, Red blood cell count; HGB, hemoglobin. Partial correlation analyses were adjusted by age, sex, comorbidities, SpO2, LVEF and LVFS. The shaded areas represent the 95% confidence intervals.
Systematic reviews have shown that while moderately high-altitude environments may reduce the risk of stroke, living above 3,500 m is associated with a significantly increased risk. This is likely linked to polycythemia[1]. Our findings indicate that adaptive hyperhemoglobinemia and changes in the number and morphology of RBC reduce cerebral blood flow velocity, offering clinical support. In the case of a compensatory elevation of RBC and hemoglobin at high altitudes, it leads to increased blood viscosity in the brain. The direct result was a reduction in the cerebral blood flow velocity[9], which is in accordance with our conventional understanding. This finding may be attributed to the relatively young age of the individuals included in our study, who had not yet displayed noticeable abnormalities in cardiac function. The Himalayan and Andes regions are the two major high-altitude regions in the world. Previous studies have also reported that the cerebral blood flow velocity of Andes highland residents is lower, which is consistent with the conclusions of our study population[6]. One possible explanation is that changes in cerebral blood flow at high altitudes may be influenced by factors such as RBC indices and blood viscosity[10]. It is important to note that the small size of the HAN may potentially affect the accuracy of the results. Larger studies involving more participants are required to clarify whether regional or ethnic differences exist in these effects.
Researchers have empirically associated hyperhemoglobinemia with cerebrovascular blockage at high altitudes. In this study, we addressed this gap by monitoring the velocity of the cerebral blood flow using TCD. These findings reveal that relocation to high-altitude regions can lead to a relatively low flow velocity and high resistance among the population, which is attributed to compensatory erythrocyte proliferation in response to the hypoxic environment in high-altitude plateaus. This further substantiates the idea that elevated RBC levels may serve as a risk factor for cerebral ischemic disease, owing to the decrease in cerebral blood flow velocity among high-altitude populations.
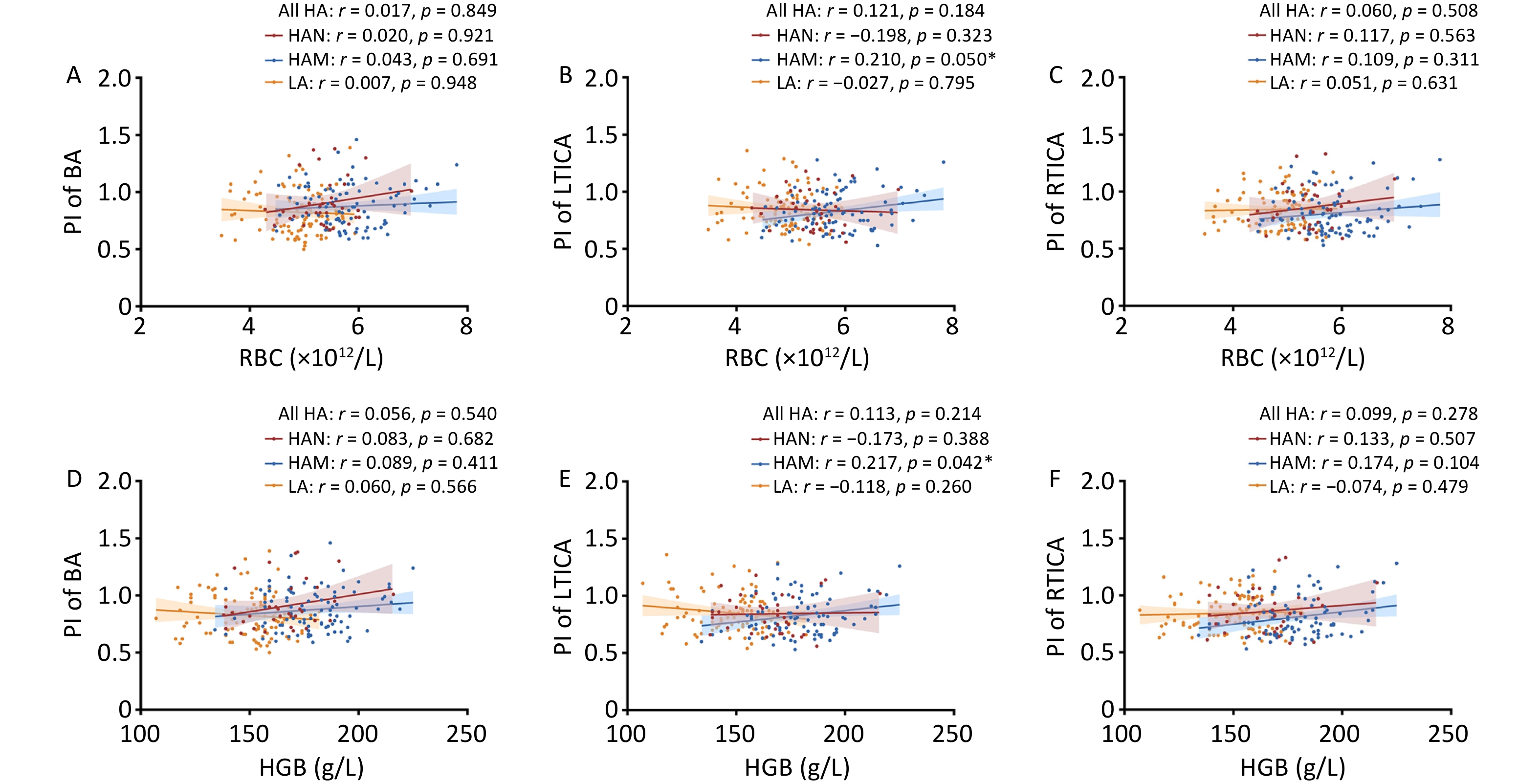
To enhance clarity, we conducted an analysis specifically focusing on the relationship between RBC, HGB, and the cerebral vessel index. We found positive correlations between RBC count and PI of the LTICA in HAM (r = 0.210, P = 0.050). However, no correlation between RBC and PI was found in any of the other groups (Figure 3A–C). HGB also did not show any correlation with the PI of the basilar artery in any group (Figure 3D). Furthermore, we observed positive correlations between HGB and the PI of the LTICA in HAM (r = 0.217, P = 0.042) (Figure 3E–F). In other words, changes in RBC could potentially influence cerebral blood flow in high-altitude migrants.
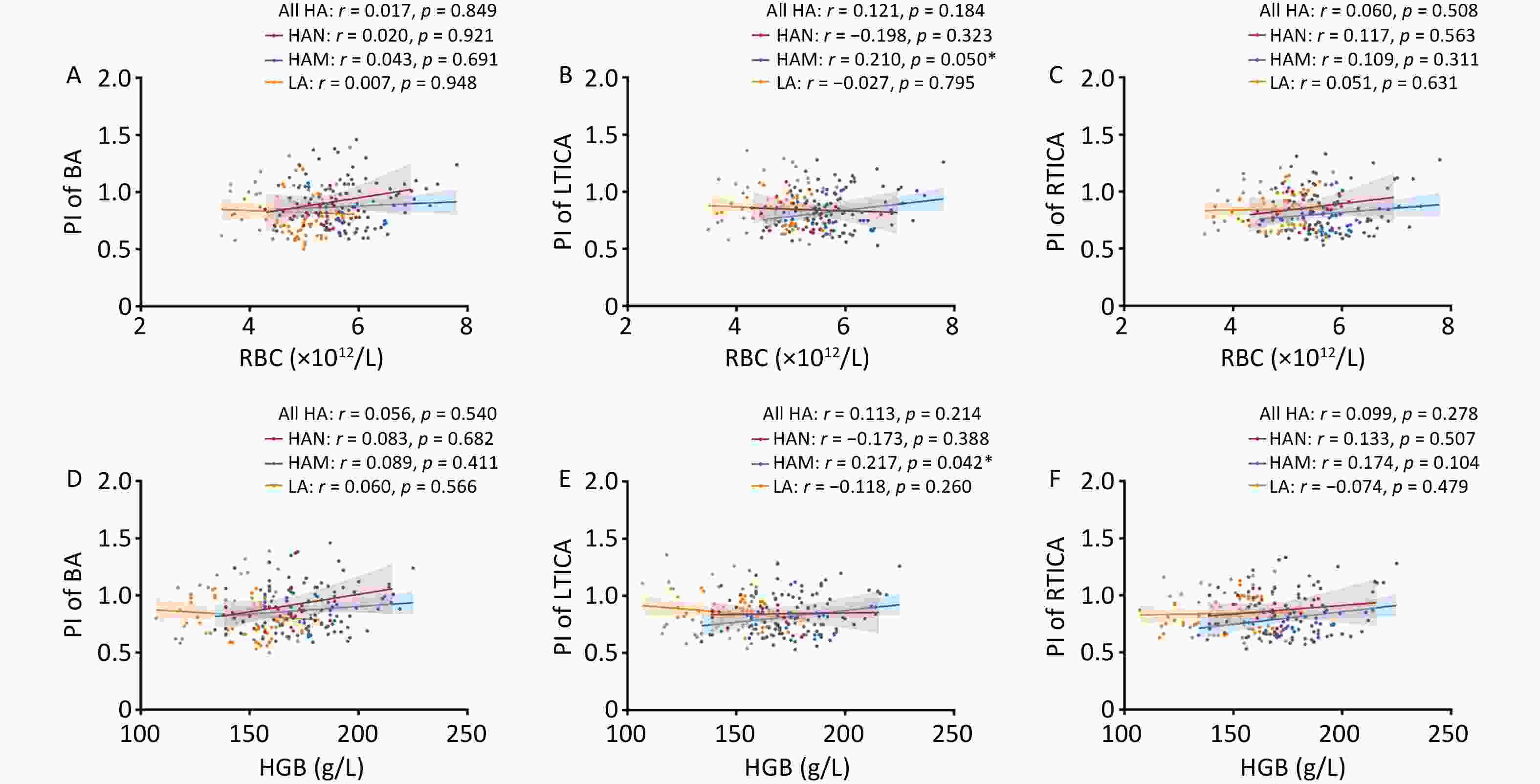
Figure 3. Correlations of red cell indices with PI of BA, LTICA and RTICA in LA, HAM, HAN and all HA. A–C Correlations of RBC with PI of BA (A), LTICA (B) and RTICA (C) in LA, HAM, HAN and all HA groups. D–F Correlations of HGB with PI of BA (D), LTICA (E) and RTICA (F) in LA, HAM, HAN and all HA groups. RBC, Red blood cell count; HGB, hemoglobin; PI, pulsatility index; BA, basilar artery; LTICA, left terminal internal carotid artery; RTICA, right terminal internal carotid artery; LA, low-altitude participants; HAM, high-altitude migrants; HAN, high-altitude natives. Partial correlation analyses were adjusted by age, sex, comorbidities, SpO2, LVEF and LVFS. The shaded areas represent the 95% confidence intervals.
Researchers have empirically associated hyperhemoglobinemia with cerebrovascular blockage at high altitudes. In this study, we addressed this gap by monitoring the velocity of the cerebral blood flow using TCD. These findings reveal that relocation to high-altitude regions can lead to a relatively low flow velocity and high resistance among the population, which is attributed to compensatory erythrocyte proliferation in response to the hypoxic environment in high-altitude plateaus. This further substantiates the idea that elevated RBC levels may serve as a risk factor for cerebral ischemic disease, owing to the decrease in cerebral blood flow velocity among high-altitude populations.
Because this was a cross-sectional study, causal relationships could not be inferred. Longitudinal follow-up studies are warranted to monitor the temporal changes in cerebral blood flow velocity and cerebral infarction incidence rates. Future studies should expand sample size to better characterize adaptive heterogeneity between HAM and HAN. Moreover, measurements may be affected by interoperator variability in the TCD technique and interindividual differences in skull anatomy. A methodological assessment of cerebral oxygenation is needed to further clarify the impact of reduced cerebral blood flow velocity on stroke risk.
In summary, our study found that red cell indices at high altitudes were correlated with cerebral blood flow velocity. This further indicates that an excessive erythrocyte count may be a potential primary risk factor for cerebral ischemic disease at high altitudes.
Associations between Red Cell Indices and Cerebral Blood Flow Velocity in High Altitude
doi: 10.3967/bes2025.131
- Received Date: 2025-04-19
- Accepted Date: 2025-08-11
The authors declare no competing or other conflicts of interests.
This study was approved by The Institutional Review Boards of Shigatse Branch, Xinqiao Hospital (2023-004) and Daping Hospital (2024-394), China.
&These authors contributed equally to this work.
| Citation: | Haolun Sun, Taiming Zhang, Dongyu Fan, Haoxiang Wang, Luran Xu, Qing Du, Jun Liang, Li Zhu, Xu Wang, Li Lei, Xiaoshu Li, Wangsheng Jin. Associations between Red Cell Indices and Cerebral Blood Flow Velocity in High Altitude[J]. Biomedical and Environmental Sciences. doi: 10.3967/bes2025.131 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: