-
Cyclic volatile methyl siloxanes (cVMSs) are widely used in industrial and consumer products because of their thermal stability, low reactivity, and reduced surface tension[1]. Their extensive use has resulted in environmental pollution globally. Recognized as very persistent and very bioaccumulative (vPvB), compounds such as octamethylcyclotetrasiloxane (D4), decamethylcyclopentasiloxane (D5), and dodecamethylcyclohexasiloxane (D6) are regulated in the European Union[2] and are monitored worldwide. cVMSs have been detected in surface and drinking water, raising concerns about human exposure through ingestion, inhalation, or dermal contact. cVMSs are known to be hepatotoxic and may potentially induce nonalcoholic fatty liver disease (NAFLD)[3]. However, the occurrence of cVMSs in drinking water in China and associated health risks remain inadequately assessed.
This study was conducted in Shanghai, a megacity with a population of 24 million. We analyzed seven cVMSs—hexamethylcyclotrisiloxane (D3), D4, D5, D6, tetradecamethylcycloheptasiloxane (D7), hexadecamethylcyclooctasiloxane (D8), and octadecamethylcyclononasiloxane (D9)—in raw and finished water samples. Samples were collected from drinking water treatment plants (DWTPs) that source water from reservoirs Q and J. The analysis was performed using liquid–liquid extraction coupled with gas chromatography–mass spectrometry (LLE-GC-MS). A comprehensive risk assessment was subsequently conducted following the U.S. Environmental Protection Agency (EPA) and European Scientific Committee on Consumer Safety (SCCS) guidelines to evaluate multi-route exposure risks across various age groups. Furthermore, we employed HepG2 cells as an in vitro model to examine cVMSs mixtures’ potential to induce hepatocytic lipid accumulation under normal as well as high-fat (HF) culture conditions. This study provides essential data for human health risk assessments and aids in developing future strategies to safeguard drinking water quality.
Water samples (both raw and finished) were collected from three DWTPs in Shanghai in November 2023 during the dry season. DWTP A utilizes reservoir Q (Yangtze River), while DWTP B and C draw water from reservoir J (Huangpu River). All three DWTPs employed advanced treatment processes that incorporated ozone oxidation and activated carbon filtration, with DWTP C utilizing ultrafiltration membrane technology. A detailed schematic of the water treatment process is shown in Supplementary Figure S1.
Water samples were collected at each sampling site over three consecutive days, with simultaneous preparation of field blanks for quality control purposes. Water samples were kept at 4 °C and were analyzed within 96 hours of collection. Detailed sampling protocols are provided in Supplementary Text S1.
Water samples were extracted using liquid–liquid extraction. We spiked 200 mL of the sample with the surrogate standard, M4Q, and extracted three times using 20 mL of hexane/ethyl acetate (1:1, v/v). After dehydration, the extract was concentrated to 0.2 mL under a gentle nitrogen stream and immediately analyzed using GC-MS. The specifications of all the chemicals used are listed in Supplementary Table S1, and detailed experimental procedures, instrument parameters, and ion chromatograms are provided in Supplementary Texts S2–S3, Supplementary Table S2, and Supplementary Figures S2–S3. The method was validated in terms of linearity, method detection limits (MDLs) and method quantification limits (MQLs), precision, and accuracy.
The U.S. EPA-recommended methods [Risk Assessment Guidance for Superfund (RAGS)] were used to calculate the average daily dose (ADD) under the central tendency exposure (CTE) as well as reasonable maximum exposure (RME) scenarios using the 50th and 95th percentiles of the exposure factors, respectively. The calculation formulas are provided below, and the values of the exposure factors are listed in Supplementary Tables S3–S5.
$$ {ADD}_{oral}=\left({C}_{w}\times IR\times EF\times ED\right)/(BW\times AT) $$ (1) where ADDoral is the average daily dose via ingestion (mg·kg-1·d-1);
Cw is the concentration (ng/L) of cVMSs in the finished water used in this study;
IR is the daily intake rates of drinking water (L/day);
EF is the exposure frequency (day/year), which was assumed to be 365 days in this study;
ED is exposure duration (year);
BW is body weight (kg);
AT is the average time (day) (non-carcinogenic risk AT = ED × 365).
$$ {ADD}_{inh}={(C}_{air}\times InhR\times AE\times ET\times EF\times ED)/(BW\times AT) $$ (2) $$ {C}_{air}=\left[{(Y}_{s}\left(t\right)+{Y}_{s}\left(i\right)\right]/2 $$ (3) $$ {Y}_{s}(t)=\{\left[1-\mathrm{e} ^{\land} (-bt)\right]\times \mathrm{a}\}/\mathrm{b} $$ (4) $$ a=\{{C}_{w}\times {Q}_{L}\times \left[1-e^\left(-N\right)\right]\}/{V}_{s} $$ (5) $$ b=\left\{\left[{Q}_{L}\times \left(1-\mathrm{e}^{\land} (-N)\right)/H\right]+{Q}_{Gs}\right\}/{V}_{s} $$ (6) $$ N={K}_{OL}A/{Q}_{L} $$ (7) $$ 1/{K}_{OL}A=1/{K}_{L}A+1/({K}_{G}A\times H) $$ (8) where ADDinh is the average daily exposure by inhalation (mg·kg-1·d-1);
Cair is the concentration in air, calculated from Little’s[4] theoretical equation for the cVMSs concentration in the bathroom (ng/L);
AE is the absorption efficiency and takes the value of 100%;
InhR is the inhalation rates(m3/day);
ET is exposure time (min/day);
Ys (i) is the initial cVMSs concentration in the shower room (assumed to be 0 ng/L);
Ys (t) is the concentration of cVMSs (ng/L) in the shower room at time t (min);
QL is the volumetric flow rates of water (min/L);
a and b are factors;
t is the contact time (min);
N is a dimensionless coefficient;
H is Henry's constant (dimensionless);
QGS is the volumetric flow rates of air (L/min);
KOL, KL, and KG are the overall, liquid-, and gas-phase mass-transfer coefficients, respectively, and A is the interfacial area available for mass transfer between water and air (it is recommended that the ratio of the gas-to liquid-phase mass transfer coefficients for a shower system be 17).
$$ \begin{split} ADD_{der}= & [2FA\times K_P\times C_w\times \sqrt{(6\tau_{event}\times t_{event)/\pi}}\times \\ & EV\times EF\times ED\times SA]/(BW\times AT) \end{split} $$ (9) $$ \mathrm{log}{K}_{p}=-2.80+0.66\mathrm{log}{K}_{OW}-0.0056MW\left({r}^{2}=0.66\right) $$ (10) $$ {\tau }_{event}=\left({l}_{sc}^{2}\right)/\left({6D}_{sc}\right)={0.105\times 10}^{\left(0.0056MW\right)} $$ (11) where ADDder is the average daily dose for dermal contact (mg·kg-1·d-1);
FA is fraction absorbed water (dimensionless);
Kp is the dermal permeability coefficient of the compound in water (cm·hr-1);
Kow is the octanol/water partition coefficient (dimensionless);
MW is the molecular weight (g·mole-1);
τevent is the substance delay time per event (min·event-1);
lsc is the apparent thickness of the stratum corneum (cm); assuming lsc = 10-3 cm, τevent can be evaluated using equation (11);
Dsc is the effective diffusion coefficient of a chemical through the stratum corneum (cm2/hr);
tevent is event duration (min·event-1);
EV is event frequency (events·day-1);
SA is skin surface area available for contact (cm2).
Margin of safety (MoS) values for cVMSs in drinking water were calculated according to the methodology recommended by the European SCCS[5,6]. The calculation formulae are as follows:
$$ \text{MoS}=\text{systemic\;POD/SED} $$ (12) $$ \text{SED}=\text{ADD} \times \text{Absorption} /10^{6} $$ (13) where POD is the point of departure (mg·kg-1·d-1);
SED is the systemic exposure dose (mg·kg-1·d-1).
The POD values and pathway-specific absorption rates (%) are listed in Supplementary Table S6.
In the cell-based experiments, the HepG2 human hepatocellular carcinoma cell line was used to establish a HF culture condition (positive control) via treatment with sodium oleate and sodium palmitate (70 μmol/L:35 μmol/L). Under both normal and HF culture conditions, cells were treated with a cVMSs mixture at 50–500 times the drinking water concentration for 24–72 h to evaluate lipogenic toxicity. Details of the solution preparation, exposure, and preparation of the sodium oleate and sodium palmitate mixtures are presented in Supplementary Texts S4–S5 and Supplementary Table S7. DNA content was measured using the NucBlue assay (Ex/Em = 360/460 nm) to evaluate cell viability, whereas triglyceride levels were determined using the AdipoRed reagent (Ex/Em = 485/572 nm) and normalized against the corresponding DNA content. Cell viability and normalized triglyceride content were calculated using the following equations:
$$ \begin{split} & cell\;viability=\left[FI_{DNA\left(experimental\;group\right)}-FI_{DNA\left(blank\;control\;group\right)}\right]/ \\ & \left[FI_{DNA\left(control\;group\right)}-FI_{DNA\left(blank\;control\;group\right)}\right] \end{split} $$ (14) $$ \begin{split} &relative\;triglyceride\;content=[FI_{\mathrm{T}\mathrm{G}\left(experimental\;group\right)} \\ & -FI_{\mathrm{T}\mathrm{G}\left(blank\;control\;group\right)}]/ \{ \text{Cell\; viability} \times \\ & \left[FI_{\mathrm{T}\mathrm{G}\left(controlgroup\right)} -FI_{\mathrm{T}\mathrm{G}\left(blankcontrolgroup\right)}\right]\} \end{split} $$ (15) where FI stands for fluorescence intensity.
Statistical analyses were conducted using SPSS 20.0, with data presented as mean ± standard error (mean ± SE). Group comparisons were performed using one-way analysis of variance (ANOVA), Dunnett's t-test and Dunnett's t3 test (α = 0.05). Graphical representations were generated using GraphPad Prism 8.0 and Origin 2024. Concentrations below the method detection limit were assigned a value half of the detection limit for statistical analysis.
To minimize background contamination, three precautionary measures were implemented: (1) using freshly opened reagents with minimal target compound background; (2) replacing silicone septums with aluminum foil for sample vials; and (3) thoroughly rinsing all glassware with n-hexane and acetone followed by baking at 200 °C. Furthermore, all sample concentrations were corrected by subtracting the field blank values to account for potential contamination from both the GC-MS system and environmental sources.
In this study, LLE was employed, yielding absolute recoveries of 71.1%–121.8% for all the target compounds (D3–D9). The optimized method was rigorously validated (Supplementary Table S8), demonstrating excellent linearity (R2 = 0.997–0.999) across a concentration range of 0–100 ng/L. The MDLs ranged from 0.09–1.43 ng/L. The spiked recoveries were between 93.74% and 122.26%, with relative standard deviations (RSDs) ranging from 1.21%–10.28% (all below 15%). The average recovery rate of the M4Q surrogate was 105.96% (RSD = 3.15%).
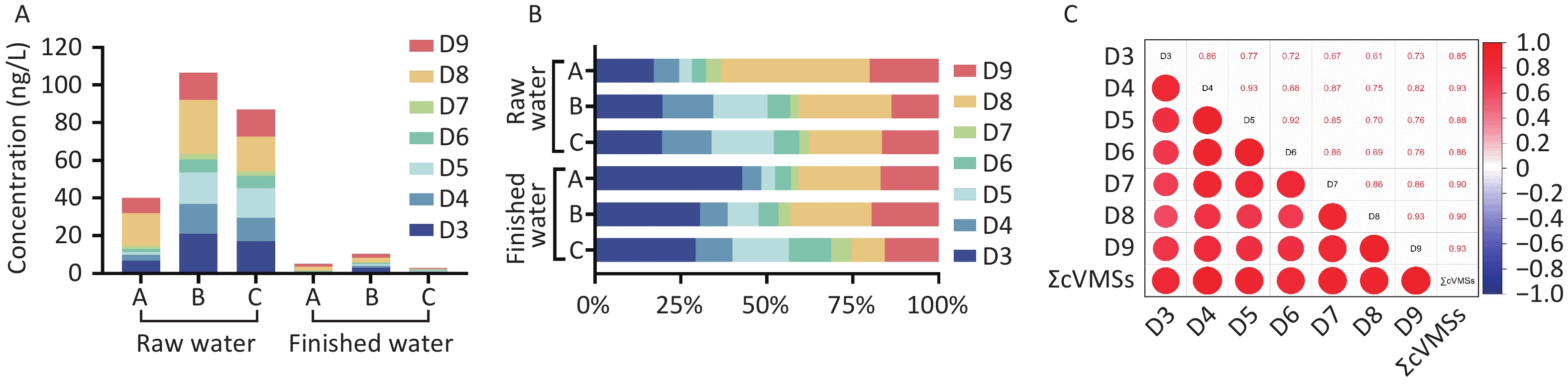
In this study, 27 raw water samples collected from three DWTPs in Shanghai showed the universal detection of cVMSs (Supplementary Table S9). The detection frequencies were 85.19% for D3, 96.30% for D4, and 85.19% for D6, whereas D5, D7, D8, and D9 were detected in 100% of samples (Supplementary Table S10). Total cVMSs (∑cVMSs) concentrations in the raw water samples ranged from 29.78 to 157.16 ng/L (mean: 77.89 ng/L; Supplementary Table S9). Significantly higher concentrations were observed in DWTP B samples (mean: 106.73 ng/L) and DWTP C samples (mean: 86.97 ng/L) compared to DWTP A samples (mean: 39.97 ng/L; P < 0.001). These differences likely reflect variations in source water quality, as DWTP A draws water from reservoir Q (Yangtze River source), whereas DWTP B and C utilize reservoir J (Huangpu River source). The mean concentration of ∑cVMSs in this study was comparable to or lower than those reported in surface waters from other regions (Supplementary Table S11).
Among the seven cVMSs analyzed, D8, D9, and D3 were the predominant compounds in raw water, with mean concentrations ranging from 17.26–28.63 ng/L, 8.02–14.72 ng/L, and 6.86–21.01 ng/L, respectively, collectively accounting for > 50% of ∑cVMSs (Figure 1). This finding aligns with that of a recent study documenting the predominance of higher molecular weight cVMSs (D8 and D9) in the surface waters of the Lower Yangtze River Basin[7]. The elevated concentrations of these compounds may reflect the intensive industrial activity in the Yangtze River Delta region and the widespread use of related products.
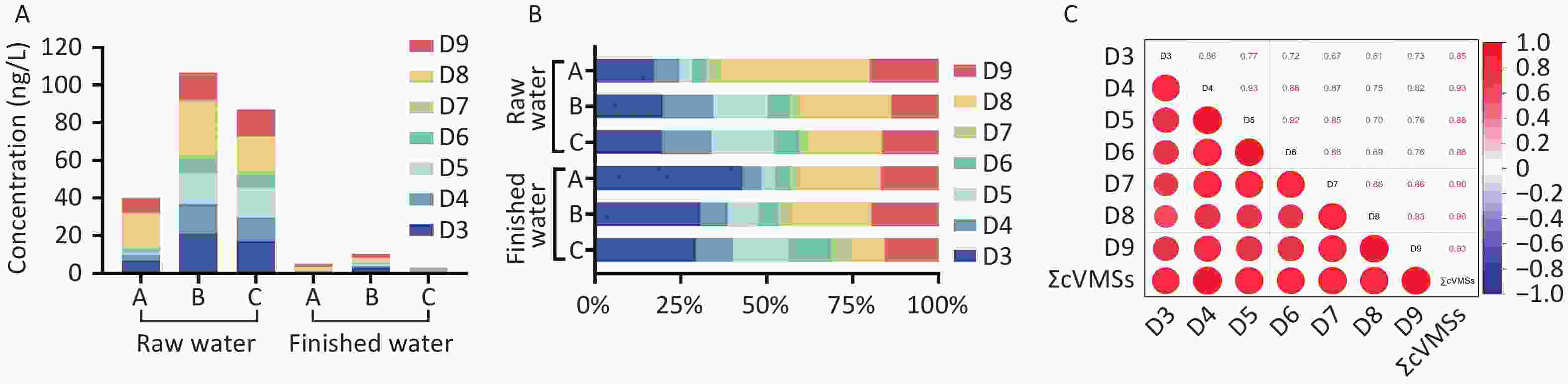
Figure 1. Concentration (A) and composition (B) of cVMSs in raw water and finished water from DWTPs in Shanghai, and correlation of cVMSs in water samples (C). cVMSs, cyclic volatile methyl siloxanes; DWTPs, drinking water treatment plants.
The detection frequencies of the seven cVMSs (18.52%–70.37%) were significantly lower in raw water (P < 0.001; Supplementary Table S10). ∑cVMSs in finished water ranged from 2.99 to 10.37 ng/L (Supplementary Table S9), representing a 67%–91% reduction compared to raw water concentrations (P < 0.001; Supplementary Table S12). The lowest concentration was observed in DWTP C (2.99 ng/L), which may be attributed to its additional ultrafiltration membrane treatment. D8, D9, and D3 were the most abundant cVMSs in the finished water, whereas D7 had the lowest concentration. This distribution pattern closely mirrored the relative abundance observed in the raw water samples. The cVMSs concentrations measured in this study were lower than those reported for tap water in Vietnam[8].
Significant positive correlations were observed among D3–D9 and between individual cVMSs and ΣcVMSs (R = 0.61–0.93; P < 0.01) across all water samples. Stronger correlations were evident between the adjacent compounds (Figure 1, Supplementary Table S13). This finding is consistent with those previously reported in Vietnam[8] and Sweden[9]. This correlation may reflect their shared emission sources and comparable environmental fate, and interconversion among cVMSs (e.g., D5 → D4 → D3)[9]. Further research is necessary to better understand the environmental behavior of high-molecular-weight cVMSs (D7–D9).
This study employed U.S. EPA-recommended methods to estimate the ingestion, inhalation, and dermal route exposure to cVMSs (Figure 2). Some of these cVMSs are volatile organic compounds (VOCs) and have properties such as low boiling points (< 250 °C; Supplementary Table S14) and skin permeability (Kp). However, because of the limitations of available parameters, inhalation and dermal exposure assessments were conducted only for D4, D5, and D6. Exposure assessments were based on Chinese population exposure factors and measured cVMSs concentrations in Shanghai's finished waters (Supplementary Tables S15–S16). Oral ADDs were highest for D8, D9, and D3. Mixed cVMSs oral ADDs ranged from 0.079–0.232 ng/kg-bw/day (CTE) to 1.58–6.37 ng/kg-bw/day (RME). Mixed cVMSs inhalation ADDs ranged from 0.03–0.11 ng/kg-bw/day (CTE) to 0.42–1.47 ng/kg-bw/day (RME). Dermal exposure levels were 0.29–0.66 ng/kg-bw/day (CTE) and 2.79–6.05 ng/kg-bw/day (RME), higher than other routes. Children exhibited higher exposure levels than adults in this study. The average daily oral exposure in this study was slightly lower than that reported in Vietnam (0.409 ng/kg-bw/day for adults and 0.412 ng/kg-bw/day for children)[8].
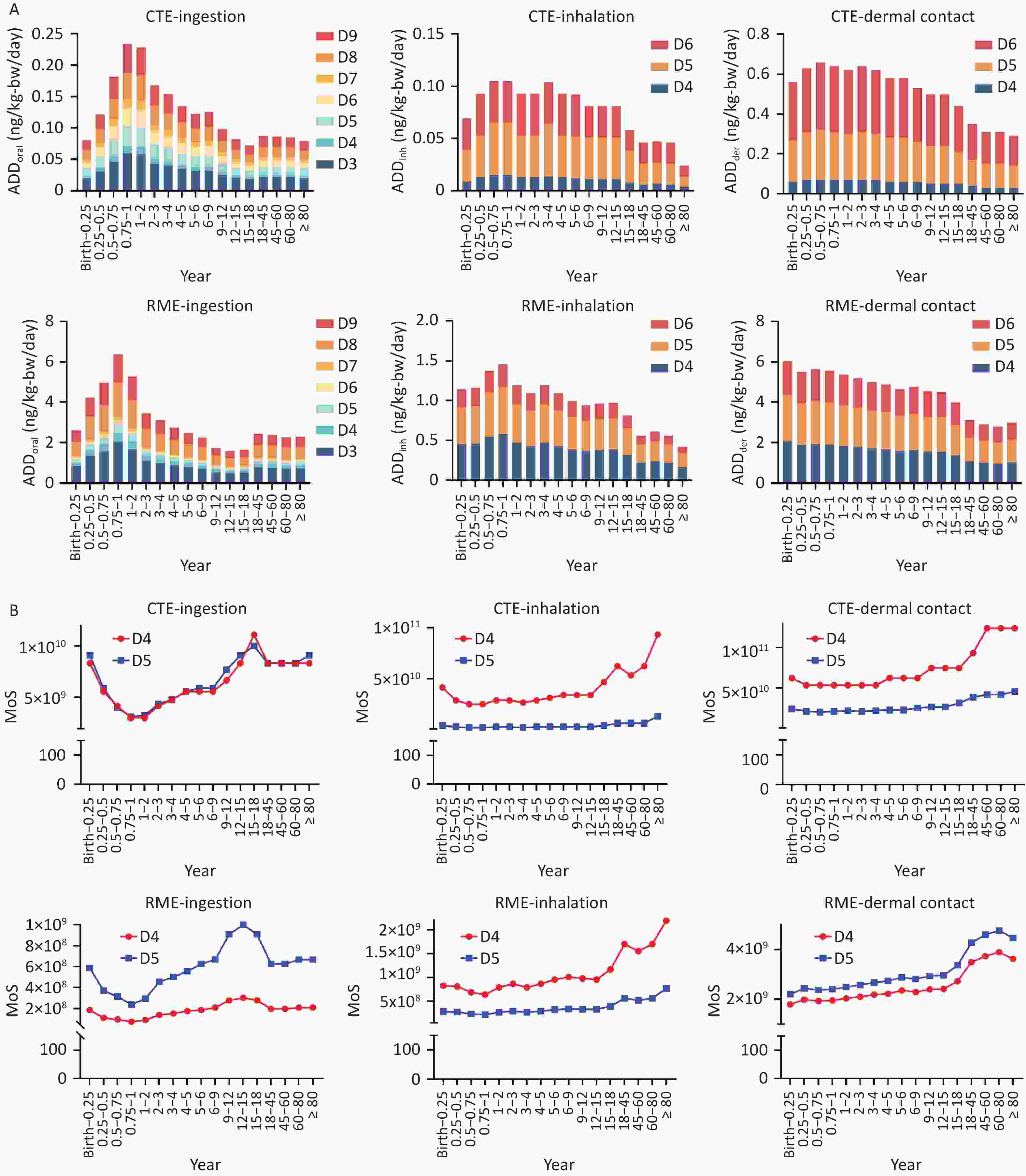
Figure 2. Estimated average daily exposure to cVMSs (A) and estimated MoS values of cVMSs (B) via ingestion, inhalation, and dermal contact from drinking water in three DWTPs across different age groups. The daily exposure and the health risks were estimated under both CTE and RME scenarios. cVMSs, cyclic volatile methyl siloxanes; DWTPs,drinking water treatment plants; MoS, margin of safety; CTE, central tendency exposure; RME, reasonable maximum exposure.
Following the SCCS-recommended methodology[5,6], we calculated the MoS for D4 and D5 via three exposure routes (ingestion, inhalation, and dermal contact) (Figure 2, Supplementary Tables S17 and S18). Under the CTE scenario, the MoS via three exposure routes ranged from 3.02 × 109 to 1.24 × 1011. Under the RME scenario, the MoS values ranged from 7.39 × 107 to 4.76 × 109. All values exceeded 100, indicating a negligible health risk. However, the assessment did not cover all cVMSs species, nor did it consider volatilization during boiling, seasonal variations, or other potential exposure sources. These limitations may introduce uncertainties into risk estimates.
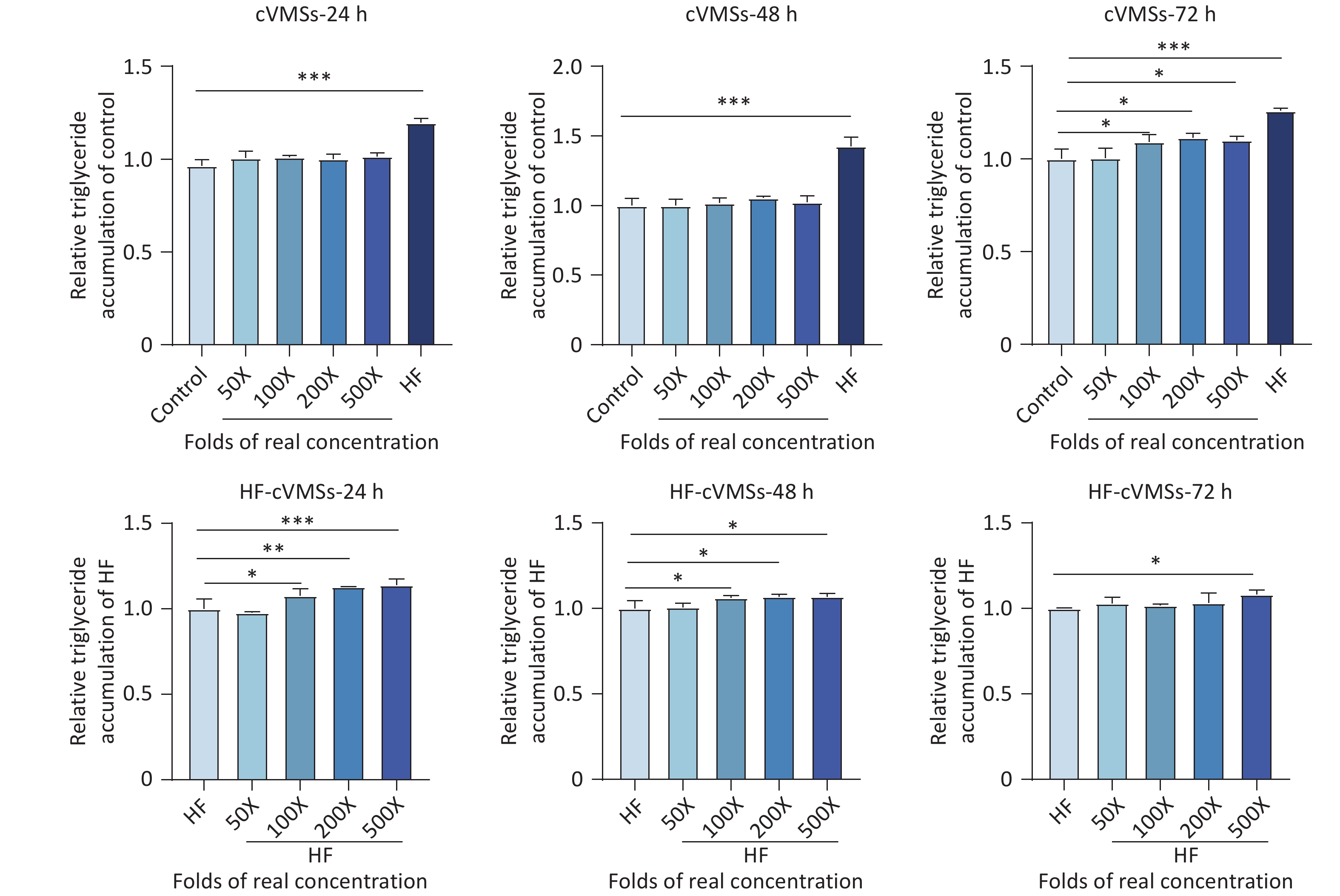
A recent epidemiological study demonstrated the association between cVMSs exposure and NAFLD among Chinese adults[3], and to further explore the potential link, an in vitro model employing HepG2 cells was established. No significant cytotoxicity (P > 0.05) was observed under either normal or HF culture conditions after 24–72 h exposure to cVMSs mixtures at concentrations of 50–500 times the actual levels (Supplementary Figure S4). Under normal culture conditions, lipid accumulation increased significantly (1.09-fold, P < 0.05) in HepG2 cells after 72-hour exposure to 100 times the actual concentration (Figure 3). Under the HF culture conditions, lipid accumulation increased in a dose-dependent manner. A 24-hour exposure to 500 times the actual concentration resulted in a significant 1.14-fold increase in lipid accumulation (P < 0.05). Notably, these effects were more pronounced and manifested earlier than under normal culture conditions, suggesting a potentially elevated health risk for populations with underlying metabolic disorders such as obesity. The underlying mechanisms may involve oxidative stress and inflammatory responses[10]. Further studies are needed to investigate the toxicokinetics of cVMSs in humans.
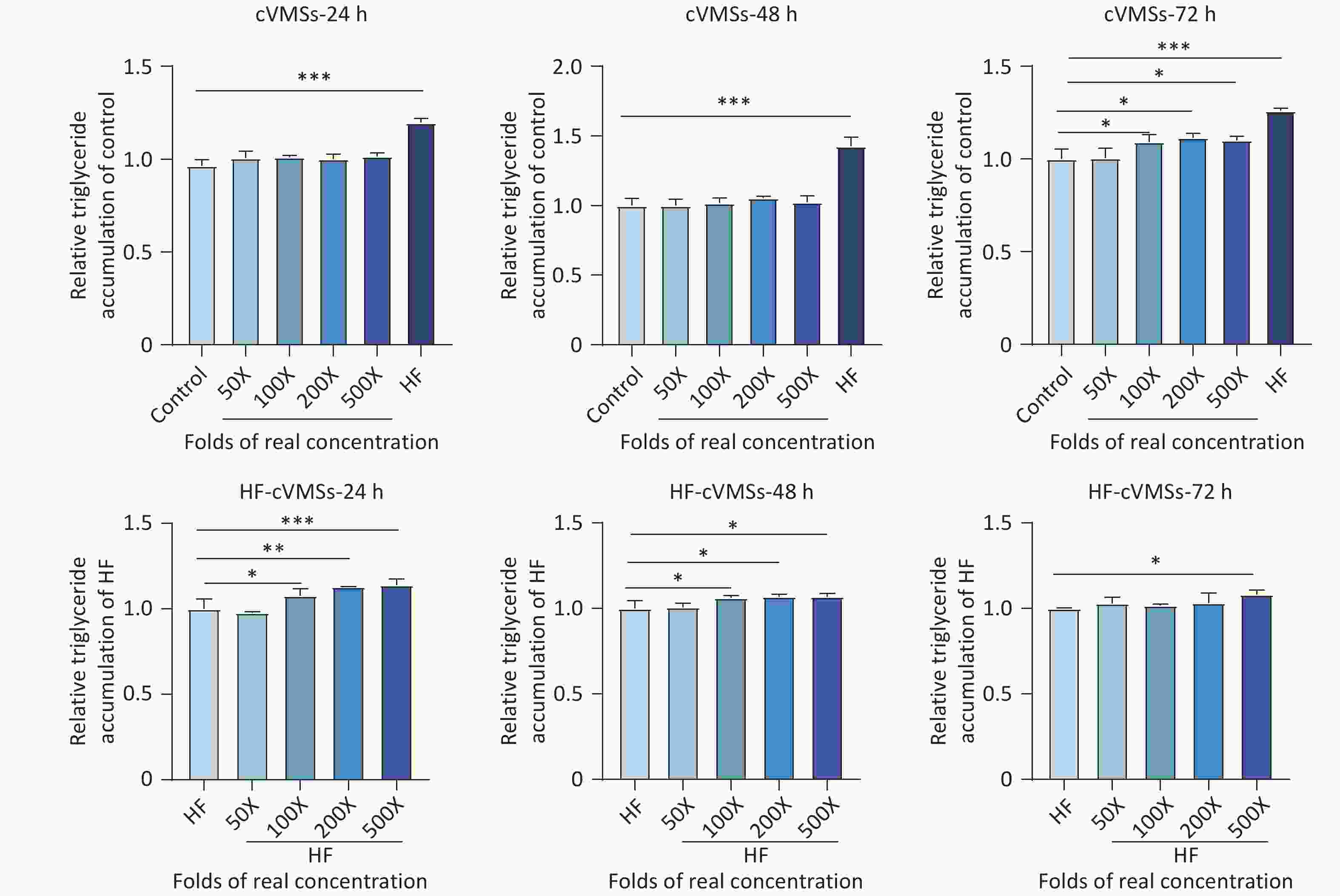
Figure 3. Lipid accumulation effects of mixed exposure to cVMSs in drinking water in Shanghai on HepG2 cells under normal and HF culture conditions after 24, 48, and 72 h exposure to cVMSs at varying folds of realistic levels (n = 3, *P < 0.05, **P < 0.01, ***P < 0.001 compared with control or HF group). cVMSs, cyclic volatile methyl siloxanes.
In this study, we developed and validated a novel analytical method for the simultaneous quantification of seven cVMSs in drinking water. Our investigation revealed detectable concentrations of cVMSs in both raw and finished water samples collected from DWTPs across Shanghai's major water sources, with significantly higher contamination levels in reservoir J than in reservoir Q. Furthermore, the ultrafiltration treatment process demonstrated superior efficiency in cVMSs removal. Despite the low health risks from drinking water, cVMSs may exacerbate NAFLD, particularly in obese or HF diet individuals. This study provides valuable scientific evidence for optimizing water treatment technologies and establishing regulatory guidelines for cVMSs in drinking water.
Distribution, Health Risk and Hepatotoxic Implications of Cyclic Volatile Methylsiloxanes in Drinking Water in Shanghai, China
doi: 10.3967/bes2025.138
- Received Date: 2025-07-05
- Accepted Date: 2025-10-16
The authors declare no competing interests.
This study did not involve human or animal subjects. Therefore, no ethical approval was required.
&These authors contributed equally to this work.
| Citation: | Chunlei Wang, Yongqing Diao, Chuyi Chen, Jielan Hu, Yuxin Li, Xi Yu, Xia Wang. Distribution, Health Risk and Hepatotoxic Implications of Cyclic Volatile Methylsiloxanes in Drinking Water in Shanghai, China[J]. Biomedical and Environmental Sciences. doi: 10.3967/bes2025.138 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: