-
Circadian rhythm is a self-sustaining endogenous oscillation that serves as an internal timekeeping mechanism adapted to the Earth’s 24-h rotational schedule. It exists ubiquitously in nearly all organisms, from prokaryotes to mammals, and regulates diverse physiological and behavioral processes by synchronizing them with environmental fluctuations[1]. Previous reports indicated that circadian rhythms exist in biological individuals and cells cultured in vitro[2]. The mammalian circadian rhythm system consists of a central pacemaker located in the suprachiasmatic nucleus (SCN) of the hypothalamus, which coordinates peripheral rhythms through the sympathetic and parasympathetic nervous systems[3]. This hierarchical mechanism uses neural populations as optimal models for circadian rhythm research. At the cellular level, the circadian rhythm is genetically controlled by conserved transcription-translation feedback loops, including the circadian locomotor output cycles kaput (CLOCK), brain and muscle ARNT-Like 1 (BMAL1), period (PER), and cryptochrome (CRY), which regulate downstream genes and other circadian genes that comprise secondary transcription-translation feedback loops[4].
Ionizing radiation (IR) such as X-rays and carbon ion beams (CIB) radiation induces genotoxic stress and distinct DNA damage response pathways[5]. According to a few studies focusing on IR, the expression of circadian genes is altered both in vivo and in vitro by IR, as is the behavioral rhythm of animals. Although the nervous system plays a critical role in circadian rhythms[6], the impact of IR on neural cells remains unclear. Moreover, as a high linear energy transfer (LET) particle radiation, CIB induces more severe radiobiological damage than low-LET photon radiation, whereas differences in circadian rhythm after X-rays and CIB have not been explored. In this study, mouse hippocampal neuronal cells (HT22 cells) and microglia (BV2 cells) were exposed to 2 Gy X-rays or CIB. Circadian rhythm dynamics were analyzed by quantitative detection of mRNA and protein expression profiles, and subcellular localization. The results showed that IR disrupted PER1 expression while promoting the nuclear import of PER1, thus establishing IR as an exogenous modulator of circadian rhythms.
To characterize circadian rhythms in vitro, the mRNA profiles of core circadian genes (Per1, Cry1, Clock, and Bmal1) were quantified in BV2 and HT22 cells. As the circadian rhythm and cell cycle are mutually regulated in a coupled manner, to avoid the influence of cell cycle regulatory factors, various methods of cell cycle synchronization have been chosen to stabilize the cell rhythm prior to radiation treatment[7,8]. The results showed that the combination of serum starvation plus 1 μmol/L Dex for 24 h elevated the proportion of HT22 and BV2 cells in both the G0/G1 phase and S phase while reduced the proportion of cells in the G2/M phase (Supplementary Figure S1A). In addition, serum shock was induced using 50% horse serum, generating a circadian rhythm by simulating the entrainment effect in vivo through differences in blood-borne signaling factors in the serum. A similar cell cycle distribution was observed in cells subjected to serum shock (Supplementary Figure S2B). Based on synchronization efficacy, combinatorial treatment of serum starvation plus 1 μmol/L Dex was selected for subsequent circadian rhythm studies, with the end time of the treatment defined as zeitgeber time 0 (ZT0) to standardize temporal reference. All subsequent time points following the release from synchronization are shown in ZT. Transcription profiles of core circadian genes revealed distinct temporal dynamics in synchronized HT22 and BV2 cells across ZT0-ZT30. In particular, Per1 and Cry1 exhibited stable and significant periodic oscillations. Per1 expression exhibited an initial 6-h suppression phase followed by progressive recovery and increased steadily until ZT30 (Supplementary Figure S1C and 1D). Cell-specific Cry1 regulation was observed: HT22 cells showed a transient increase at ZT6, followed by a rapid decline, while BV2 cells displayed sustained suppression until ZT12 before rebounding. The transcription profiles of the core circadian genes in the serum shock group were similar to those in the serum starvation plus Dex combinatorial treatment group (Supplementary Figure S2C).
Owing to the robust circadian oscillations exhibited by Per1 and Cry1, coupled with their nuclear translocation characteristics, we focused on these two core circadian genes. Synchronized HT22 and BV2 cells (via the serum starvation/Dex combinatorial protocol) were irradiated with 2 Gy X-rays or CIB at ZT0, Per1 and Cry1 mRNA, and protein expression dynamics were quantified across six ZTs (ZT0-ZT30) to assess IR-induced circadian dysregulation (Figure 1A). Quantitative analysis revealed a conserved Per1 expression phase in HT22 cells across different groups (Figure 1B). The increase in Per1 transcription was faster in the irradiated groups, and a significant difference was detected only at ZT24 in both the CIB and X-ray groups. Notably, the increase of Per1 in the CIB group was much greater than that in the X-ray group at ZT24 (Figure 1B). The transcription of Cry1 exhibited similar oscillations in the different groups, with no substantial difference in mRNA levels in HT22 cells (Figure 1B). In irradiated BV2 cells, the phases of Per1 and Cry1 mRNA were similar to those in the non-irradiated group, with significantly elevated mRNA levels of in Per1 at ZT18 and an unperturbed amplitude of Cry1 (Figure 1C). Additionally, we confirmed the effect of IR on circadian gene transcription in the serum shock system (Supplementary Figure S2A). The results showed that the expression of Per1 in HT22 and BV2 cells did not change until ZT6 and then increased, with IR-upregulated expression at ZT18 in HT22 and ZT30 in BV2 cells. (Supplementary Figure S2D). This result was similar to that observed under serum starvation conditions. In summary, X-rays and CIB irradiation failed to affect the phase but significantly changed the amplitude of Per1 transcription in HT22 and BV2 cells, especially at 18–24 h after irradiation, and CIB caused greater perturbation compared to X-rays.
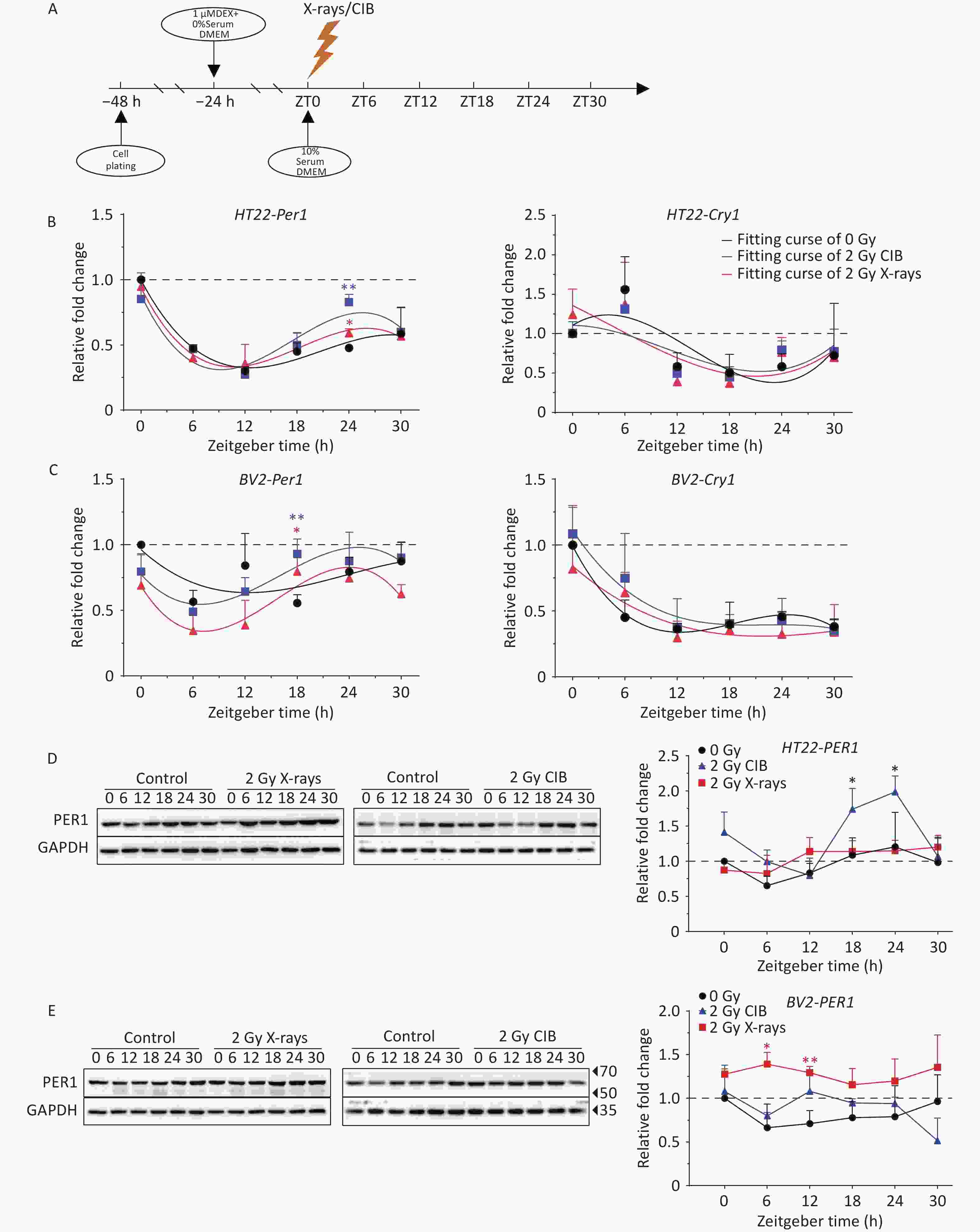
Figure 1. IR influences the mRNA and protein levels of PER1. (A) Scheme of IR action in HT22 and BV2 cells subjected to serum starvation and dexamethasone treatment. (B–C) mRNA levels of circadian genes (Per1 and Cry1) in synchronized HT22 (B) and BV2 cells (C) treated with 2 Gy X-rays and 2 Gy CIB were measured using quantitative PCR. (D–E) Protein levels of PER1 in synchronized HT22 (D) and BV2 cells (E) treated with 2 Gy X-rays and 2 Gy CIB were measured by western blotting. The data are shown as the means ± S.D., n = 3. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by two-tailed Student’s t-test indicate statistical significance. IR, ionizing radiation; CIB, carbon ion beams.
Subsequently, total cellular proteins were extracted to detect temporal changes in protein expression. The levels of PER1 in irradiated HT22 cells were higher than those in unirradiated cells, whereas a significant increase was detected at ZT18 and ZT24 in the CIB groups. In addition, the extent of increase in the CIB group was greater than that in the X-ray group, which was consistent with the trend observed for Per1 mRNA (Figure 1B and 1D). In BV2 cells, the trend of PER1 protein in the non-irradiated group was consistent with that of Per1 mRNA (Figure 1C and 1E). PER1 protein expression increased after irradiation with 2 Gy X-rays and CIB. However, significant upregulation was detected only at ZT6 and ZT12 in the X-rays irradiated group (Figure 1E). Collectively, IR significantly altered PER1 protein expression dynamics, with expression alterations consistent with Per1 transcription patterns.
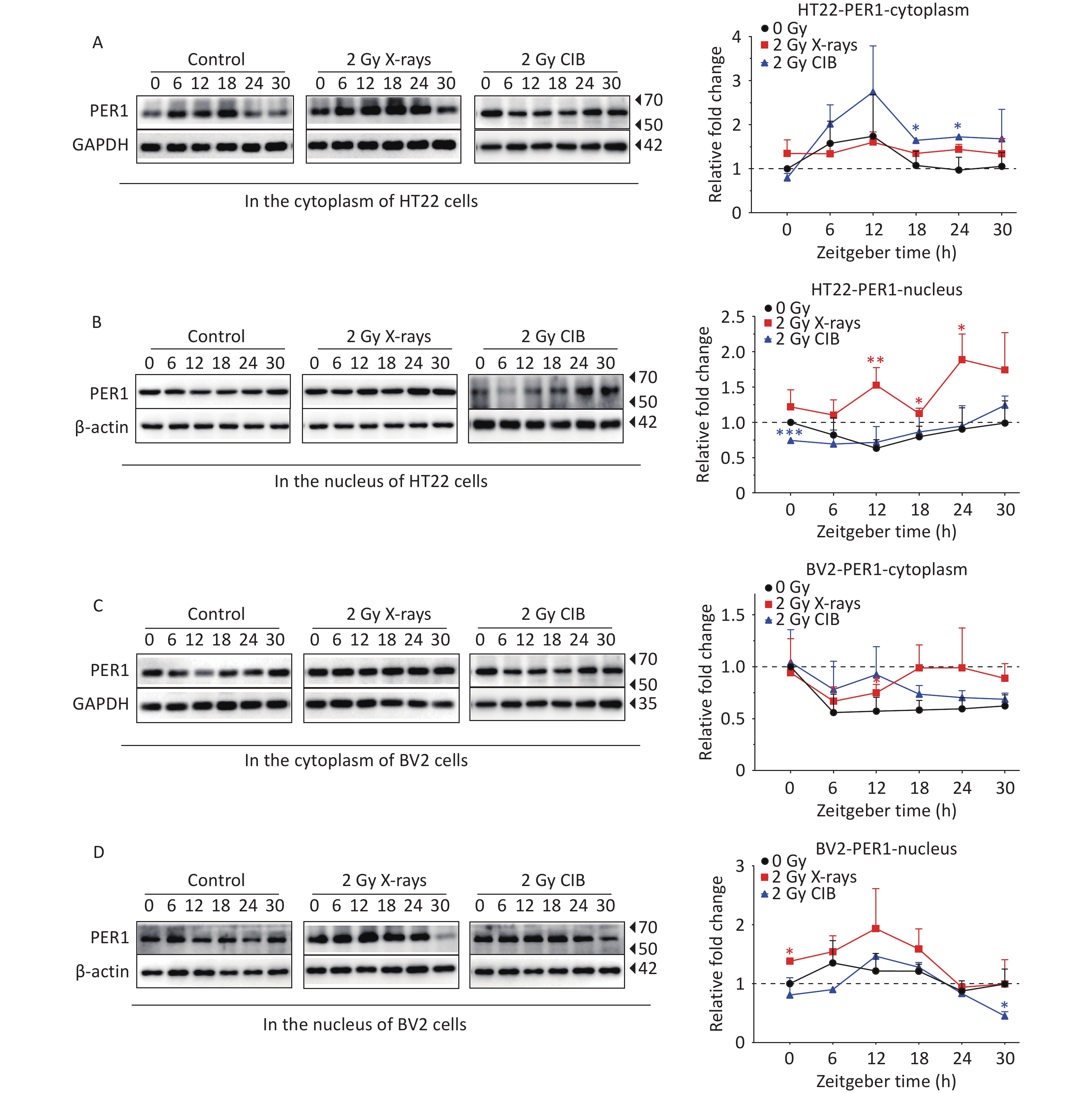
As the activation and nuclear transfer of PER1 play a non-negligible role in transcription-translational feedback loops, coupled with the unified upregulation at both the mRNA and protein levels after IR treatment, we further analyzed PER1 subcellular localization through western blotting and fluorescence. PER1 levels in HT22 cells treated with X-rays were much higher than those in the control cells, and significant increases in PER1 in the nucleus were detected at ZT12, ZT18, and ZT24. CIB irradiation also upregulated the expression PER1 with significant increases in the cytoplasm at ZT18 and ZT24 in HT22 cells (Figure 2A and 2B). In BV2 cells exposed to X-rays, PER1 was significantly upregulated at ZT12 in the cytoplasm and at ZT0 in the nucleus. PER1 was also upregulated in the cytoplasm and nucleus of CIB-treated BV2 cells treated with CIB; however, the difference was not significant (Figure 2C and 2D). In summary, X-ray irradiation increased the levels of cytoplasmic and nuclear PER1 in both HT22 and BV2 cells. In HT22 cells, the cytoplasmic PER1 phase after either X-ray or CIB irradiation was similar to that in the non-irradiated group (Figure 2A and 2C). However, the phase alteration of nuclear PER1 differed significantly from that in the cytoplasm of HT22 cells after exposure to 2 Gy X-rays or 2 Gy CIB (Figure 2B). The alteration in the expression phase was the greatest at ZT12. In BV2 cells, the expression levels of cytoplasmic PER1 in both the X-ray and CIB irradiated groups were similar to those in the unirradiated group. Similar to HT22 cells, we found that the expression of PER1 in the nucleus of BV2 cells increased in the irradiated groups but decreased in the unirradiated group from ZT6 to ZT12. PER1 expression in the nucleus increased markedly from ZT6 to ZT12 in the irradiated group, especially in BV2 cells treated with 2 Gy CIB and decreased slightly in the unirradiated group. Therefore, ZT12 was selected to verify the intracellular distribution of PER1. A stable PER1-GFP reporter cell line was established by genomic integration of PER1 fused to GFP in HT22 cells. We observed the subcellular localization of PER1 at ZT12 and analyzed the intensity of PER1-GFP in the nucleus and total cellular PER1-GFP. The results showed that both 2 Gy X-ray (Supplementary Figure 3A) and 2 Gy CIB irradiation (Supplementary Figure 3B) significantly elevated the nuclear-to-total PER1-GFP ratio. Immunofluorescence in HT22 cells confirmed these observations (Supplementary Figure 3C), demonstrating that 2 Gy X-rays enhanced PER1 nuclear translocation. Taken together, these results confirm that IR promotes the nuclear import of PER1 in HT22 cells.
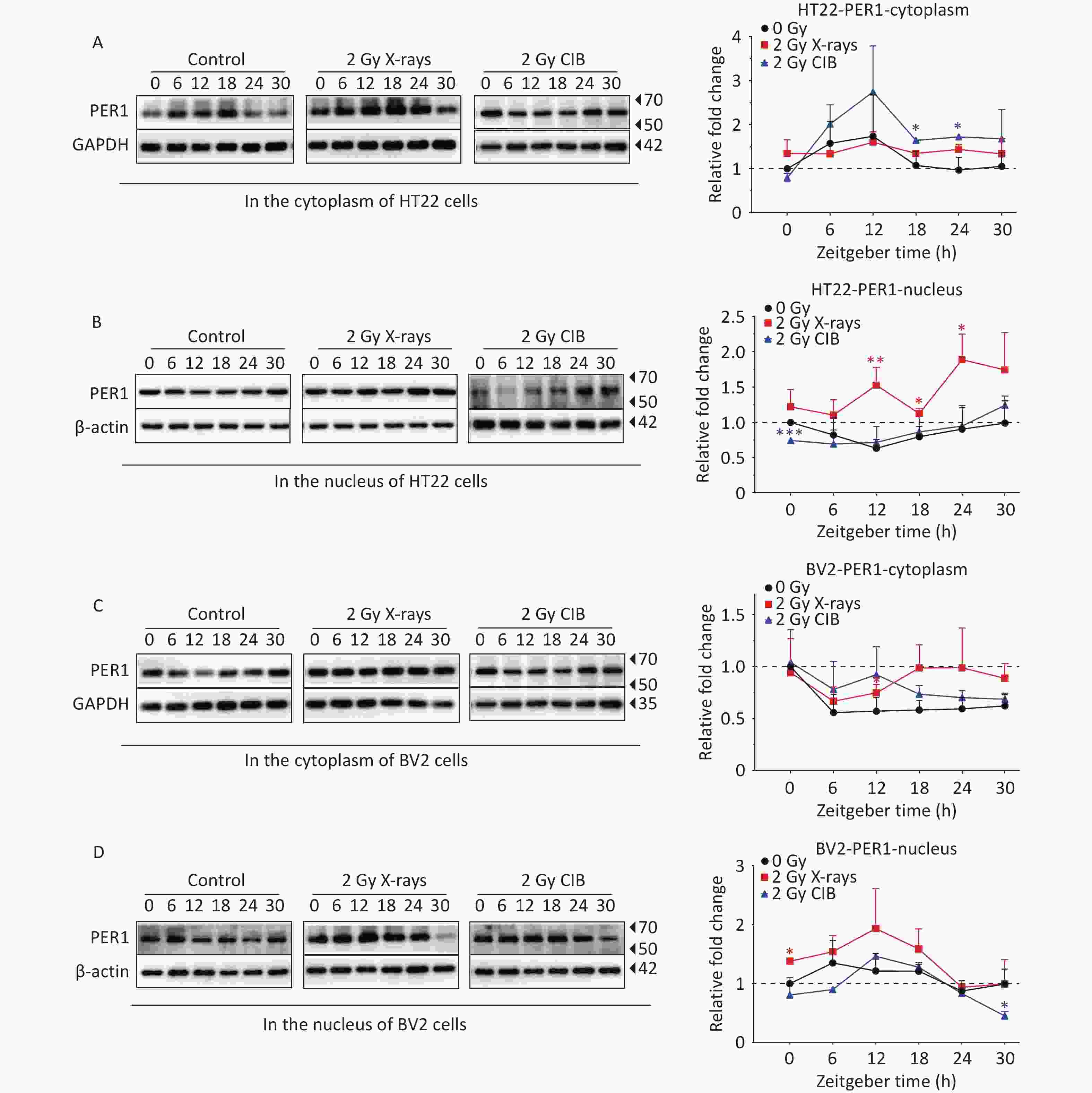
Figure 2. IR changes the protein levels of PER1 in cytoplasm and nucleus. (A–B) Protein levels of PER1 in the cytoplasm (A) and nucleus (B) of synchronized HT22 cells treated with IR from 2 Gy X-rays and 2 Gy CIB were measured by western blotting. (C–D) Protein levels of PER1 in the cytoplasm (C) and nucleus (D) of synchronized BV2 cells treated with 2 Gy X-rays and 2 Gy CIB were measured by western blotting. The data are shown as mean ± S.D., n=3. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by two tailed Student’s t-test represents statistical significance. IR, ionizing radiation; CIB, carbon ion beams.
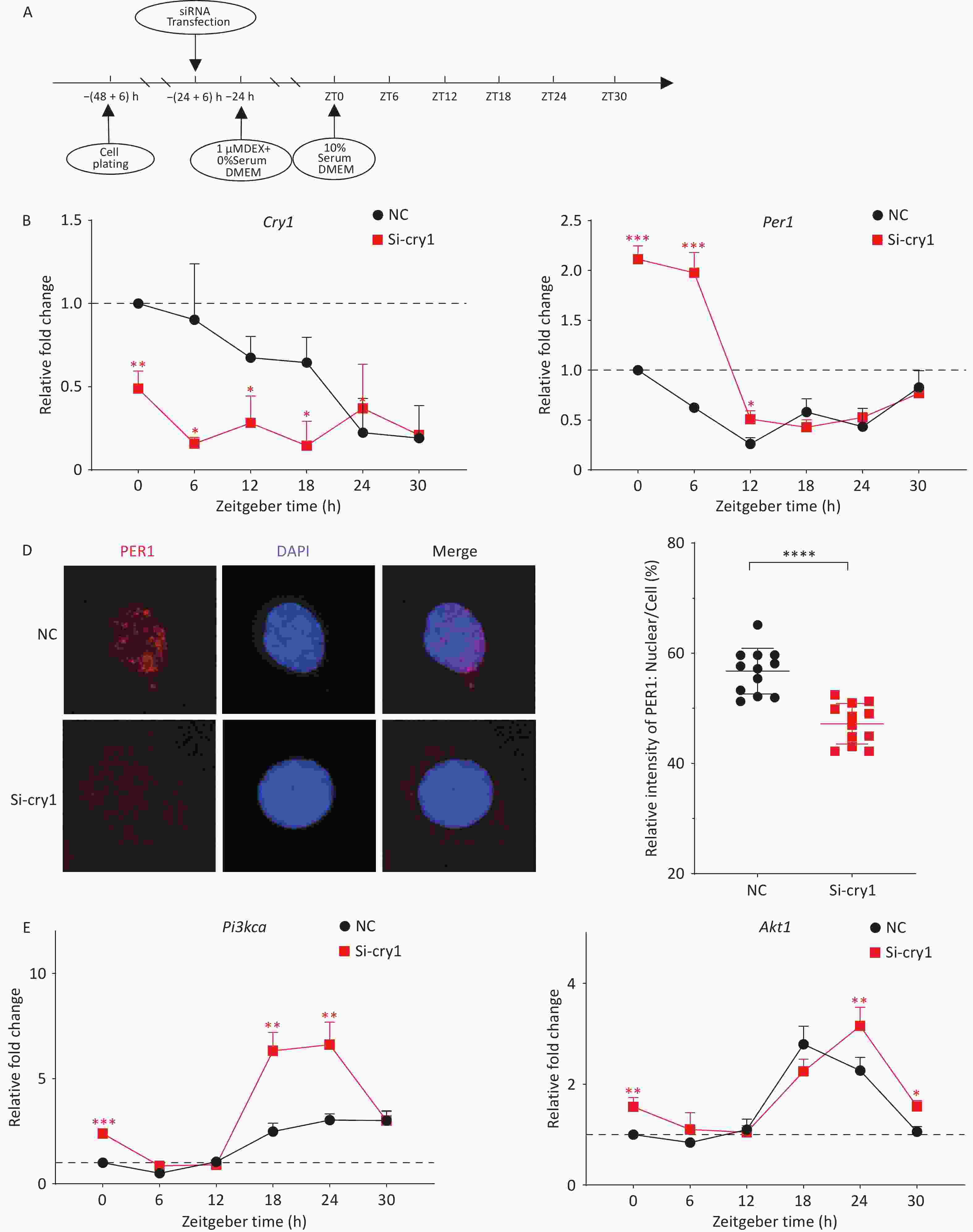
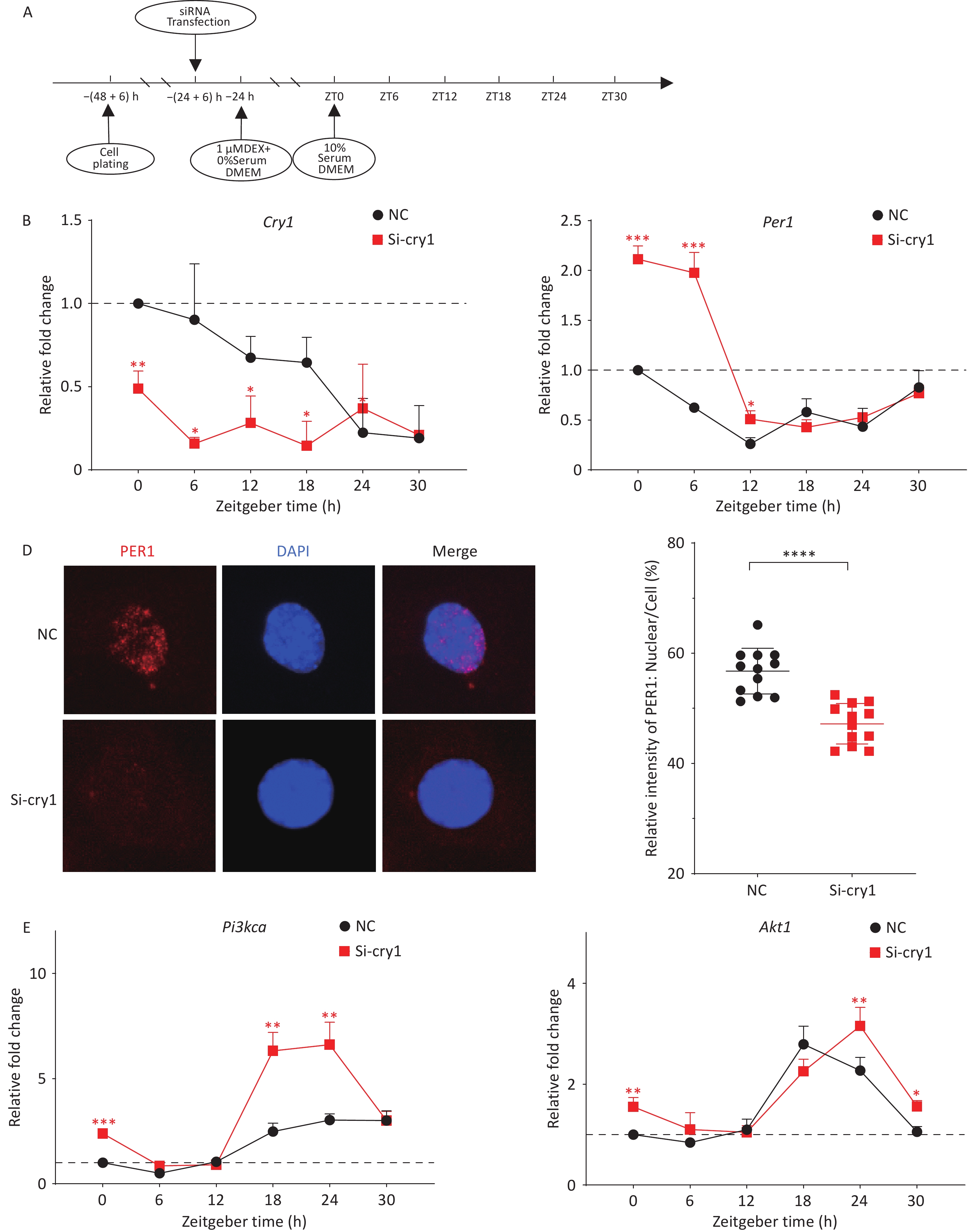
Figure 3. Nuclear imports of PER1 regulates the downstream PI3K/AKT pathway. (A) Scheme for siRNA transfection of HT22 cells. (B–C) mRNA levels of the circadian genes Cry1 (B) and Per1 (C) in synchronized HT22 cells transfected with siRNAs were measured using quantitative PCR. (D) PER1 in the nucleus of HT22 cells treated with si-Cry1 (PER1, red; DAPI, blue). Immunofluorescence was performed using the indicated antibodies. (E) mRNA levels of Pi3kca and Akt1 in synchronized HT22 cells transfected with siRNAs were measured using quantitative PCR. The data are shown as the means ± S.D., n = 3. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by two-tailed Student’s t test indicate statistical significance.
The circadian gene Per1 functions as a negative regulatory component of core transcription-translation feedback loops. Following nuclear translocation, PER1-CRY1 protein complexes suppress CLOCK-BMAL1 transcriptional activity, thereby inhibiting the autoregulatory expression of downstream genes. To specifically inhibit the nuclear import of PER1 without reducing its expression, Cry1 was knocked down using siRNAs (Figure 3B). As expected, PER1 nuclear accumulation was significantly attenuated at circadian time ZT12 (Figure 3D). Notably, quantitative analysis revealed Per1 mRNA accumulation during the early circadian phase (ZT0-12) (Figure 3C). To explore whether the nuclear import of the PER-CRY heterodimer could regulate the expression of the downstream genes Pi3k/Akt, the mRNA levels of Pi3kca and Akt1 were detected. Compared to the corresponding controls, the mRNA levels of Pi3kca and Akt1 in si-Cry1-HT22 cells were significantly greater at ZT0 (Figure 3E). Furthermore, the expression levels of the downstream genes Pi3kca and Akt1 increased significantly from ZT18 to ZT24 because of a phase shift as Cry1 mRNA levels decreased. Taken together, these results showed that inhibition of the nuclear import of the PER1-CRY1 heterodimer, even with increased PER1, promoted continued expression and activation of the PI3K/AKT downstream pathway, indicating the critical role of PER1 trafficking in the regulatory mechanism. Notably, this enhancement was more pronounced in Pi3kca than in Akt1. Previous studies have shown that reduced PER1 levels lead to significantly increased PI3K and p-AKT levels, whereas AKT levels are not significantly altered[9]. The PI3K/AKT pathway induces cell proliferation, migration, and invasion in multiple tumor types[10]. The effect of PER1 on the PI3K/AKT pathway was recently demonstrated. PER1 overexpression significantly inhibits the PI3K/AKT pathway and tumor progression, while the opposite effect was observed in PER1-knockdown OSCC cells. However, the mechanism by which PER1 regulates the PI3K/AKT pathway has not yet been elucidated. This study specifically examines PER1 nuclear translocation as a potential regulatory mechanism. Contrary to the established findings, CRY1 knockdown induced PER1 upregulation rather than inhibition of PI3K/AKT signaling. Immunofluorescence analysis revealed impaired nuclear accumulation of PER1 under these experimental conditions. These results suggest that the regulatory function of PER1 depends not only on its expression level, but also on its subcellular localization.
Taken together, these results provide evidence that IR upregulates the expression level and enhances the nuclear import of PER1 in mouse brain neural cells. Furthermore, our findings establish nuclear import of PER1 as a critical regulatory mechanism and provide novel insights into oncological research through circadian protein dynamics and subcellular trafficking.
Ionizing Radiation Alters Circadian Gene Per1 Expression Profiles and Intracellular Distribution in HT22 and BV2 Cells
doi: 10.3967/bes2025.139
The authors declare no conflicts of interest.
This study did not involve animal experiments or human experiments.
| Citation: | Zhiang Shao, Yuan Wang, Pei Qu, Zhouhang Zheng, Yixuan Li, Wei Wang, Qingfeng Wu, Dan Xu, Jufang Wang, Nan Ding. Ionizing Radiation Alters Circadian Gene Per1 Expression Profiles and Intracellular Distribution in HT22 and BV2 Cells[J]. Biomedical and Environmental Sciences. doi: 10.3967/bes2025.139 |








 Quick Links
Quick Links
 DownLoad:
DownLoad: