-
Aging is a progressive, physiological impairment involving various organs and tissues. Increase in oxidative stress drives the pathophysiology of various diseases, including aging. An excessive increase in reactive oxygen species (ROS) levels damages cellular lipids, proteins, and DNA, inhibiting their normal functions and resulting in cell aging and death[1-3].Moreover, oxidative stress triggers the pathogenesis of age-associated or neurodegenerative diseases[4-5].
D-galactose is a reducing sugar that accelerates aging by decreasing the activities of antioxidant enzymes and impairing the functions of macromolecules and cells, especially neurons[6]. Accelerated aging using D-galactose is a long-standing preclinical model[7]. It induces oxidative stress and tissue injury, leading to cellular apoptosis, cognitive impairment, mitochondrial dysfunction, and a decline in the function of antioxidant defense systems[8-10]. Mitochondrial dysfunction extensively leads to aging and age-associated degeneration[11].
Dietary antioxidants and mitochondrial nutrients delay aging and ameliorate age-associated diseases[12-13].Certain bioactive peptides combat oxidative stress and memory deficits[14-15]. Accordingly, hydrolysis of proteins is a feasible approach to produce biologically active peptides[16]. Antioxidant peptides with potent free radical-scavenging activities prevent oxidative tissue damage and delay aging.
Silybum marianum L. Gaernt is an annual or biennial Mediterranean plant that has been used to treat liver diseases for over 2000 years. Flavonolignans, collectively known as silymarin, are abundantly found in this plant. It is mainly present in the shell and kernel of the seed, and contains protein and oil[17-19]. Protein by-products of silymarin biosynthesis in seed kernels are rich in essential amino acids that are highly valuable[20-21].Furthermore, S. marianum protein hydrolysate (SMPH) has in vitro antioxidant effects[22]. Oligopeptides in S. marianum are reported to inhibit liver mitochondrial injury induced by hydroxyl ions in mice[23].Natural antioxidants are preferred because they are considered safer than synthetic antioxidants. Therefore, development of protein hydrolysates as natural antioxidants or neuroprotective agents has gained importance[14-16]. We previously demonstrated antioxidant activities of SMPH in vitro. In this study, to further establish its antioxidant and anti-aging potential in vivo, we used a model of accelerated aging by chronically injecting ICR mice with D-galactose.
-
D-galactose was purchased from Amresco Inc. (USA). Rhodamine 123 was purchased from Beyotime Institute of Biotechnology (Haimen, China). Total antioxidant capacity (T-AOC), and levels of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), monoamine oxidase (MAO), malondialdehyde (MDA), Na+-K+-ATPase, and Ca2+-Mg2+-ATPase were determined by commercial kits purchased from Nanjing Jiancheng Institute of Biological Engineering (Nanjing, China). ELISA kits for quantifying 8-OHdG, caspase-3, and Bcl-2 were purchased from Shanghai Jianglai Bioscience Co. Ltd. (Shanghai, China). 1, 6-diphenylhexa-1, 3, 5-triene (DPH) was purchased from Sigma (USA).
-
Seeds of S. marianum were obtained from Jiangsu Zhongxing Pharm. Co. Ltd., and identified by Prof. Jun Chen (School of Pharmacy, Jiangsu University). Proteins from the seeds were isolated in our laboratory by alkali extraction and acid precipitation[24]. Briefly, S. marianum seeds were powdered, defatted with hexane, and dispersed in 16 parts of deionized alkaline water (pH 11) at 50 ℃ for 1 h. The slurry was centrifuged (1, 500 ×g, 30 min), and the supernatant was adjusted to pH 5.5 and centrifuged again. Proteins in the supernatant were precipitated, re-dispersed in deionized water (pH 7), and freeze-dried. This freeze-dried protein fraction (protein content: 84.53% ± 0.52%) was hydrolyzed by Neutrase® at a substrate:enzyme ratio of 60:1 (w/w) under optimal conditions (pH 7, 55 ℃ for 2 h). The hydrolysis was thermally stopped using a boiling water bath for 10 min. After cooling to room temperature, the SMPH obtained was centrifuged (6, 500 ×g, 4℃, 15 min) and freeze-dried. The composition of SMPH by molecular weight was as follows: < 1 kD, 64.74%; 1-3 kD, 9.52%; 3-10 kD, 13.46%; ≥ 10 kD, 12.28%.
-
ICR mice (female, 3-month-old) were housed in an animal house at the Laboratory Animal Research Center of Jiangsu University, Zhenjiang, China. The license number of the mice was SCXK (SU) 2013-0011. After acclimation for a week, the mice were randomized into 5 groups (n = 10/group). Mice in group 1 were injected saline (vehicle control group), and the other groups of mice received daily intraperitoneal injection of D-galactose (positive control group) at a dose of 500 mg/kg for 7 weeks. Simultaneously, mice in groups 3, 4, and 5 were orally administered SMPH at doses of 400, 800, and 1, 200 mg/kg, respectively, daily for 7 weeks; mice in groups 1 and 2 were orally administered the same volume of distilled water. During the entire experimental period (including acclimation), the mice were allowed unrestricted access to standard rodent diet and water. All procedures were approved by the Laboratory Animal Management Committee of Jiangsu University and adhered to guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. After 7 weeks, the mice were humanely euthanized, and the brains and livers were promptly removed and stored at -80 ℃ until analysis. Blood samples were collected and centrifuged (1, 000 ×g, 15 min, 4 ℃) to separate the sera. Brain and liver homogenates (10%) were centrifuged (1, 500 ×g, 15 min, 4 ℃) and the supernatants were used for biochemical analysis.
-
Liver mitochondrial fraction was prepared according to the method described by Tang et al.[25], with some modifications. Briefly, the liver was homogenized with ice-cold isolation buffer (0.25 mol/L sucrose, 5 mmol/L Tris-HCl, and 1 mmol/L EDTA, pH 7.4) and centrifuged (1, 000 ×g, 10 min). The supernatant was subjected to high-speed centrifugation (8, 000 ×g, 15 min, 4 ℃) to obtain the mitochondrial pellet. After washing and centrifuging (8, 000 ×g) twice, the pellet was re-suspended in assay-specific buffers.
-
The sera were assayed for glucose, cholesterol, triglycerides, high-density lipoprotein cholesterol (HDLC), and low-density lipoprotein cholesterol (LDLC) levels, using an Olympus AU2700 clinical chemistry analyzer (Olympus Inc., Japan).
-
Commercially available kits were used to determine the levels of protein, SOD, GSH-Px, T-AOC, MDA, MAO, Na+-K+-ATPase, and Ca2+-Mg2+-ATPase. 8-OHdG, caspase-3, and Bcl-2 levels were determined using ELISA kits. All procedures were carried out according to the manufacturers' instructions.
-
The ΔΨm was measured according to the method described by Zhu et al.[26]. Briefly, the mitochondrial suspension was incubated with a rhodamine 123 fluorescence probe for 20 min at 37 ℃. After sedimentation, the mitochondria were washed twice and resuspended in phosphate-buffered saline. Fluorescence intensity was recorded using a Cary Eclipse spectrophotofluorometer (America Varian Technology China Ltd.) at ex/em: 505/534 nm.
-
Measurement of mitochondrial membrane fluidity was carried out using a DPH probe in a Cary Eclipse spectrophotofluorometer, as described by Zhou et al.[27]. The mitochondria were incubated with DPH (10-6 mol/L) at 30 ℃ for 30 min in the dark. Fluorescence intensity was measured at ex/em: 361/431 nm at 25 ℃. Fluorescence polarization (P) was calculated as P = (Ivv-GIvh) ÷ (Ivv+ GIvh), where G is the grating correction factor, equal to Ihv/Ihh; I is the intensity of light; and v and h denote vertical and horizontal polarizer orientations, respectively. The first and the second terms refer to the excitation and emission light, respectively. Microviscosity (η) was calculated using the equation η = 2P ÷ (0.46 -P). The η value inversely correlates with the membrane fluidity.
-
The liver was isolated, washed with physiological saline, and cut into small blocks (1 mm3) that were immediately fixed in a solution containing 2.5% formaldehyde-glutaraldehyde and post-fixed in 1% osmium tetroxide solution. Further, they were serially dehydrated in ethanol. After replacing ethanol with propylene oxide, the blocks were embedded in an epoxy resin, and ultrathin sections were obtained. The sections were double-stained with uranyl acetate and lead citrate, and observed under a Tecnai12 TEM.
-
Statistical analysis was performed using SPSS, version 17.0. The values were analyzed by one-way analysis of variance (ANOVA), followed by Duncan's multiple comparisons test. All the results are expressed as mean ± SD, and differences were considered statistically significant at P < 0.05.
-
No significant (P > 0.05) differences in the levels of glucose, HDLC, and LDLC were observed among the five groups (Table 1). However, the levels of cholesterol and triglycerides were significantly (P < 0.05) higher in positive control mice than in vehicle control mice. SMPH significantly decreased the level of triglycerides at 400 mg/kg. However, the level of cholesterol in positive control mice was significantly decreased only at a dose of 800 mg/kg of SMPH. Since pharmacological effects of a complex mixture are dependent on the concentration of its individual components, we observed that a dose of 800 mg/kg was effective in reducing cholesterol levels.
Table 1. Levels of Glucose, Cholesterol, Triglycerides, High-density Lipoprotein Cholesterol (HDLC), and Low-density Lipoprotein Cholesterol (LDLC) in the Serum
Groups Glucose
(mmol/L)Cholesterol
(mmol/L)Triglycerides
(mmol/L)HDLC
(mmol/L)LDLC
(mmol/L)Vehicle control 4.32 ± 0.79a 2.06 ± 0.22a 2.28 ± 0.71a 1.95 ± 0.22a 0.24 ± 0.04a Positive control 4.87 ± 0.71a 2.56 ± 0.58b 2.99 ± 1.08b 2.11 ± 0.43a 0.21 ± 0.06a SMPH (400 mg/kg) 4.25 ± 0.87a 2.23 ± 0.22ab 2.29 ± 0.44a 1.96 ± 0.24a 0.27 ± 0.21a SMPH (800 mg/kg) 4.23 ± 0.36a 2.11 ± 0.26a 1.62 ± 0.31a 1.94 ± 0.19a 0.19 ± 0.05a SMPH (1, 200 mg/kg) 4.70 ± 0.78a 2.31 ± 0.30ab 2.12 ± 0.50a 2.15 ± 0.35a 0.22 ± 0.05a Note. Data are expressed as mean ± SD (n = 10); different letters indicate a significant difference (P < 0.05); Positive Control: D-galactose-treated mice; SMPH: D-galactose-treated mice receiving SMPH. -
To determine whether SMPH attenuates oxidative damage in the livers of D-galactose-treated mice, we measured the activities of GSH-Px and SOD along with T-AOC and MDA level in the liver (Table 2). SOD and GSH-Px activities as well as T-AOC were significantly lower in positive-control mice than in vehicle control mice. Moreover, D-galactose treatment reduced the level of MDA. However, supplementation with SMPH effectively countered the effect of D-galactose.
Table 2. Activities of Glutathione Peroxidase (GSH-Px) and Superoxide Dismutase (SOD), Total Antioxidant Capacity (T-AOC), and Malondialdehyde (MDA) Level in the Liver
Groups SOD
(U/mg protein)GSH-Px
(U/mg protein)T-AOC
(U/mg protein)MDA
(nmol/mg protein)Vehicle control 33.27 ± 4.09a 415.91 ± 27.60a 1.02 ± 0.15a 7.14 ± 0.53ac Positive control 24.37 ± 6.05b 273.66 ± 43.57b 0.62 ± 0.13b 11.52 ± 0.29b SMPH (400 mg/kg) 32.37 ± 5.18a 388.96 ± 22.89c 0.64 ± 0.06b 6.11 ± 0.47a SMPH (800 mg/kg) 36.11 ± 5.45a 412.37 ± 33.01a 0.89 ± 0.21c 8.11 ± 0.89c SMPH (1, 200 mg/kg) 32.17 ± 3.88a 359.88 ± 28.52c 0.81 ± 0.07c 7.30 ± 1.06c Note. Data are expressed as mean ± SD (n = 10); different letters indicate a significant difference (P < 0.05); Positive Control: D-galactose-treated mice; SMPH: D-galactose-treated mice receiving SMPH. -
The activities of GSH-Px and SOD as well as T-AOC were significantly lower, and MAO and MDA levels were significantly higher in the brains of positive control mice than in the brains of vehicle control mice (Table 3). However, SMPH treatment significantly increased the activities of GSH-Px and SOD, and decreased both MAO and MDA levels in the brain. No significant differences in the levels of T-AOC were observed between mice treated with SMPH and positive control mice. Further, the levels of GSH-Px, SOD, MAO, and MDA in the brains of vehicle control mice did not significantly differ from those in the brains of SMPH-treated mice.
Table 3. Activities of Glutathione Peroxidase (GSH-Px) and Superoxide Dismutase (SOD), Total Antioxidant Capacity (T-AOC), and Levels of Monoamine Oxidase (MAO) and Malondialdehyde (MDA) in the Brain
Groups SOD
(U/mg protein)GSH-Px
(U/mg protein)T-AOC
(U/mg protein)MAO
(U/mg protein)MDA
(nmol/mg protein)Vehicle control 65.53 ± 9.16a 16.17 ± 4.11a 0.40 ± 0.08a 1.20 ± 0.27a 3.52 ± 0.25a Positive control 41.06 ± 2.64b 7.33 ± 3.14b 0.23 ± 0.07b 1.94 ± 0.31b 5.06 ± 0.64b SMPH (400 mg/kg) 62.25 ± 9.83a 17.29 ± 3.14a 0.25 ± 0.06b 1.49 ± 0.34a 3.92 ± 0.20a SMPH (800 mg/kg) 62.69 ± 3.45a 19.61 ± 3.52a 0.24 ± 0.05b 1.16 ± 0.24a 3.43 ± 0.47a SMPH (1, 200 mg/kg) 58.54 ± 7.37a 18.56 ± 2.22a 0.24 ± 0.04b 1.28 ± 0.12a 3.98 ± 0.21a Note.Data are expressed as mean ± SD (n = 10); different letters indicate a significant difference (P < 0.05); Positive Control: D-galactose-treated mice; SMPH: D-galactose-treated mice receiving SMPH. -
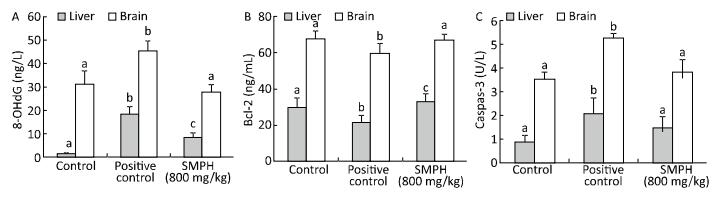
The level of Bcl-2 was significantly lower, while the levels of 8-OHdG and caspase-3 were significantly higher in positive control mice than in vehicle control mice (Figure 1). By contrast, SMPH-treated mice had significantly higher levels of Bcl-2 and lower levels of 8-OHdG and caspase-3 in the brains and livers than positive control mice. No significant differences in the levels of Bcl-2 and caspase-3 were observed between vehicle control and SMPH-treated mice.
-
The effect of SMPH on membrane fluidity was demonstrated by reduced η and P values in SMPH-treated mice relative to those in positive control mice (Table 4). η and P values in the liver mitochondrial membrane were significantly higher in positive control mice than in vehicle control mice. However, SMPH significantly decreased these values. The liver ΔΨm was significantly lower in positive control mice than in vehicle control mice. Treatment with SMPH significantly increased the ΔΨm.
Table 4. Liver Mitochondrial Membrane Potential (ΔΨm) and Fluidity
Groups ΔΨm Fluorescence polarization (P) Microviscosity (η) Vehicle control 44.46 ± 0.48a 0.16 ± 0.02a 1.05 ± 0.15a Positive control 24.94 ± 1.54b 0.32 ± 0.02b 4.49 ± 0.69b SMPH (400 mg/kg) 38.33 ± 1.61c 0.27 ± 0.03c 2.82 ± 0.62c SMPH (800 mg/kg) 39.61 ± 0.73c 0.22 ± 0.02d 1.89 ± 0.26d SMPH (1, 200 mg/kg) 39.50 ± 0.89c 0.21 ± 0.02d 1.76 ± 0.22d Note. Data are expressed as mean ± SD (n = 10); different letters indicate a significant difference (P < 0.05); Positive Control: D-galactose-treated mice; SMPH: D-galactose-treated mice receiving SMPH. -
The effect of SMPH on the activities of Na+-K+‑ATPase and Ca2+-Mg2+‑ATPase are presented in Table 5. The activities of Na+-K+‑ATPase and Ca2+-Mg2+-ATPase were significantly lower in positive control mice than in vehicle control mice. In contrast, their activities were significantly increased in SMPH-treated mice compared with those in positive control mice.
Table 5. Activities of Na+-K+-ATPase and Ca2+-Mg2+-ATPase in Liver Mitochondria
Groups Na+-K+-ATPase (U/mg protein) Ca2+-Mg2+-ATPase (U/mg protein) Vehicle control 6.92 ± 0.55a 5.35 ± 0.32a Positive control 3.06 ± 0.14b 2.62 ± 0.39b SMPH (400 mg/kg) 4.03 ± 0.26c 3.66 ± 0.87c SMPH (800 mg/kg) 4.59 ± 0.31cd 4.23 ± 0.38c SMPH (1, 200 mg/kg) 4.62 ± 0.38d 3.79 ± 0.29c Note.Data are expressed as mean ± SD (n = 10); different letters indicate a significant difference (P < 0.05); Positive Control: D-galactose-treated mice; SMPH: D-galactose-treated mice receiving SMPH. -
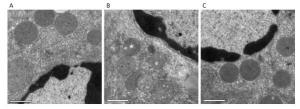
Ultrastructural changes in liver mitochondria were observed by TEM (Figure 2). The results showed that D-galactose treatment led to liver mitochondrial oxidative damage. Mitochondria in positive control mice exhibited swelling and vacuolization or disruption of mitochondrial cristae (Figure 2B). Treatment with SMPH ameliorated these morphological changes, and mitochondria in SMPH-treated mice were less swollen with intact membranes.
-
Aging is characterized by an accelerated decline in physiological performance and physical activity[28]. D-Galactose-induced aging is used as an experimental animal model to study aging and develop anti-aging strategies[29-30]. D-galactose increases the level of free radicals in animals. The free radical theory of aging suggests that an increased level of free radicals leads to age-related degenerative diseases[31-32].
These free radicals cause cell damage and death[33]. SOD, GSH-Px, and CAT are the most important antioxidant enzymes that inhibit free radical formation and are used as biomarkers of excessive ROS production. They act as the primary defense system against ROS during oxidative stress[34]. Under normal physiological conditions, SOD, CAT, and GSH-Px efficiently counteract oxidative damage induced by free radicals. However, they are overwhelmed under excessive oxidative stress conditions[35-36]. In the present study, the activities of SOD and GSH-Px were found to be significantly lower in the liver and brain of D-galactose-treated mice than in vehicle control mice; however, SMPH significantly increased their activities. Determination of T-AOC enables quantification of the non-enzymatic endogenous antioxidant potential. We observed that SMPH treatment increased the T-AOC in the liver, indicating that the function of the non-enzymatic antioxidant defense system in D-galactose-treated mice was enhanced. We previously demonstrated the in vitro antioxidant activities of SMPH. In the present study, we showed that SMPH exhibits free radical-scavenging antioxidant capacity in D-galactose-treated mice.
An increase in ROS levels induces lipid peroxidation, which is also associated with aging. Therefore, inhibition of lipid peroxidation prevents free-radical-mediated diseases[37]. Peroxidation of polyunsaturated fatty acids in biological membranes leads to the formation of MDA. Therefore, it is used as an indicator of oxidative stress damage. The levels of MDA were significantly higher in the liver and brain of positive control mice than in vehicle control mice (Tables 2 and 3). Moreover, treatment with D-galactose induced lipid peroxidation in mice by increasing the production of ROS. Similar findings have been reported in tissues of D-galactose-treated mice[38].Treatment with SMPH significantly reduced the levels of MDA in both the liver and brain of mice. These results indicated that SMPH exerts antioxidant effects by preventing ROS-induced lipid peroxidation in D-galactose-treated mice.
MAO catalyzes the degradation of neuroactive and vasoactive amines, and its activation is associated with age-related disturbances, imbalance in homeostasis, and generation of free radicals in nerve tissues[39]. In our study, the activity of brain MAO was significantly higher in positive control mice than in vehicle control mice. However, the administration of SMPH significantly prevented this increase and attenuated monoamine metabolism. As a biomarker of oxidative DNA damage, significantly higher levels of 8-OHdG were observed in the liver and brain of positive control mice than in vehicle control mice (Figure 1A). These results suggested that D-galactose induces oxidative DNA damage in mice. Treatment with SMPH significantly reduced the levels of 8-OHdG in both the liver and brain of mice, indicating that the administration of SMPH effectively inhibits oxidative DNA damage in D-galactose-treated mice.
Apoptosis is a biological process that plays a crucial role in the normal tissue development and homeostasis. In this study, D-galactose significantly increased apoptosis in the liver and brain, whereas SMPH treatment significantly reduced it (Figure 1B and 1C).
Bcl-2 is a proto-oncogene that regulates the mitochondrial apoptotic pathway[40]. In our study, we found that D-galactose decreased the levels of Bcl-2 in the liver and brain. However, SMPH treatment prevented the decline in Bcl-2 levels (Figure 1B), owing to its antioxidant property. Caspase-3 is another regulator of apoptosis and is activated by ROS, leading to neuronal dysfunction[41]. In the present study, we found that SMPH reduced caspase-3 activity in the liver and brain of D-galactose-treated mice (Figure 1C). These results suggested that SMPH regulates the expression of mitochondrial apoptosis-related proteins by mitigating oxidative stress.
Oxidative mitochondrial damage accelerates aging[42]. It is considered a hallmark of cellular aging and is the major source of ROS[43]. ROS-oxidized mitochondrial membrane lipids exhibit reduced fluidity and increased permeability[44-45]. In this study, we found that chronic treatment with D-galactose induces mitochondrial dysfunction in the liver of mice, whereas treatment with SMPH effectively ameliorated D-galactose-induced mitochondrial dysfunction (Tables 4-5, and Figure 2).
The mitochondrial membrane fluidity and potential reflect the biophysical and biochemical health of the mitochondrial membrane[46]. In this study, a significant reduction in the membrane potential and fluidity was observed in liver mitochondria when mice were treated with D-galactose. However, SMPH significantly prevented this reduction owing to its antioxidant potential, thereby preventing mitochondrial membrane damage. Mitochondrial oxidative phosphorylation is the primary source of energy for metabolism. About 40% of the total ATP is consumed by Na+-K+-ATPase and Ca2+-Mg2+-ATPase, which maintain the plasma membrane potential and intracellular Ca2+ stores[47]. In the present study, the activities of Na+-K+-ATPase and Ca2+-Mg2+-ATPase were lower in the liver mitochondria of positive control mice than in vehicle control mice. Treatment with SMPH significantly increased their activities by preventing free radical-induced membrane damage. Our results also revealed abnormal liver mitochondria in D-galactose-treated mice; the degree of liver mitochondrial damage was significantly decreased with administration of SMPH. We previously showed that oligopeptides of S. marianum inhibit liver mitochondrial injury induced by hydroxyl ions in mice. The present study showed that SMPH attenuated D-galactose-induced liver mitochondrial dysfunction. Since digestion of proteins produces free amino acids and oligopeptides that are absorbed into the blood, the protective effects of SMPH could be due to either the free amino acids or its hydrolysis product after systemic absorption to counteract D-galactose-mediated damage.
-
In conclusion, the results of this study indicated that SMPH protects against D-galactose-induced aging in mice. It increases the activities of antioxidant enzymes, attenuates lipid peroxidation, modulates the production of apoptosis-related factors, and prevents mitochondrial damage induced by D-galactose. Based on these findings, SMPH may be developed into a novel functional food material that prevents oxidative tissue damage and age-related diseases.
-
The authors declare no conflict of interest.
-
ZHU Shu Yun designed this study, collected test data, and wrote the manuscript; JIANG Ning and TU Jie participated in the writing and revising of the manuscript; YANG Jing performed the statistical analyses; ZHOU Yue assisted in the animal experiments and collected the test data. All authors have read and approved the final manuscript.
doi: 10.3967/bes2017.083
Antioxidant and Anti-aging Activities of Silybum Marianum Protein Hydrolysate in Mice Treated with D-galactose
-
Abstract:
Objective In the present study, we investigated the antioxidant and anti-aging effects of Silybum marianum protein hydrolysate (SMPH) in D-galactose-treated mice. Methods D-galactose (500 mg/kg body weight) was intraperitoneally injected daily for 7 weeks to accelerate aging, and SMPH (400, 800, 1, 200 mg/kg body weight, respectively) was simultaneously administered orally. The antioxidant and anti-aging effects of SMPH in the liver and brain were measured by biochemical assays. Transmission electron microscopy (TEM) was performed to study the ultrastructure of liver mitochondria. Results SMPH decreased triglyceride and cholesterol levels in the D-galactose-treated mice. It significantly elevated the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), and total antioxidant capacity (T-AOC), which were suppressed by D-galactose. Monoamine oxidase (MAO) and malondialdehyde (MDA) levels as well as the concentrations of caspase-3 and 8-OHdG in the liver and brain were significantly reduced by SMPH. Moreover, it increased Bcl-2 levels in the liver and brain. Furthermore, SMPH significantly attenuated D-galactose-induced liver mitochondrial dysfunction by improving the activities of Na+-K+-ATPase and Ca2+-Mg2+-ATPase as well as mitochondrial membrane potential (ΔΨm) and fluidity. TEM showed that the degree of liver mitochondrial damage was significantly decreased by SMPH. Conclusion The results indicated that SMPH protects against D-galactose-induced accelerated aging in mice through its antioxidant and anti-aging activities. -
Key words:
- Silybum marianum protein hydrolysate /
- Antioxidant /
- Anti-aging /
- D-galactose
-
Table 1. Levels of Glucose, Cholesterol, Triglycerides, High-density Lipoprotein Cholesterol (HDLC), and Low-density Lipoprotein Cholesterol (LDLC) in the Serum
Groups Glucose
(mmol/L)Cholesterol
(mmol/L)Triglycerides
(mmol/L)HDLC
(mmol/L)LDLC
(mmol/L)Vehicle control 4.32 ± 0.79a 2.06 ± 0.22a 2.28 ± 0.71a 1.95 ± 0.22a 0.24 ± 0.04a Positive control 4.87 ± 0.71a 2.56 ± 0.58b 2.99 ± 1.08b 2.11 ± 0.43a 0.21 ± 0.06a SMPH (400 mg/kg) 4.25 ± 0.87a 2.23 ± 0.22ab 2.29 ± 0.44a 1.96 ± 0.24a 0.27 ± 0.21a SMPH (800 mg/kg) 4.23 ± 0.36a 2.11 ± 0.26a 1.62 ± 0.31a 1.94 ± 0.19a 0.19 ± 0.05a SMPH (1, 200 mg/kg) 4.70 ± 0.78a 2.31 ± 0.30ab 2.12 ± 0.50a 2.15 ± 0.35a 0.22 ± 0.05a Note. Data are expressed as mean ± SD (n = 10); different letters indicate a significant difference (P < 0.05); Positive Control: D-galactose-treated mice; SMPH: D-galactose-treated mice receiving SMPH. Table 2. Activities of Glutathione Peroxidase (GSH-Px) and Superoxide Dismutase (SOD), Total Antioxidant Capacity (T-AOC), and Malondialdehyde (MDA) Level in the Liver
Groups SOD
(U/mg protein)GSH-Px
(U/mg protein)T-AOC
(U/mg protein)MDA
(nmol/mg protein)Vehicle control 33.27 ± 4.09a 415.91 ± 27.60a 1.02 ± 0.15a 7.14 ± 0.53ac Positive control 24.37 ± 6.05b 273.66 ± 43.57b 0.62 ± 0.13b 11.52 ± 0.29b SMPH (400 mg/kg) 32.37 ± 5.18a 388.96 ± 22.89c 0.64 ± 0.06b 6.11 ± 0.47a SMPH (800 mg/kg) 36.11 ± 5.45a 412.37 ± 33.01a 0.89 ± 0.21c 8.11 ± 0.89c SMPH (1, 200 mg/kg) 32.17 ± 3.88a 359.88 ± 28.52c 0.81 ± 0.07c 7.30 ± 1.06c Note. Data are expressed as mean ± SD (n = 10); different letters indicate a significant difference (P < 0.05); Positive Control: D-galactose-treated mice; SMPH: D-galactose-treated mice receiving SMPH. Table 3. Activities of Glutathione Peroxidase (GSH-Px) and Superoxide Dismutase (SOD), Total Antioxidant Capacity (T-AOC), and Levels of Monoamine Oxidase (MAO) and Malondialdehyde (MDA) in the Brain
Groups SOD
(U/mg protein)GSH-Px
(U/mg protein)T-AOC
(U/mg protein)MAO
(U/mg protein)MDA
(nmol/mg protein)Vehicle control 65.53 ± 9.16a 16.17 ± 4.11a 0.40 ± 0.08a 1.20 ± 0.27a 3.52 ± 0.25a Positive control 41.06 ± 2.64b 7.33 ± 3.14b 0.23 ± 0.07b 1.94 ± 0.31b 5.06 ± 0.64b SMPH (400 mg/kg) 62.25 ± 9.83a 17.29 ± 3.14a 0.25 ± 0.06b 1.49 ± 0.34a 3.92 ± 0.20a SMPH (800 mg/kg) 62.69 ± 3.45a 19.61 ± 3.52a 0.24 ± 0.05b 1.16 ± 0.24a 3.43 ± 0.47a SMPH (1, 200 mg/kg) 58.54 ± 7.37a 18.56 ± 2.22a 0.24 ± 0.04b 1.28 ± 0.12a 3.98 ± 0.21a Note.Data are expressed as mean ± SD (n = 10); different letters indicate a significant difference (P < 0.05); Positive Control: D-galactose-treated mice; SMPH: D-galactose-treated mice receiving SMPH. Table 4. Liver Mitochondrial Membrane Potential (ΔΨm) and Fluidity
Groups ΔΨm Fluorescence polarization (P) Microviscosity (η) Vehicle control 44.46 ± 0.48a 0.16 ± 0.02a 1.05 ± 0.15a Positive control 24.94 ± 1.54b 0.32 ± 0.02b 4.49 ± 0.69b SMPH (400 mg/kg) 38.33 ± 1.61c 0.27 ± 0.03c 2.82 ± 0.62c SMPH (800 mg/kg) 39.61 ± 0.73c 0.22 ± 0.02d 1.89 ± 0.26d SMPH (1, 200 mg/kg) 39.50 ± 0.89c 0.21 ± 0.02d 1.76 ± 0.22d Note. Data are expressed as mean ± SD (n = 10); different letters indicate a significant difference (P < 0.05); Positive Control: D-galactose-treated mice; SMPH: D-galactose-treated mice receiving SMPH. Table 5. Activities of Na+-K+-ATPase and Ca2+-Mg2+-ATPase in Liver Mitochondria
Groups Na+-K+-ATPase (U/mg protein) Ca2+-Mg2+-ATPase (U/mg protein) Vehicle control 6.92 ± 0.55a 5.35 ± 0.32a Positive control 3.06 ± 0.14b 2.62 ± 0.39b SMPH (400 mg/kg) 4.03 ± 0.26c 3.66 ± 0.87c SMPH (800 mg/kg) 4.59 ± 0.31cd 4.23 ± 0.38c SMPH (1, 200 mg/kg) 4.62 ± 0.38d 3.79 ± 0.29c Note.Data are expressed as mean ± SD (n = 10); different letters indicate a significant difference (P < 0.05); Positive Control: D-galactose-treated mice; SMPH: D-galactose-treated mice receiving SMPH. -
[1] . Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell, 2005; 120, 483-495. doi: 10.1016/j.cell.2005.02.001 [2] . Oyewole AO, Birch-Machin MA. Mitochondria-targeted antioxidants. Faseb Journal, 2015; 29, 4766-71. doi: 10.1096/fj.15-275404 [3] . Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell B, 2007; 39, 44-84. doi: 10.1016/j.biocel.2006.07.001 [4] . Golden TR, Melov S. Mitochondrial DNA mutations, oxidative stress, and aging. Mech Ageing Dev, 2001; 122, 1577-89. doi: 10.1016/S0047-6374(01)00288-3 [5] . Jomova K, Vondrakova D, Lawson M, et al. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell Biochem, 2010; 345, 91-104. doi: 10.1007/s11010-010-0563-x [6] . Cui X, Zuo PP, Zhang Q, et al. Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice:protective effects of R-alpha-lipoic acid. J Neurosci Res, 2006; 84, 647-54. doi: 10.1002/(ISSN)1097-4547 [7] . Cui X, Wang LN, Zuo PP, et al. D-galactose-caused life shortening in Drosophila melanogaster and Musca domestica is associated with oxidative stress. Biogerontology, 2004; 5, 317-25. doi: 10.1007/s10522-004-2570-3 [8] . Kumar A, Prakash A, Dogra S. Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced by D-galactose in mice. Food Chem Toxicol, 2010; 48, 626-32. doi: 10.1016/j.fct.2009.11.043 [9] . Anand KV, Jaabir MSM, Thomas PA, et al. Protective role of chrysin against oxidative stress in d-galactose-induced aging in an experimental rat model. Geriatr Gerontol Int, 2012; 12, 741-50. doi: 10.1111/ggi.2012.12.issue-4 [10] . Lu J, Zheng YL, Luo L, et al. Quercetin reverses D-galactose induced neurotoxicity in mouse brain. Behav Brain Res, 2006; 171, 251-60. doi: 10.1016/j.bbr.2006.03.043 [11] . Judge S, Leeuwenburgh C. Cardiac mitochondrial bioenergetics, oxidative stress, and aging. Am J Physiol Cell Physiol, 2007; 292, C1983-92. doi: 10.1152/ajpcell.00285.2006 [12] . Navarro A, Boveris A. Mitochondrial nitric oxide synthase, mitochondrial brain dysfunction in aging, and mitochondriatargeted antioxidants. Adv Drug Deliver Rev, 2008; 60, 1534-44. doi: 10.1016/j.addr.2008.05.002 [13] . Szeto HH. Mitochondria-targeted peptide antioxidants:novel neuroprotective agents. AAPS J, 2006; 8, E521-31. doi: 10.1208/aapsj080362 [14] . Leo EEM, Fernández JJA, Campos MRS. Biopeptides with antioxidant and anti-inflammatory potential in the prevention and treatment of diabesity disease, Biomed Pharmacother, 2016; 83, 816-26. doi: 10.1016/j.biopha.2016.07.051 [15] . Torres-Fuentes C, Contreras MM, Recio I, et al. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem, 2015; 180, 194-202. doi: 10.1016/j.foodchem.2015.02.046 [16] . Su GW, Zhao TT, Zhao YQ, et al. Effect of anchovy (Coilia mystus) protein hydrolysate and its Maillard reaction product on combating memory-impairment in mice. Food Res Int, 2016; 82, 112-20. doi: 10.1016/j.foodres.2016.01.022 [17] . Abenavoli L, Capasso R, Milic N, et al. Milk thistle in liver diseases:Past, present, future. Phytother Res, 2010; 24, 1423-32. doi: 10.1002/ptr.v24:10 [18] . Loguercio C, Festi D. Silybin and the liver:From basic research to clinical practice. World J. Gastroentero, 2011; 17, 2288-301. https://www.wjgnet.com/1007-9327/full/v17/i18/2288-T5.htm [19] . Xu DF, Zhang WM, Shi JS, et al. Advance in the study and utilization of domestic resoure of Silybum marianum. Food Res Dev, 2007; 28, 157-61. (In Chinese) doi: 10.1007/s10668-015-9698-y [20] . Zhu SY, Dong Y, Chen XD, et al. Protein and amino acid composition of milk thistle meal and functional properties. Journal of the Chinese Cereals and Oils Association, 2011; 26, 71-4. (In Chinese) http://d.wanfangdata.com.cn/Periodical_zglyxb201108016.aspx [21] . Zhu SY, Dong Y, Tu J, et al. Amino acid composition and in vitro digestibility of protein isolates from Silybum marianum. J Food Agric Environ, 2013; 11, 136-40. http://world-food.net/amino-acid-composition-and-in-vitro-digestibility-of-protein-isolates-from-silybum-marianum/ [22] . Zhu SY, Dong Y, Zhang HH, et al. Enzymatic Hydrolysis of Milk Thistle Cake Protein and Antioxidation of Hydrolysate. Journal of the Chinese Cereals and Oils Association, 2011; 26, 68-72. (In Chinese) http://www.currentscience.ac.in/Volumes/113/03/0500.pdf [23] . Zhu SY, Sha S, Qin YY, et al. Protective effect of Silybum marianum oligopeptides on mice liver mitochondria injury. Journal of the Chinese Cereals and Oils Association, 2015; 30, 97-101. (In Chinese) http://en.cnki.com.cn/Article_en/CJFDTotal-ZLYX201501020.htm [24] . Zhu SY, Dong Y. Optimized technology for extracting milk thistle cake protein by response surface methodology. Science and Technology of Food Industry, 2011; 32, 256-8. (In Chinese) doi: 10.1007/s00253-013-5002-y [25] . Tang YH, Gao C, Xing MY, et al. Quercetin prevents ethanol-induced dyslipidemia and mitochondrial oxidative damage. Food Chem Toxicol, 2012; 50, 1194-200. doi: 10.1016/j.fct.2012.02.008 [26] . Zhu SY, Dong Y, Tu J, et al. Silybum marianum oil attenuates oxidative stress and ameliorates mitochondrial dysfunction in mice treated with D-galactose. Pharmacogn Mag, 2014; 10, S92-9. doi: 10.4103/0973-1296.127353 [27] . Zhou XM, Cao YL, Dou DQ. Protective effect of ginsenoside-Re against cerebral ischemia/reperfusion damage in rats. Biol Pharm Bull, 2006; 29, 2502-5. doi: 10.1248/bpb.29.2502 [28] . Paradies G, Petrosillo G, Paradies V, et al. Mitochondrial dysfunction in brain aging:role of oxidative stress and cardiolipin. Neurochem Int, 2011; 58, 447-57. doi: 10.1016/j.neuint.2010.12.016 [29] . Ho SC, Liu JH, Wu RYY. Establishment of the mimetic aging effect in mice caused by D-galactose. Biogerontology, 2003; 4, 15-8. doi: 10.1023/A:1022417102206 [30] . Shen YX, Xu SY, Wei W, et al. Melatonin reduces memory changes and neural oxidative damage in mice treated with D-galactose. J Pineal Res, 2002; 32, 173-8. doi: 10.1034/j.1600-079x.2002.1o850.x [31] . Zhang XL, An LJ, Bao YM, et al. D-galactose administration induces memory loss and energy metabolism disturbance in mice:protective effects of catalpol. Food Chem Toxicol, 2008; 46, 2888-94. doi: 10.1016/j.fct.2008.05.032 [32] . Kumar A, Dogra S, Prakash A. Effect of carvedilol on behavioral, mitochondrial dysfunction, and oxidative damage against D-galactose induced senescence in mice. N-S Arch Pharmacol, 2009; 380, 431-41. doi: 10.1007/s00210-009-0442-8 [33] . Shayganni E, Bahmani M, Asgary S, et al. Inflammaging and cardiovascular disease:management by medicinal plants. Phytomedicine, 2016; 23, 1119-26. doi: 10.1016/j.phymed.2015.11.004 [34] . Ren Y, Yang XS, Niu XW, et al. Chemical characterization of the avenanthramide-rich extract from oat and its effect on D-galactose-induced oxidative stress in mice. J Agr Food Chem, 2011; 59, 206-11. doi: 10.1021/jf103938e [35] . Nasri H, Rafieian-Kopaei M. Medicinal plants and antioxidants:Why they are not always beneficial? Iran J Public Public Health, 2014; 43, 255-7. https://www.ncbi.nlm.nih.gov/pubmed/26060753 [36] . Rafieian-Kopaei M, Baradaran A, Rafieian M. Oxidative stress and the paradoxical effects of antioxidants. J Res Med Sci, 2013; 18, 628. https://www.ncbi.nlm.nih.gov/pubmed/24516501 [37] . Abuja PM, Albertini R. Methods for monitoring oxidative stress, lipid peroxidation and oxidation resistance of lipoproteins. Clin Chim Acta, 2001; 306, 1-17. doi: 10.1016/S0009-8981(01)00393-X [38] . Khan MZ, Atlas N, Nawaz W. Neuroprotective effects of Caralluma tuberculata on ameliorating cognitive impairment in a D-galactose-induced mouse model. Biomed Pharmacother, 2016; 84, 387-94. doi: 10.1016/j.biopha.2016.09.055 [39] . Tian Y, Zou B, Yang L, et al. High molecular weight persimmon tannin ameliorates cognition deficits and attenuates oxidative damage in senescent mice induced by D-galactose. Food Chem Toxicol, 2011; 49, 1728-36. doi: 10.1016/j.fct.2011.04.018 [40] . Garcia-Saez AJ. The secrets of the Bcl-2 family. Cell Death and Differ, 2012; 19, 1733-40. doi: 10.1038/cdd.2012.105 [41] . Schon EA, Manfredi G. Neuronal degeneration and mitochondrial dysfunction. J Clin Invest, 2003; 111, 303-12. doi: 10.1172/JCI200317741 [42] . Bhat AH, Dar KB, Anees S, et al. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight, Biomed Pharmacother, 2015; 74, 101-10. doi: 10.1016/j.biopha.2015.07.025 [43] . Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol, 2000; 10, 369-77. doi: 10.1016/S0962-8924(00)01803-1 [44] . Harman D. The free radical theory of aging. Antioxid Redox Sign, 2003; 5, 557-61. doi: 10.1089/152308603770310202 [45] . Xu MY, Wang P, Sun YJ, et al. Joint toxicity of chlorpyrifos and cadmium on the oxidative stress and mitochondrial damage in neuronal cells. Food Chem Toxicol, 2017; 103, 246-52. doi: 10.1016/j.fct.2017.03.013 [46] . Li JX, Tong CWC, Xu DQ, et al. Changes in membrane fluidity and lipid peroxidation of skeletal muscle mitochondria after exhausting exercise in rats. Eur J Appl Physiol, 1999; 80, 113-7. doi: 10.1007/s004210050566 [47] . Acharya MM, Katyare SS. Structural and functional alterations in mitochondrial membrane in picrotoxin-induced epileptic rat brain. Exp Neurol, 2005; 192, 79-88. doi: 10.1016/j.expneurol.2004.11.004 -





 下载:
下载:



 Quick Links
Quick Links