-
Iodine is essential for fetal brain development. Iodine deficiency (ID) in pregnancy may be associated with neurodevelopmental deficits in offspring. There is an increasing need for iodine during pregnancy[1]. Maternal minor iodine-deficient supply can lead to marginal ID. Marginal ID is regarded as the state in which the median urinary iodine concentration is 100-150 μg/L, which comprises a large percentage of women of reproductive age with low levels of circulating TT4 but relatively normal circulating levels of free triiodothyronine (FT3), free thyroxine, and thyroid stimulating hormone (TSH)[2]. At present, marginal ID, as a milder form of ID, is an important public health issue worldwide.
Thyroid hormones (TH) participate in normal development of the central nervous system. TH deficiency can lead to abnormal changes in brain development, including cerebellar disorders. Some studies, including ours, revealed postnatal neuronal abnormalities in the TH-deficient rat cerebellum. Bergmann glia cells (BGs) and Purkinje cells (PCs) are TH target cells during development of the cerebellum[3]. Maintaining the unique morphology of BGs and its scaffolds contributes to the survival of PCs. Damaging BGs affects growth and function of PCs due to the interactions between glial cells and neurons. It is necessary to clarify glial-neuronal functions to understand the mechanism of TH influence of cerebellar development and function.
Glutamate transporters and glutamate receptors on BGs are important targets for cell-cell glutamate release during synaptic transmission[4]. Glutamate transporters play an important role removing glutamate. Two classes of glial cell glutamate transporters, such as glutamate and aspartate transporter (GLAST) and glutamate transporter 1 (GLT1), are mainly expressed on BGs in the cerebellum, especially near glutamatergic synapses of PCs[5]. Disrupting glutamate dynamics by inhibiting glial transporter function affects modulation of excitatory and inhibitory transmission to PCs. PCs and BGs express the glutamate receptor subunits glutamate receptor 1 (GluR1) and glutamate receptor 4 (GluR4). This subunit combination results in assembly of an AMPA receptor (AMPAR). Ca2+ signaling in BGs through activation of AMPAR is a mechanism for glial membranes to detect and associate with synaptic sites. Therefore, the glutamate transporter and glutamate receptors play an important role in neuron function. However, there is little evidence to suggest that interactions between glial cells and neurons are affected in offspring following exposure to maternal marginal ID. Thus, the aim of this study was to investigate the effects of maternal marginal ID on the interactions between BGs and PCs, and the underlying mechanisms involved.
Female Wistar rats (130-150 g) were randomly assigned to three groups of control group, marginal ID group, and severe ID group. Animal use was approved by the Animal Use and Care Committee at China Medical University. Each group was administered the ID diet (iodine content 60 ± 1.5 ng/g, measured by As3+-Ce4+ catalytic spectrophotometry) and deionized water supplemented with potassium iodide. The final concentrations of iodine in the deionized water were 183, 117, and 0 μg/L for the control, marginal ID, and severe ID groups, respectively. Female rats were fed the specific diet for 3 months and were then mated with normal male rats (male:female = 2:1). Each group consisted of 12-14 pregnant rats. Each litter was culled to 8-9 pups on postnatal day (PN) 4 (same number of males and females in each group, where possible). Heart blood samples were obtained from eight pups in each group on PN7, PN14, and PN21 for the TH analysis. Marginal ID led to subtle alterations in circulating TH levels, mainly T4, but did not affect FT3 or TSH levels[6].
The preserved cerebella collected on PN7, PN14, and PN21 were embedded in paraffin and sectioned into 6-μm-thick sagittal sections. After deparaffinization and a preincubation, the slices were incubated with rabbit anti-calbindin-D-28K antibody (Sigma-Aldrich, St, Louis, MO, USA) and mouse anti-GFAP antibody (Millipore Corp., Bedford, MA, USA), and then with second antibody conjugated to the fluorescent markers FITC and Rhodamine (Zhongshan Biotechnology, Beijing, China). The slices were captured under a fluorescence microscope (BX61 + DP-71, Olympus/IPP, Tokyo, Japan). Then, the images from lobules 4-5 were obtained under the same conditions at a magnification of 400×. The contact points between GFAP-positive fibers and calbindin-positive cells at the interface between external granular layer (EGL) and molecular layer (ML) were counted per 100 μm.
The pups' cerebella were removed for Western blot on PN7, PN14, and PN21. The protein in each sample was estimated by the Pierce BCA Protein Assay Kit (Thermo Scientific, Waltham, MA, USA). The membranes were incubated with primary antibody rabbit anti-GLAST (Cell Signaling Technology, Danvers, MA, USA), rabbit anti-GLT-1 (Cell Signaling Technology), rabbit anti-GluR1 (Millipore Corp.), and rabbit anti-GluR4 (Santa Cruz Biotechnology) overnight at 4 ℃. Then, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Zhongshan Biotechnology). The blots were developed with the high-performance luminol substrate solution (PexBio, Beijing, China). Protein bands were subsequently quantified with an image analysis program (Gel Image System Ver. 4.00).
All statistical analyses were carried out using SPSS software (SPSS Inc., Chicago, IL, USA). All experiments were performed in triplicate, and data are presented as mean ± standard error. A P-value < 0.05 was considered significant. A one-way analysis of variance followed by Student-Newman-Keuls test was used to compare the treatment and control groups.
Marginal ID can lead to mild long-term potentiation impairments, a slight reduction in spine number, and dendritic morphology of the pyramidal neurons in the hippocampal CA1 region in rat offspring[6]. Our previous study also showed that maternal marginal ID can lead to slightly damaged development of BGs (submitted). Furthermore, we explored the structural relationship between BGs and developing PCs, as much less attention has been paid to this question. Therefore, in the present study, we focused on the postnatal neuronal-glial interactions in the cerebellum, an excellent experimental model of motor coordination.
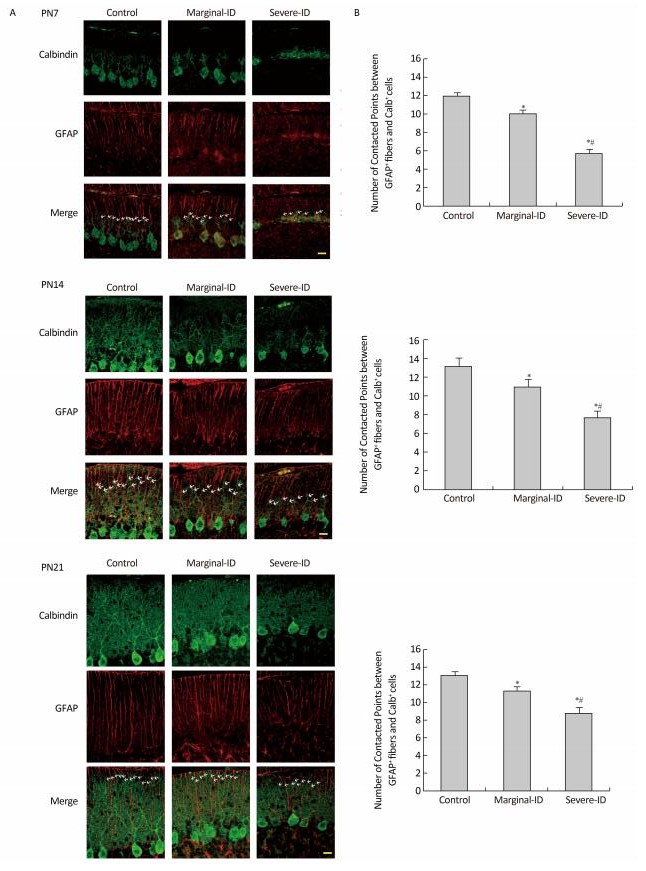
Vertical dendritic tips protruding into the EGL were parallel to and closely associated with BG fibers around the border between the ML and EGL (Figure 1A). The number of contact points between BG fibers and PC dendrites decreased significantly on PN7, PN14, and PN21 in the severe ID group (Figure 1B; P < 0.05). A slight reduction in the marginal ID group (Figure 1B; P < 0.05) was observed. BG fibers connected closely with PC dendrites and provided a structural basis to for PC dendritic growth, and played an important role in synaptic function, plasticity, and dendritic spine development of PCs. Studies have shown that maintaining the special structure and scaffolds of BGs contributes to the survival of PCs[4]. Thus, disrupting the BG scaffolding can result in PC degeneration. Delta/Notch-like EGF-related receptor (DNER) is expressed on Purkinje dendrites, and is a ligand for the Notch receptor on BGs. Activation of Notch by binding DNER on PCs appears to drive BG maturation[7]. Therefore, BGs and PCs affect each other. Thus, we speculate that marginal ID affects the interactions between BGs and PCs, and further influences their communication in the cerebellum. Some studies have demonstrated no gender difference in the damage caused by ID[8], including our previous studies. Thus, the number of males and females was equal in our experiments to ensure a balance.
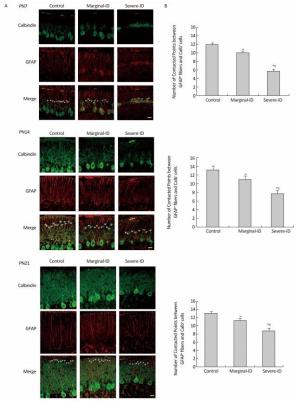
Figure 1. Marginal ID and severe ID reduced the number of contacted points between cerebellar PCs and BGs on PN7, PN14, and PN21. Representative photomicrographs show fluorescent staining of anti-Calbindin-D-28K (green) and anti-GFAP (red) on PN7, PN14, and PN21 (A). The merged images show overlapping localization. Scale bar = 20 μm. The bar graphs (B) show the quantification of the numbers of contacted points between GFAP positive fibers and calbindin positive cells around the border between the ML and EGL following marginal ID and severe ID group in the cerebellum on PN7, PN14, and PN21. Each bar represents the mean ± SEM for the groups. *Indicates a significant difference from the control group, P < 0.05; #Indicates a significant difference from the marginal ID group, P < 0.05.
Microdomains on BGs enclose Purkinje neuron synapses, and represent the major site for communication between cells. Stimulating both the climbing fiber and parallel fiber inputs to Purkinje neurons results in the generation of complex inward currents in BGs[4]. About half of the total current can be blocked by AMPA receptor antagonists, and the remainder can be blocked by inhibitors of glutamate transporters, which is similar to parallel fibers. Therefore, glutamate transporters and AMPARs are the principal targets for glutamate released during synaptic transmission on BGs.
GLAST and GLT-1 are two glial cell glutamate transporters. The GLAST protein level in the marginal ID group was significantly downregulated compared with that in the control group on PN21 (Figure 2; P < 0.05). Levels tended to decrease on P7 and P14. GLT-1 was downregulated in rats exposed to marginal ID and severe ID compared with the control group on PN7 and PN14 (Figure 2; P < 0.05). Overall, our results show that marginal ID disturbed GLAST and GLT-1 expression on PN7, PN14, and PN21. GLAST and GLT-1 play an important role removing excessive glutamate from the synaptic cleft, and they balance the glutamate steady-state and restrain excited toxicity to prevent nerve damage. GLAST and GLT-1 are closely linked to a variety of neurodegenerative diseases[9]. Because disturbance of these two glutamate transporters is associated with a variety of neurological disorders, it is important to pay close attention to glutamate transporters, as they are ideal for treating a variety of nervous system diseases. Our results suggest that abnormal expression of GLAST and GLT-1 in the marginal ID group may have disturbed the glutamate transporters on BGs and affected nerve conduction of PCs in the cerebellum.
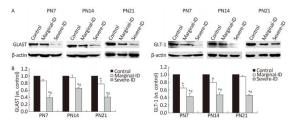
Figure 2. Marginal ID and severe ID affected GLAST and GLT-1 of cerebellum. The upper bands (A) depict representative findings subjected to developmental marginal ID and severe ID for rats. The lower bar graphs show the results of the semi-quantitative measurement of GLAST and GLT-1 (B) on PN7, PN14, and PN21. Each bar represents the mean ± SEM for the groups. Mean expression in the marginal ID and severe ID groups is shown as the fold change compared with mean expression in the control group that has been ascribed an arbitrary value of 1. Within each time point, *indicates a significant difference from the control group, P < 0.05; #Indicates a significant difference from the marginal ID group, P < 0.05.
GluR1 and GluR4 are the two glutamate receptor subunits expressed in BGs and PCs. GluR1 and GluR4 were significantly downregulated in rats in the marginal and severe ID groups on PN21 compared with the control group (Figure 3; P < 0.05). GluR1 and GluR4 protein levels decreased significantly in the severe ID group on PN7 and PN14. GluR1 expression undergoes a developmental switch from neurons to glia. This process appeared to correlate with the degree of PC dendritic growth and being enwrapped by BGs. It is very possible that BGs influence the final composition of AMPA receptors in PCs, either by soluble factors released by the glia or by local contact with the glial cell plasma membrane. Astrocytes and neurons may share similar targeting mechanisms to transport AMPA receptors from intracellular compartments to the glial cell plasma membrane[10]. This subunit combination results in assembly of an AMPAR that is inwardly rectifying and Ca2+ permeable. This composition also makes AMPAR of BGs vulnerable to polyamine block. This property has been used to confirm that AMPAR currents resulting from synaptic activity are due to activation of channels expressed by BGs, rather than a secondary effect of neuronal AMPAR activation. The polyamine 1-napthyl-acetylspermine inhibits AMPAR currents in BGs, which are evoked by climbing fiber and parallel fiber stimulation. AMPAR in BGs helps glial membranes detect and associate with synaptic sites by activating Ca2+ signaling[4]. Therefore, AMPAR is very important for cerebellar PC synaptic growth. Our data show that marginal ID disturbed GluR1 and GluR4 expression on PN7, PN14, and PN21. The disturbance in the GluR1 and GluR4 levels may be attributed to a decrease in the frequency of the interaction between BGs and PCs following marginal ID, which is the possible mechanism to inhibit the glial-neuronal relationship in the cerebellum.
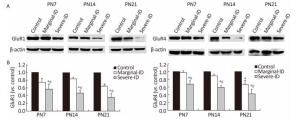
Figure 3. Marginal ID and severe ID affected GluR1 and GluR4 of cerebellum. The upper bands (A) depict representative findings subjected to developmental marginal ID and severe ID for rats. The lower bar graphs show the results of the semi-quantitative measurement of GluR1 and GluR4 (B) on PN7, PN14, and PN21. Each bar represents the mean ± SEM for the groups. Mean expression in the marginal ID and severe ID groups is shown as the fold change compared with mean expression in the control group that has been ascribed an arbitrary value of 1. Within each time point, *indicates a significant difference from the control group, P < 0.05; #Indicates a significant difference from the marginal ID group, P < 0.05.
In summary, we observed a slight limitation during the interaction between BGs and PCs caused by marginal ID in the rat cerebellum. Expression of the glutamate transporter and glutamate receptor, which may be involved in cell-cell morphology and organization, was altered. In conclusion, we speculate that maternal marginal ID slightly affected the interactions between cerebellar BGs and PCs in offspring, which may involve the glutamate transporter and glutamate receptor.
doi: 10.3967/bes2017.126
Effects of Maternal Marginal Iodine Deficiency on Interactions between Cerebellar Bergmann Glia Cells and Purkinje Cells in Rat Offspring
-
Abstract: Iodine deficiency (ID) during early pregnancy has an adverse effect on children's psychomotor and motor function but the mechanism has not been clarified. Therefore, our aim was to study the effect of maternal marginal ID on cerebellar neurodevelopment and the underlying mechanism. After obtaining marginal ID rats, we examined interactions between Bergmann glia cells (BGs) and Purkinje cells (PCs) using immunofluorescence and expression of the glutamate transporter and receptor by western blot. Our results showed that marginal ID reduced the number of contacted points between BGs and PCs, and disturbed expression of the glutamate transporter and receptor. Our results support the hypothesis that marginal ID inhibits interactions of BGs-PCs, which may be involved in abnormal regulation of the glutamate transporter and receptor.
-
Figure 1. Marginal ID and severe ID reduced the number of contacted points between cerebellar PCs and BGs on PN7, PN14, and PN21. Representative photomicrographs show fluorescent staining of anti-Calbindin-D-28K (green) and anti-GFAP (red) on PN7, PN14, and PN21 (A). The merged images show overlapping localization. Scale bar = 20 μm. The bar graphs (B) show the quantification of the numbers of contacted points between GFAP positive fibers and calbindin positive cells around the border between the ML and EGL following marginal ID and severe ID group in the cerebellum on PN7, PN14, and PN21. Each bar represents the mean ± SEM for the groups. *Indicates a significant difference from the control group, P < 0.05; #Indicates a significant difference from the marginal ID group, P < 0.05.
Figure 2. Marginal ID and severe ID affected GLAST and GLT-1 of cerebellum. The upper bands (A) depict representative findings subjected to developmental marginal ID and severe ID for rats. The lower bar graphs show the results of the semi-quantitative measurement of GLAST and GLT-1 (B) on PN7, PN14, and PN21. Each bar represents the mean ± SEM for the groups. Mean expression in the marginal ID and severe ID groups is shown as the fold change compared with mean expression in the control group that has been ascribed an arbitrary value of 1. Within each time point, *indicates a significant difference from the control group, P < 0.05; #Indicates a significant difference from the marginal ID group, P < 0.05.
Figure 3. Marginal ID and severe ID affected GluR1 and GluR4 of cerebellum. The upper bands (A) depict representative findings subjected to developmental marginal ID and severe ID for rats. The lower bar graphs show the results of the semi-quantitative measurement of GluR1 and GluR4 (B) on PN7, PN14, and PN21. Each bar represents the mean ± SEM for the groups. Mean expression in the marginal ID and severe ID groups is shown as the fold change compared with mean expression in the control group that has been ascribed an arbitrary value of 1. Within each time point, *indicates a significant difference from the control group, P < 0.05; #Indicates a significant difference from the marginal ID group, P < 0.05.
-
[1] Zimmermann MB, Gizak M, Abbott K, et al. Iodine deficiency inpregnant women in Europe. Lancet Diabetes Endocrinol, 2015; 3, 672-4. doi: 10.1016/S2213-8587(15)00263-6 [2] Versloot PM, Schröder-van der Elst JP, van der Heide D, et al. Effects of marginal iodine deficiency on thyroid hormone production, distribution and transport in nonpregnant and near term pregnant rats. Eur J Endocrinol, 1998; 138, 713-8. doi: 10.1530/eje.0.1380713 [3] Fauquier T, Chatonnet F, Picou F, et al. Purkinje cells and Bergmann glia are primary targets of the TRα1 thyroid hormone receptor during mouse cerebellum postnatal development. Development, 2014; 141, 166-75. doi: 10.1242/dev.103226 [4] Bellamy TC. Interactions between Purkinje neurones and Bergmann glia. Cerebellum, 2006; 5, 116-26. doi: 10.1080/14734220600724569 [5] Cvetanovic M. Decreased expression of glutamate transporter GLAST in Bergmann glia is associated with the loss of Purkinje neurons in the spinocerebellar ataxia type 1. Cerebellum, 2015; 14, 8-11. doi: 10.1007/s12311-014-0605-0 [6] Min H, Dong J, Wang Y, et al. Marginal Iodine Deficiency Affects Dendritic Spine Development by Disturbing the Function of Rac1 Signaling Pathway on Cytoskeleton. Mol Neurobiol, 2017; 54, 437-49. doi: 10.1007/s12035-015-9657-5 [7] Eiraku M, Tohgo A, Ono K, et al. DNER acts as a neuron-specific Notch ligand during Bergmann glial development. Nat Neurosci, 2005; 8, 873-80. doi: 10.1038/nn1492 [8] Ghanbari M, Ghasemi A. Maternal hypothyroidism: An overview of current experimental models. Life Sci, 2017; 187, 1-8. doi: 10.1016/j.lfs.2017.08.012 [9] Kim K, Lee SG, Kegelman TP, et al. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J Cell Physiol, 2011; 226, 2484-93. doi: 10.1002/jcp.v226.10 [10] Douyard J, Shen L, Huganir RL, et al. Differential neuronal and glial expression of GluR1 AMPA receptor subunit and thescaffolding proteins SAP97 and 4. 1N during rat cerebellar development. J Comp Neurol, 2007; 502, 141-56. doi: 10.1002/(ISSN)1096-9861 -




 下载:
下载:



 Quick Links
Quick Links