-
Exposure to microgravity results in postflight cardiovascular dysfunction and orthostatic intolerance in astronauts, which poses a threat to the health of the astronauts and safety during spaceflight. Although much progress has been made in this field, the underlying mechanism remains to be established.
Studies on ground-based rodent animals have reported region-specific vascular structural and functional remodeling in hindlimb unweighting (HU) rat arteries[1-4]. Both nitric oxide (NO) and endothelin-1 in endothelial cells are modified in a simulated microgravity setting[5]. The levels of endothelial nitric oxide synthase and nitrate/nitrite content in cerebral arteries increase[6, 7], which was also confirmed in human umbilical vein endothelial cells and human microvessel endothelial cells exposed to microgravity simulated by a random positioning machine and rotating wall vessel[8, 9]. Calcium influx and efflux are modulated by large-conductance calcium-activated K+ channels (BKCa) and L-type Ca2+ channels in HU rat cerebral vascular smooth muscle cells (VSMCs), which regulate vascular tension[10, 11]. Plasma membrane calcium-regulating channels, sarco/endoplasmic reticulum Ca2+ ATPase, ryanodine receptors, and the inositol 1,4,5-trisphosphate receptor (IP3R) regulate intracellular Ca2+ homeostasis in VSMCs[12]. The influx of extracellular Ca2+ and the release of Ca2+ from intracellular calcium stores, such as the sarcoplasmic reticulum (SR) and mitochondria regulate cellular Ca2+ homeostasis[13]. However, the mechanism of regulating Ca2+ homeostasis in VSMCs exposed to microgravity or simulated microgravity remains unclear.
Reactive oxygen species (ROS) increase cytoplasmic Ca2+ concentration ([Ca2+]) by facilitating Ca2+ release from the endoplasmic reticulum (ER)/SR through the IP3R[14, 15]. Mitochondrial-derived ROS enhance angiotensin II-triggered vascular contraction by elevating Ca2+ and IP3 levels[16]. Mitochondria regulate Ca2+ through fission and fusion mediated by mitofusion 1/2 (MFN1/2), dynamin-related protein 1 (DRP1), and fission protein 1 (FIS1)[17-20]. Our previous studies have detected cellular oxidative stress and mitochondrial oxidative injury in HU rat cerebral VSMCs[21-23] and inhibiting NADPH oxidase improves cerebrovascular reactivity in HU rats[7]; however, whether these factors are associated with the regulation of Ca2+ homeostasis and vascular remodeling is unclear. To better understand the molecular mechanisms of vascular remodeling during microgravity, the present study was designed to investigate the roles and mechanism of mitochondrial oxidative stress during Ca2+ homeostasis and the regulation of cerebrovascular contraction in HU rat cerebral arteries.
-
The handling and treatment of animals were according to the Guiding Principles for the Care and Use of Animals in the Physiological Sciences and were approved by the Chinese guidelines for experimental animals. The care and use of experimental rats were supervised and approved by the Animal Ethical Committee of Chinese PLA General Hospital.
-
HU was used to simulate microgravity in rats as described in our previous studies[6, 24]. Briefly, male Sprague-Dawley rats were randomly assigned to four groups: control (CON), HU, mitoTEMPO-treated HU (HU + MT), and mitoTEMPO-treated control (CON + MT). To simulate the cardiovascular effect of microgravity, the HU rats were maintained in about a −30° head-down tilt position and housed individually with their hindlimbs unloaded under a 12:12-h light-dark cycle at 23 ± 1 °C with food and water available ad libitum. The control rats were housed in identical Plexiglas cages, except that the tail suspension device was removed. HU + MT and CON + MT rats received distilled water containing mitoTEMPO (Alexis Biochemicals, San Diego, CA, USA) provided at a rate of 0.7 mg/(kg·day) by gavage. The rats in the other groups received an equal volume of vehicle (distilled water). The rats were killed by exsanguination via the abdominal aorta 3 weeks later. The cerebral arteries were rapidly removed and placed in cold Krebs buffer solution containing 118.3 mmol/L NaCl, 14.7 mmol/L KCl, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4·7H2O, 2.5 mmol/L CaCl2·2H2O, 25 mmol/L NaHCO3, 11.1 mmol/L dextrose, and 0.026 mmol/L EDTA (pH 7.4). VSMCs were dissociated from cerebral arteries as described previously[11].
-
The samples were homogenized in 10 mmol/L HEPES lysis buffer (320 mmol/L sucrose, 1 mmol/L EDTA, 1 mmol/L DTT, 10 μg/mL leupeptin, and 2 μg/mL aprotinin, pH 7.40) at 0–4 °C. The homogenate was centrifuged at 12,000 ×g for 10 min at 4 °C. Protein concentrations were determined with a bicinchoninic acid assay kit (Pierce, Rockford, IL, USA). The extracts were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. The membranes were incubated with antibodies against IP3R, MFN1, MFN2, DRP1, FIS1, or GADPH at 4 °C overnight (Abcam, Cambridge, UK). Then, the membranes were incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibodies for 2 h. The blots were washed and developed with an enhanced chemiluminescent system (Amersham Biosciences, Uppsala, Sweden) according to the manufacturer’s protocol.
-
Cerebral VSMCs were washed three times in 0.1 mmol/L phosphate buffered solution (PBS), followed by fixation in 1% osmium tetroxide at 4 °C for 3 h. The samples were washed three times in 0.1 mmol/L PBS again and gradually dehydrated in alcohol at different concentrations at 4 °C. After replacing the solution with propylene oxide and embedding using the SPI-Chem Embedding Kit (SPI, Chicago, IL, USA), the samples were fixed at 70 °C for 8 h. The sections (70 nm) were prepared with a Leica EM UC6 ultramicrotome (Leica Microsystems, Wetzlar, Germany), placed on EM grids, and stained with 3% uranyl acetate-lead citrate solution (SPI), followed by washing, drying, and imaging using a JEM1230 TEM (JEOL, Tokyo, Japan).
-
Paraffin-embedded sections (3 μm) of the basilar arteries were mounted on Thermo Scientific SuperFrost™ plus slides and dried overnight. Briefly, the arterial sections were incubated with primary IP3R antibody (Abcam). After washing with PBS, the horseradish peroxidase was developed with 3,3-diaminobenzadine (Roche Diagnostics, Mannheim, Germany) as the chromogen substrate. The sections were rinsed, dehydrated in ethanol, cleared in xylene, and mounted. IP3R staining in the cerebrovascular sections was observed under a microscope.
-
Cytoplasmic, mitochondrial, and SR Ca2+ concentrations were determined using a flow cytometry system with the Ca2+ indicators Fluo-4 AM, X-Rhod-1 AM, and Fluo-5N, respectively. Isolated cerebral VSMCs were placed in Hank’s balanced salt solution (Sigma-Aldrich, St. Louis, MO, USA) and incubated with Fluo-4 AM (5 μmmol/L), X-Rhod-1 AM (5 μmmol/L), and Fluo-5N (10 μmmol/L) for 60 min at 37 °C. The Ca2+ content in individual groups of VSMCs was analyzed with the BD LSRFortessaTM flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
-
The patch-clamp technique and whole-cell patch-clamp recordings were used to analyze the KV and BKCa currents. Extracellular fluid (150 mmol/L choline chloride, 5 mmol/L KCl, 2 mmol/L CaCl2, 1 mmol/L MgCl2, 10 mmol/L HEPES, 1 mmol/L CdCl2, and 10 mmol/L D-glucose; adjusted to pH 7.4 with KOH; 320 mOsm) was continuously perfused at a rate of 0.2 mL/min. The tip of the patch-clamp electrode was about 1–2 μm, and resistance was 6–10 MΩ. The internal fluid in the glass electrode was 120 mmol/L potassium gluconate, 20 mmol/L KCl, 2 mmol/L MgCl2, 10 mmol/L EGTA, 10 mmol/L HEPES, 5 mmol/L Na2 ATP, and 1 mmol/L CaCl2; adjusted to pH 7.2 with KOH; osmotic pressure 320 mOsm). The clamping voltage was set to −80 mV, depolarization voltage was in steps of 10 mV from −70 mV to 70 mV (pulse duration of 400 ms), and whole-cell KV current was recorded every 2s. The signals were processed and recorded with an EPC-10 amplifier (HEKA Elektronik, Lambrecht, Germany). To separate the BKCa and KV currents from the total current, the BKCa channel inhibitors TEA (1 mmol/L) and CTX (100 nmol/L) were added to the extracellular fluid. The current densities and open probabilities of BKCa and KV were calculated.
-
Cerebral vascular reactivity was measured as described previously[25]. Briefly, basilar arteries (2 mm long) were carefully dissected free from the brain and mounted on two 40 μm stainless wires in the jaws of the Dual Wire Myograph System (Danish Myo Technology A/S, Hinnerup, Denmark) to record changes in isometric force. After the arteries were mounted and prepared, they were challenged with KCl (10–100 mmol/L) or cumulative concentrations (10−9–10−5 mol/L) of 5-hydroxytryptamine (5-HT). Cumulative concentration response curves were generated.
-
The results are expressed as mean ± standard error. GraphPad Prism 5.0 software (GraphPad Software Inc., La Jolla, CA, USA) was used for the statistical analysis. Protein expression and mRNA levels were compared using two-way analysis of variance (ANOVA) followed by the unpaired Student’s t-test. The isometric force measurement data were analyzed by two-way ANOVA. A P-value of < 0.05 was considered significant.
-
Microgravity simulated by HU resulted in a significantly lower soleus muscle mass (P < 0.001). Soleus muscle-to-body mass ratios decreased significantly (P < 0.001) in the HU and HU + MT rats, which confirmed the efficacy of simulated microgravity by HU. The data are summarized in Table 1.
Table 1. Body mass (g), soleus mass (mg), and soleus: body mass ratio (mg/g) of rats from the control, mitoTEMPO-treated control, hindlimb unweighting, and mitotempo-treated hindlimbunweighting groups (n = 8 in each group)
Group Initial mass (g) Final mass (g) Soleus mass (mg) Soleus: body mass (mg/g) CON 198.60 ± 1.45 345.85 ± 10.47 134.54 ± 3.62 0.39 ± 0.01 HU 196.73 ± 2.07 336.79 ± 10.74 65.28 ± 1.85*** 0.19 ± 0.01*** CON + MT 199.55 ± 2.67 338.57 ± 8.17 138.48 ± 2.82 0.41 ± 0.01 HU + MT 199.87 ± 1.80 341.89 ± 11.26 74.29 ± 2.12*** 0.22 ± 0.01*** Note. CON, control; HU, hindlimb unweighting; MT, mitoTEMPO; CON+MT, mitoTEMPO-treated control; HU + MT, mitoTEMPO-treated HU. Values are mean ± standard error. ***P < 0.001 vs. control. -
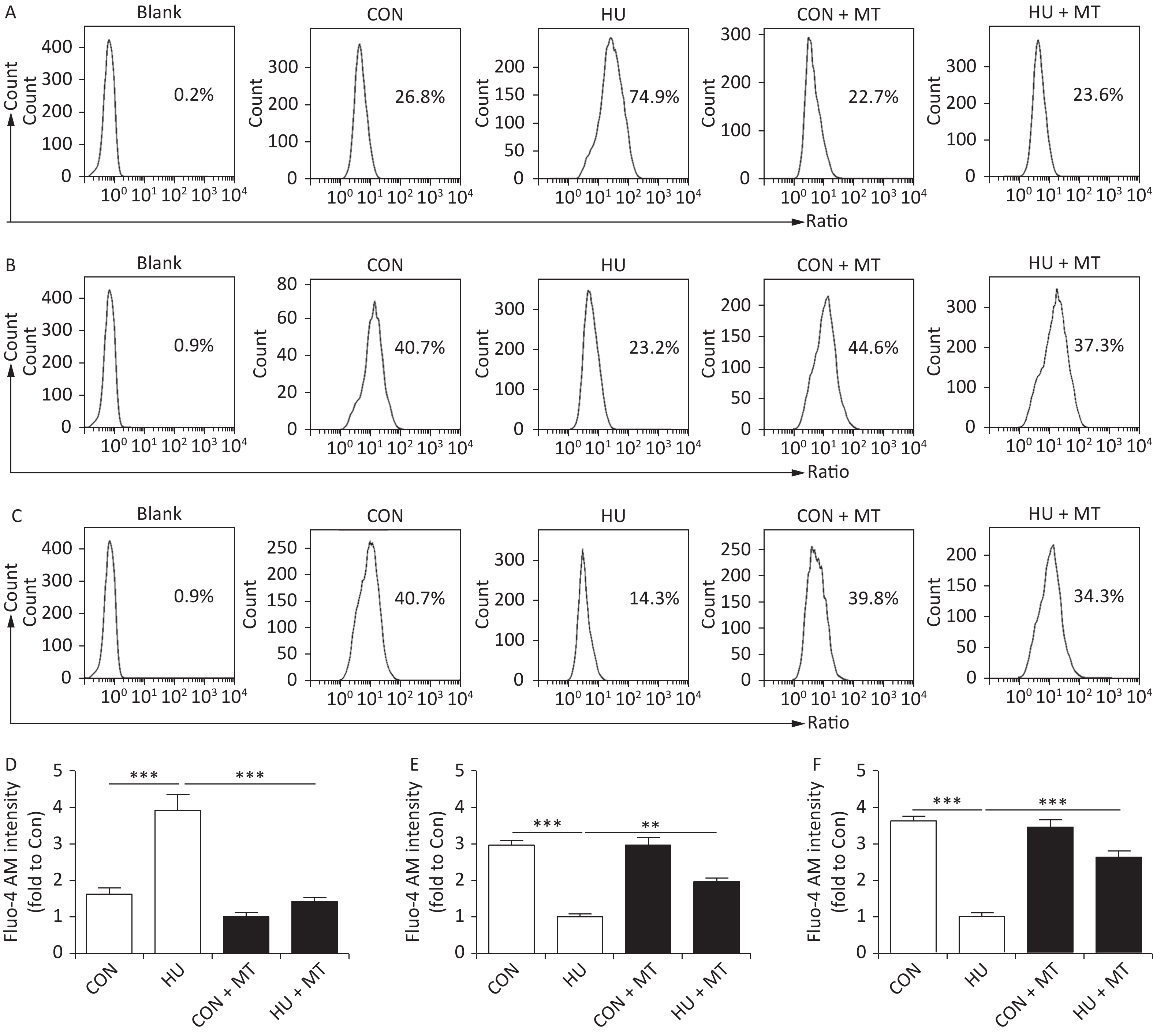
Cytoplasmic, mitochondrial, and SR Ca2+ distribution and content in cerebral VSMCs are shown in Figure 1. After HU, cytoplasmic Ca2+ content significantly increased (P < 0.001) (Figure 1A, 1D) with a significant decrease of Ca2+ in mitochondria (Figure 1B, 1E) and the SR (Figure 1C, 1F) (P < 0.001) compared with CON rat cerebral VSMCs. The chronic treatment with mitoTEMPO restored cytoplasmic (P < 0.001), mitochondrial (P < 0.01), and SR (P < 0.001) Ca2+ distribution and content in HU + MT rat cerebral VSMCs.
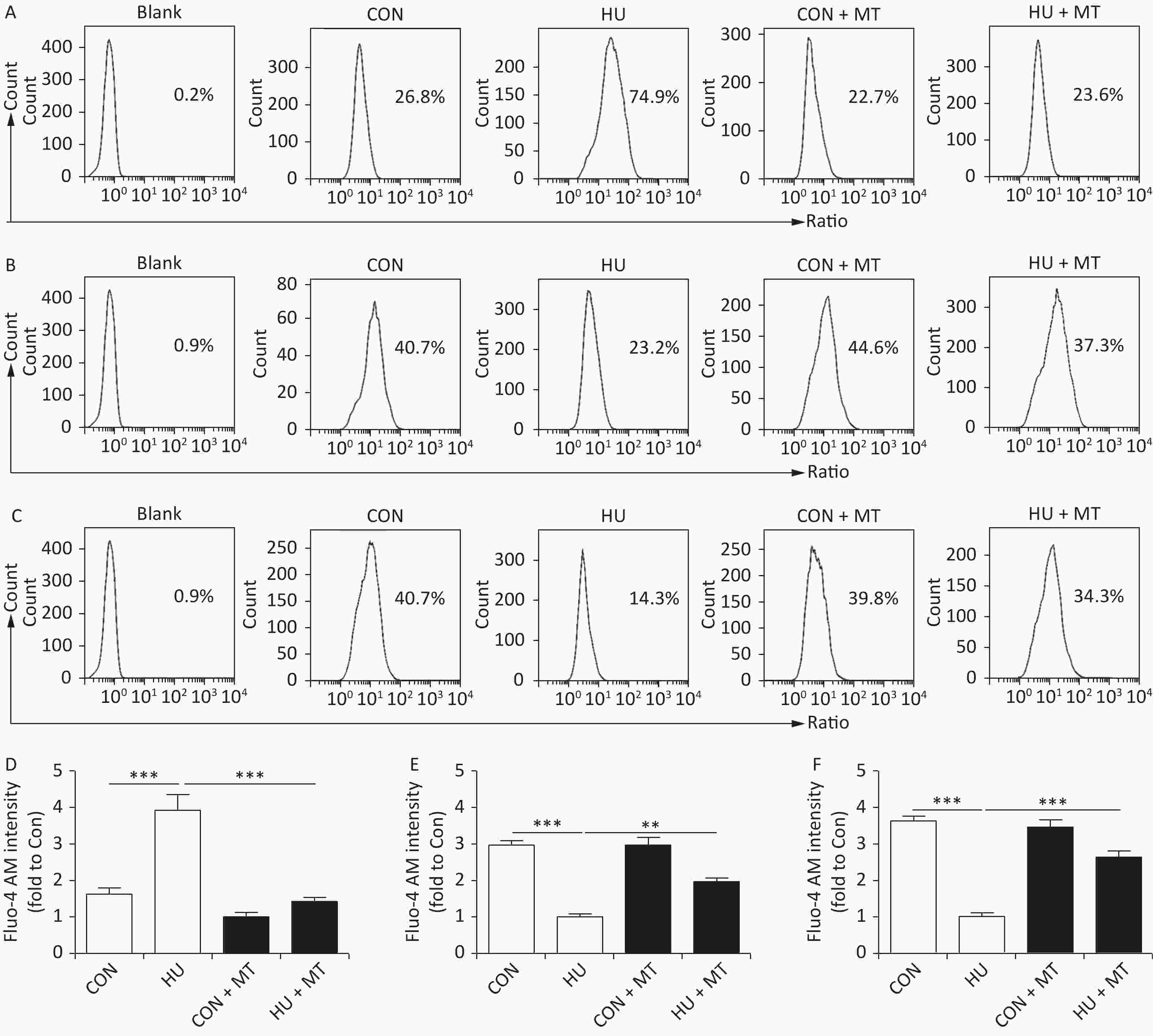
Figure 1. The effects of mitoTEMPO on cytoplasmic (A, D), mitochondrial (B, E), and sarcoplasmic reticulum (C, F) Ca2+ distribution in rat cerebral vascular smooth muscle cells. CON, control; HU, hindlimb unweighting; MT, mitoTEMPO; CON + MT, mitoTEMPO-treated control; HU + MT, mitoTEMPO-treated HU. Values are mean ± standard error. **P < 0.01 and ***P < 0.001.
-
We analyzed mitochondrial fusion and fission to investigate the mechanism of cytoplasmic, mitochondrial, and SR Ca2+ redistribution (Figure 2). TEM (Figure 2A) showed more long and narrow mitochondria in the HU rat cerebral VSMCs, while more elliptical mitochondria were observed in the CON, CON + MT, and HU + MT rat cerebral VSMCs (mitochondria are marked by white arrows), indicating that HU enhanced mitochondrial fission and that treatment with mitoTEMPO attenuates mitochondrial fission. The MFN1/2 protein and mRNA levels (Figure 2B, 2F and Figure 2C, 2G) in HU rat cerebral VSMCs decreased significantly compared to those in the CON rats (P < 0.001). The DRP1/FIS1 protein and mRNA levels (Figure 2D, 2H and Figure 2E, 2I) were significantly higher in HU rat cerebral arteries than those in the CON rats (P < 0.01 for protein and P < 0.001 for mRNA). Chronic treatment with mitoTEMPO significantly upregulated the expression of MFN1/2 (P < 0.05 for MFN1 mRNA; P < 0.001 for MFN1/2 protein and MFN2 mRNA) but decreased the expression of FIS1/DRP1 (P < 0.01 for protein and P < 0.001 for mRNA) compared to the HU.
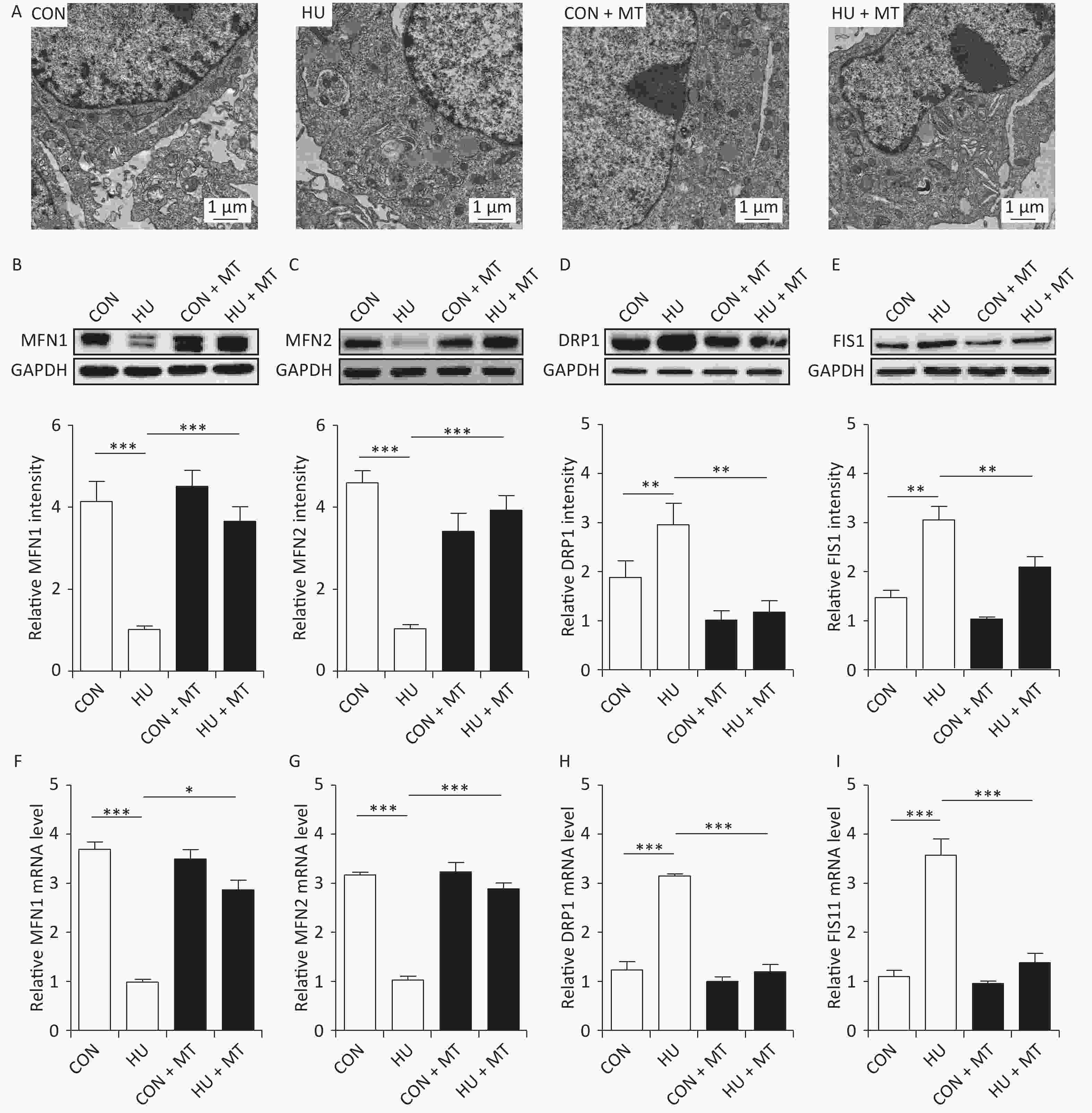
Figure 2. Effects of mitoTEMPO on mitochondrial fission and fusion (A) and the protein and mRNA levels of mitofusion 1 (MFN1) (B, F) and mitofusion 2 (MFN2) (C, G), dynamin-related protein 1 (DRP1) (D, H), and fission protein 1 (FIS1) (E, I) in rat cerebral vascular smooth muscle cells. CON, control; HU, hindlimb unweighting; MT, mitoTEMPO; CON + MT, mitoTEMPO-treated control; HU + MT, mitoTEMPO-treated HU. Values are mean ± standard error. *P < 0.05, **P < 0.01, and ***P < 0.001.
-
To investigate whether cytoplasmic Ca2+ redistribution was IP3R-dependent, we determined the abundance of the IP3R in HU rat cerebral arteries (Figure 3). IP3R protein (P < 0.001) and mRNA (P < 0.001) levels increased significantly (Figure 3A and 3B) in HU rat cerebral arteries compared to the CON. Immunohistochemical staining revealed that the IP3R was more positive in HU rat cerebral VSMCs than that in the CON (Figure 3C). Chronic treatment with mitoTEMPO partially restored the enhanced expression of the IP3R after HU.
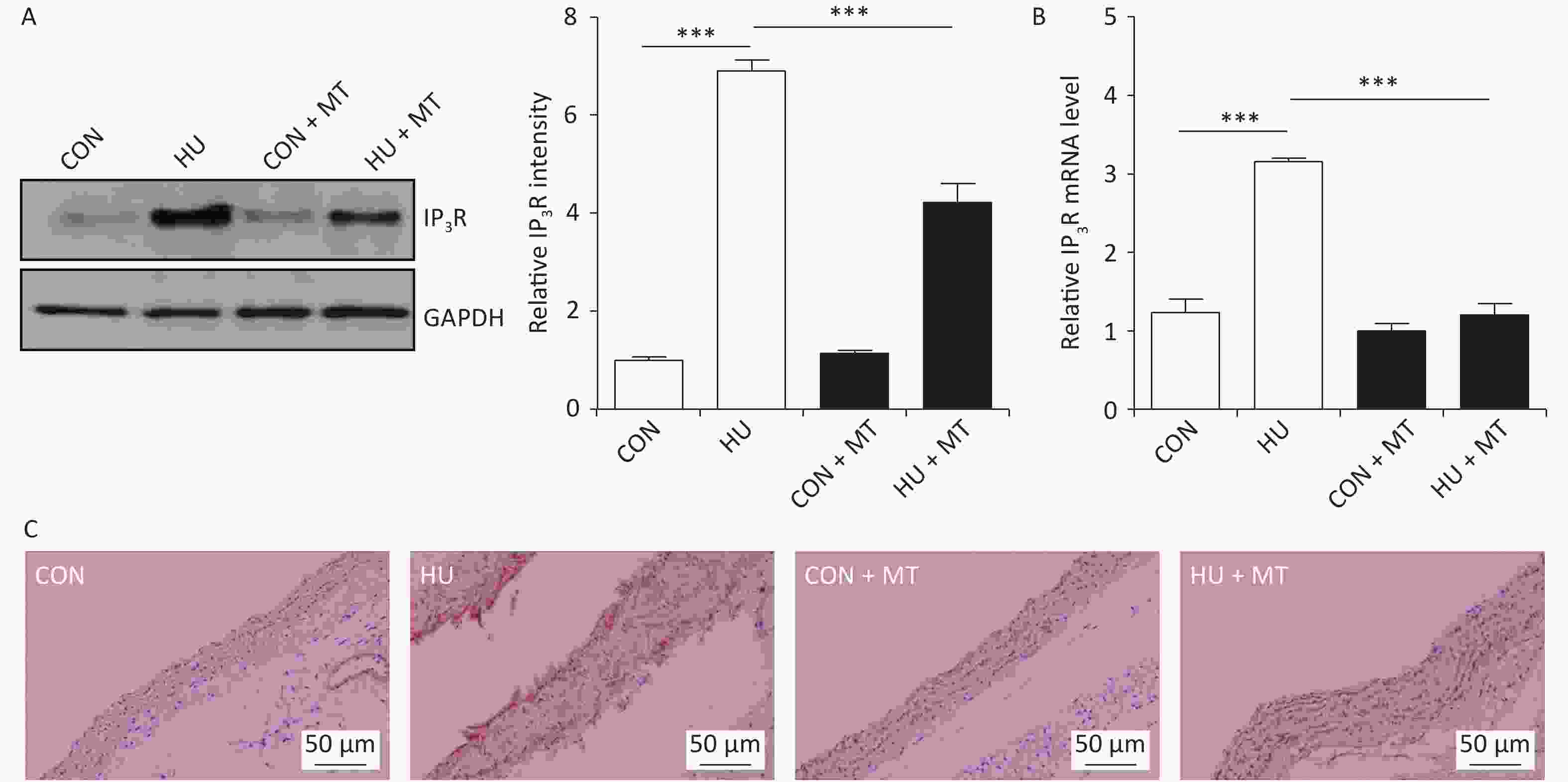
Figure 3. The effects of mitoTEMPO on protein (A) and mRNA (B) levels of the inositol 1,4,5-trisphosphate receptor (IP3R) and immunohistochemistry for IP3R (C) in rat cerebral vascular smooth muscle cells. CON, control; HU, hindlimb unweighting; MT, mitoTEMPO; CON + MT, mitoTEMPO-treated control; HU + MT, mitoTEMPO-treated HU. Values are mean ± standard error. ***P < 0.001.
-
To investigate whether cytoplasmic Ca2+ redistribution induced by mitochondrial oxidative stress was associated with changes in plasma membrane K+ channels, we analyzed total K+ current, current densities, and open probabilities (Po) of the KV and BKCa channels (Figure 4). Total K+ current decreased (Figure 4A), whereas current densities and open probabilities of KV (Figure 4B and 4C) and BKCa (Figure 4D and 4E) decreased and increased, respectively, compared to control rats, in HU rat cerebral VSMCs, which were restored by chronic treatment with mitoTEMPO.
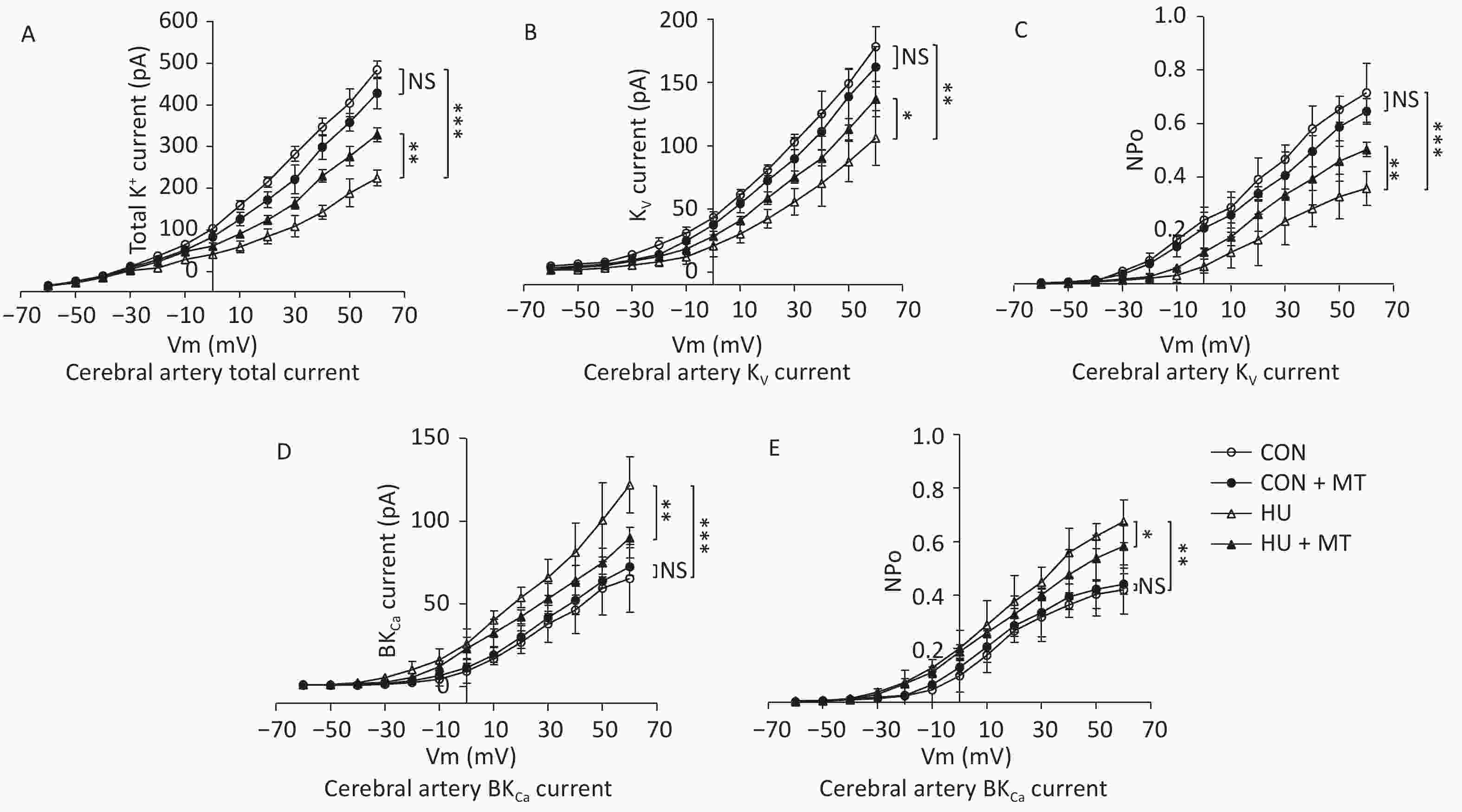
Figure 4. Effects of mitoTEMPO on total K+ current (A), current activation, and opening probabilities of voltage-gated potassium (KV) channels (B, C), and Ca2+-activated K+ (BKCa) channels (D, E) in rat cerebral vascular smooth muscle cells. CON, control; HU, hindlimb unweighting; MT, mitoTEMPO; CON + MT, mitoTEMPO-treated control; HU + MT, mitoTEMPO-treated HU. Values are mean ± standard error. *P < 0.05, **P < 0.01, and ***P < 0.001.
-
To investigate whether the changes in cytoplasmic Ca2+ were associated with cerebrovascular contraction, we studied the cerebrovascular contraction to vasoconstrictors (Figure 5). Cumulative increases in KCl and 5-HT concentrations induced concentration-dependent vasoconstriction in basilar arteries from the four groups. Three-week HU significantly enhanced the maximal contractile responses to KCl and 5-HT in rat basilar arteries (P < 0.05) compared to the CON, which was attenuated by the chronic mitoTEMPO treatment (P < 0.05).
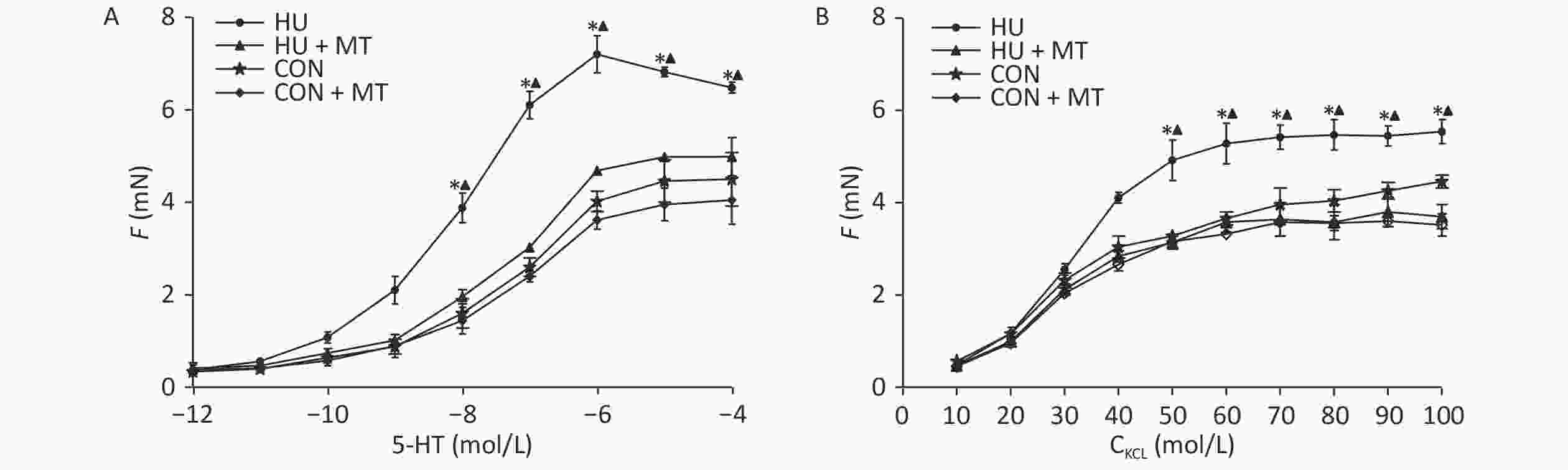
Figure 5. Effects of mitoTEMPO on vasoconstriction to cumulative KCl or 5-hydroxytryptamine (5-HT) in rat basilar arteries. CON, control; HU, hindlimb unweighting; MT, mitoTEMPO; CON + MT, mitoTEMPO-treated control; HU + MT, mitoTEMPO-treated HU. Values are mean ± standard error. *P < 0.05 for HU vs. CON, ▲P < 0.05 for HU vs. HU + MT.
-
The major findings of the present study are: (1) mitochondrial oxidative stress augmented HU rat cerebrovascular contraction to vasoconstrictors by regulating Ca2+ distribution and content in VSMCs; (2) mitochondrial oxidative stress regulated Ca2+ homeostasis by controlling IP3R abundance, Ca2+ storage, mitochondrial fusion/fission, and changes in plasma membrane ion channels (KV and BKCa) in HU rat cerebral VSMCs.
Exposure to microgravity results in postflight cardiovascular deconditioning and orthostatic intolerance in astronauts. Structural and functional remodeling of the cardiovascular system have been implicated in this process. We and other authors have found increased myogenic tone, enhanced vasoconstriction, and attenuated endothelium-dependent relaxation in HU rat cerebral arteries[3, 4, 6, 25]. We previously reported mitochondrial oxidative stress and ER stress in rat cerebral VSMCs[21-23, 26]. However, whether and how mitochondrial oxidative stress regulates HU rat cerebrovascular reactivity remains unclear. The dynamic changes in cytoplasmic Ca2+ are a critical mechanism regulating vascular tone and contraction. During microgravity, large-conductance calcium-activated K+ (BKCa) and L-type voltage-dependent Ca2+ (CaL) channels were activated in VSMCs, which are associated with apoptosis[10, 11]. The CaL currents densities increased significantly in 4-week HU rat cerebral VSMCs, and therefore induced significantly higher cytoplasmic Ca2+ and enhanced vasoconstriction[27]. KV channels are expressed in blood vessels and are key regulators of vascular tension. An increase in cytoplasmic Ca2+ concentration inactivates KV channels and induces vascular contraction[28]. The BKCa channels in the vasculature located close to the IP3Rs in the SR and the IP3Rs stimulate the opening of BKCa channels dependent on Ca2+ released from IP3Rs as in VSMCs[29]. In the current study, KV and BKCa current densities and open probabilities, which are both important Ca2+-regulating plasma ion channels, decreased and increased respectively; however, these changes were restored by the mitochondrial-targeted antioxidant mitoTEMPO. This means that mitochondrial oxidative stress regulates cytoplasmic Ca2+ concentration through the activities of plasma ion channels, which play roles in Ca2+ homeostasis in VSMCs exposed to microgravity or simulated microgravity.
The processes influencing cytoplasmic Ca2+ are important regulators of vascular function under physiological and pathophysiological settings. The mitochondria and SR are the major Ca2+ stores in VSMCs, which regulate Ca2+ homeostasis in intracellular organelles by Ca2+ sequestering activities[13]. The close connection between mitochondria and the SR plays an important role in mitochondrial dynamics, ATP production, lipid synthesis, and Ca2+ signaling[30, 31]. Mitochondria regulate intracellular Ca2+ content through Ca2+ uptake from SR via the SR-mitochondrial membrane[32]. The rate of mitochondrial Ca2+ uptake is affected by the IP3R and MFN2[33, 34], which maintain the balance between extracellular and intracellular [Ca2+][13]. The IP3R is a pivotal Ca2+ release channel located perinuclearly and peripherally in the SR[35]. ER/SR stress facilitates Ca2+ release from the SR through the IP3R[14], which causes Ca2+ uptake by mitochondria. Increased mitochondrial Ca2+ influx leads to mitochondrial Ca2+ overload and opening of the mitochondrial permeability transition pore, eventually triggering cell death[36, 37]. Overloading of Ca2+ within the mitochondrial matrix inhibits ATP production but promotes mitochondrial ROS production[38, 39]. In the current study, increased IP3R protein abundance and decreased SR Ca2+ in HU rat cerebral VSMCs suggested that IP3R-dependent Ca2+ release from the SR was activated and increased cytoplasmic Ca2+ contributed to enhance vasoconstriction.
The elevated ROS levels in mitochondria and the SR send feedback for SR membrane Ca2+ release by stimulating the IP3R[40, 41], and increased ROS production enhances the rate of mitochondrial Ca2+ uptake and has a destructive effect on mitochondria, leading to changes in the state of mitochondria from fusion to fission by inducing the expression of DRP1 and FIS1[19, 42]. Mitochondrial fusion provides the proper environment for Ca2+ regulation and cellular metabolism, which is important for Ca2+ homeostasis in VSMCs[19, 43]. MFN1 and MFN2 play critical roles maintaining the correct mitochondrial and the SR-mitochondria connection, respectively[33, 44]. MFN2, which is located in the outer mitochondrial membrane and SR membrane, tethers the SR to mitochondria and mediates Ca2+ signal transfer between the two organelles together with the IP3R[45]. Once the mitochondrial dynamics have shifted from fusion to fission, cell death occurred caused by over-expression of FIS1, which depends on SR-mitochondria; Ca2+ signal transfer[46] and DRP1-induced mitochondrial fragmentation[47]. The Ca2+ redistribution leads to increased cytoplasmic Ca2+ and an enhanced contractile response to vasoconstrictors. Consistent with these findings, our present study showed that microgravity simulated by HU changed the morphological characteristics of the mitochondria from elliptical to long and narrow, along with upregulated expression of the mitochondrial fission proteins (DRP1 and FIS1) and decreased levels of the fusion proteins (MFN1 and MFN2), which were reversed by a mitochondrial-targeted antioxidant. This finding suggests that mitochondrial oxidative stress during simulated microgravity shifted the mitochondrial state from fusion to fission. Together with our previous findings that microgravity simulated by HU is associated with oxidative stress and mitochondrial oxidative stress in cerebral VSMCs[21, 22, 48], we infer that Ca2+ and ROS overloading in mitochondria during simulated microgravity leads to mitochondrial fission by regulating the expression of mitochondrial fission and fusion proteins. Therefore, mitochondrial oxidative stress induced by 3-weeks of HU stimulated Ca2+ release from the SR by upregulating IP3R expression, impairing mitochondrial Ca2+ uptake, and undergoing mitochondrial fission, which resulted in the accumulation of Ca2+ in the cytoplasm and enhanced vasoconstriction.
Interestingly, our recent study reported that ER stress induces activation of the iNOS/NO-NF-κB/IκB pathway and plays a key role inducing endothelial inflammation and apoptosis[49]. Whether SR stress affects calcium signaling in cerebral VSMCs during microgravity simulation needs further investigation. Although we clarified that changes in calcium homeostasis caused by mitochondrial oxidative stress contributed to enhance vasoconstriction during simulated microgravity, we did not investigate the effects of mitochondrial oxidative stress on SR function. Further studies are needed to investigate functional alterations and the underlying mechanism of mitochondria and the SR during microgravity.
In conclusion, mitochondrial oxidative stress induced by simulated microgravity increased cytoplasmic Ca2+ by impairing mitochondrial fusion/fission-dependent Ca2+ uptake, enhancing IP3R-dependent SR Ca2+ release, and regulating the functions of plasma KV and BKCa channels. A mitochondrial-targeted antioxidant that alleviated mitochondrial oxidative stress restored Ca2+ homeostasis and vascular contraction to the agonists. The current results demonstrate that mitochondrial oxidative stress contributes to vasoconstriction by regulating calcium homeostasis in rat cerebrovascular smooth muscle cells exposed to simulated microgravity.
-
Guarantor of integrity of the entire study: LIU Zi Fan. Study concept and design: ZHANG Ran and LI Xin. Experimental studies: LIU Zi Fan, WANG Hai Ming, JIANG Min, WANG Lin, XIE Man Jiang, LIN Le Jian, ZHAO Yun Zhang, SHAO Jun Jie, and ZHOU Jing Jing. Data analysis: LIU Zi Fan, WANG Hai Ming, and ZHANG Ran. Manuscript preparation: LIU Zi Fan and WANG Hai Ming. Manuscript editing: LIU Zi Fan and WANG Hai Ming. Manuscript review: ZHANG Ran and LI Xin. All authors have read and approved the final version of the manuscript.
-
The authors declare no competing financial interests.
doi: 10.3967/bes2021.001
Mitochondrial Oxidative Stress Enhances Vasoconstriction by Altering Calcium Homeostasis in Cerebrovascular Smooth Muscle Cells under Simulated Microgravity
-
Abstract:
Objective Exposure to microgravity results in postflight cardiovascular deconditioning in astronauts. Vascular oxidative stress injury and mitochondrial dysfunction have been reported during this process. To elucidate the mechanism for this condition, we investigated whether mitochondrial oxidative stress regulates calcium homeostasis and vasoconstriction in hindlimb unweighted (HU) rat cerebral arteries. Methods Three-week HU was used to simulate microgravity in rats. The contractile responses to vasoconstrictors, mitochondrial fission/fusion, Ca2+ distribution, inositol 1,4,5-trisphosphate receptor (IP3R) abundance, and the activities of voltage-gated K+ channels (KV) and Ca2+-activated K+ channels (BKCa) were examined in rat cerebral vascular smooth muscle cells (VSMCs). Results An increase of cytoplasmic Ca2+ and a decrease of mitochondrial/sarcoplasmic reticulum (SR) Ca2+ were observed in HU rat cerebral VSMCs. The abundance of fusion proteins (mitofusin 1/2 [MFN1/2]) and fission proteins (dynamin-related protein 1 [DRP1] and fission-mitochondrial 1 [FIS1]) was significantly downregulated and upregulated, respectively in HU rat cerebral VSMCs. The cerebrovascular contractile responses to vasoconstrictors were enhanced in HU rats compared to control rats, and IP3R protein/mRNA levels were significantly upregulated. The current densities and open probabilities of KV and BKCa decreased and increased, respectively. Treatment with the mitochondrial-targeted antioxidant mitoTEMPO attenuated mitochondrial fission by upregulating MFN1/2 and downregulating DRP1/FIS1. It also decreased IP3R expression levels and restored the activities of the KV and BKCa channels. MitoTEMPO restored the Ca2+ distribution in VSMCs and attenuated the enhanced vasoconstriction in HU rat cerebral arteries. Conclusion The present results suggest that mitochondrial oxidative stress enhances cerebral vasoconstriction by regulating calcium homeostasis during simulated microgravity. -
Key words:
- Microgravity /
- Mitochondrial oxidative stress /
- Calcium homeostasis /
- Vasoconstriction
注释: -
Figure 1. The effects of mitoTEMPO on cytoplasmic (A, D), mitochondrial (B, E), and sarcoplasmic reticulum (C, F) Ca2+ distribution in rat cerebral vascular smooth muscle cells. CON, control; HU, hindlimb unweighting; MT, mitoTEMPO; CON + MT, mitoTEMPO-treated control; HU + MT, mitoTEMPO-treated HU. Values are mean ± standard error. **P < 0.01 and ***P < 0.001.
Figure 2. Effects of mitoTEMPO on mitochondrial fission and fusion (A) and the protein and mRNA levels of mitofusion 1 (MFN1) (B, F) and mitofusion 2 (MFN2) (C, G), dynamin-related protein 1 (DRP1) (D, H), and fission protein 1 (FIS1) (E, I) in rat cerebral vascular smooth muscle cells. CON, control; HU, hindlimb unweighting; MT, mitoTEMPO; CON + MT, mitoTEMPO-treated control; HU + MT, mitoTEMPO-treated HU. Values are mean ± standard error. *P < 0.05, **P < 0.01, and ***P < 0.001.
Figure 3. The effects of mitoTEMPO on protein (A) and mRNA (B) levels of the inositol 1,4,5-trisphosphate receptor (IP3R) and immunohistochemistry for IP3R (C) in rat cerebral vascular smooth muscle cells. CON, control; HU, hindlimb unweighting; MT, mitoTEMPO; CON + MT, mitoTEMPO-treated control; HU + MT, mitoTEMPO-treated HU. Values are mean ± standard error. ***P < 0.001.
Figure 4. Effects of mitoTEMPO on total K+ current (A), current activation, and opening probabilities of voltage-gated potassium (KV) channels (B, C), and Ca2+-activated K+ (BKCa) channels (D, E) in rat cerebral vascular smooth muscle cells. CON, control; HU, hindlimb unweighting; MT, mitoTEMPO; CON + MT, mitoTEMPO-treated control; HU + MT, mitoTEMPO-treated HU. Values are mean ± standard error. *P < 0.05, **P < 0.01, and ***P < 0.001.
Figure 5. Effects of mitoTEMPO on vasoconstriction to cumulative KCl or 5-hydroxytryptamine (5-HT) in rat basilar arteries. CON, control; HU, hindlimb unweighting; MT, mitoTEMPO; CON + MT, mitoTEMPO-treated control; HU + MT, mitoTEMPO-treated HU. Values are mean ± standard error. *P < 0.05 for HU vs. CON, ▲P < 0.05 for HU vs. HU + MT.
Table 1. Body mass (g), soleus mass (mg), and soleus: body mass ratio (mg/g) of rats from the control, mitoTEMPO-treated control, hindlimb unweighting, and mitotempo-treated hindlimbunweighting groups (n = 8 in each group)
Group Initial mass (g) Final mass (g) Soleus mass (mg) Soleus: body mass (mg/g) CON 198.60 ± 1.45 345.85 ± 10.47 134.54 ± 3.62 0.39 ± 0.01 HU 196.73 ± 2.07 336.79 ± 10.74 65.28 ± 1.85*** 0.19 ± 0.01*** CON + MT 199.55 ± 2.67 338.57 ± 8.17 138.48 ± 2.82 0.41 ± 0.01 HU + MT 199.87 ± 1.80 341.89 ± 11.26 74.29 ± 2.12*** 0.22 ± 0.01*** Note. CON, control; HU, hindlimb unweighting; MT, mitoTEMPO; CON+MT, mitoTEMPO-treated control; HU + MT, mitoTEMPO-treated HU. Values are mean ± standard error. ***P < 0.001 vs. control. -
[1] Convertino VA. Mechanisms of microgravity induced orthostatic intolerance: implications for effective countermeasures. J Gravit Physiol, 2002; 9, 1−13. [2] Hargens AR, Watenpaugh DE. Cardiovascular adaptation to spaceflight. Med Sci Sports Exerc, 1996; 28, 977−82. doi: 10.1097/00005768-199608000-00007 [3] Zhang LF. Vascular adaptation to microgravity: what have we learned? J Appl Physiol, 2001; 91, 2415−30. doi: 10.1152/jappl.2001.91.6.2415 [4] Zhang LF. Region-specific vascular remodeling and its prevention by artificial gravity in weightless environment. Eur J Appl Physiol, 2013; 113, 2873−95. doi: 10.1007/s00421-013-2597-8 [5] Maier JAM, Cialdai F, Monici M, et al. The impact of microgravity and hypergravity on endothelial cells. BioMed Res Int, 2015; 2015, 434803. [6] Zhang R, Bai YG, Lin LJ, et al. Blockade of AT1 receptor partially restores vasoreactivity, NOS expression, and superoxide levels in cerebral and carotid arteries of hindlimb unweighting rats. J Appl Physiol, 2009; 106, 251−8. doi: 10.1152/japplphysiol.01278.2007 [7] Zhang R, Ran HH, Ma J, et al. NAD(P)H oxidase inhibiting with apocynin improved vascular reactivity in tail-suspended hindlimb unweighting rat. J Physiol Biochem, 2012; 68, 99−105. doi: 10.1007/s13105-011-0123-1 [8] Shi F, Wang YC, Zhao TZ, et al. Effects of simulated microgravity on human umbilical vein endothelial cell angiogenesis and role of the PI3K-Akt-eNOS signal pathway. PLoS One, 2012; 7, e40365. doi: 10.1371/journal.pone.0040365 [9] Versari S, Villa A, Bradamante S, et al. Alterations of the actin cytoskeleton and increased nitric oxide synthesis are common features in human primary endothelial cell response to changes in gravity. Biochim Biophys Acta, 2007; 1773, 1645−52. doi: 10.1016/j.bbamcr.2007.05.014 [10] Xie MJ, Ma YG, Gao F, et al. Activation of BKCa channel is associated with increased apoptosis of cerebrovascular smooth muscle cells in simulated microgravity rats. Am J Physiol Cell Physiol, 2010; 298, C1489−500. doi: 10.1152/ajpcell.00474.2009 [11] Xue JH, Zhang LF, Ma J, et al. Differential regulation of L-type Ca2+ channels in cerebral and mesenteric arteries after simulated microgravity in rats and its intervention by standing. Am J Physiol Heart Circ Physiol, 2007; 293, H691−701. doi: 10.1152/ajpheart.01229.2006 [12] Islam MS. Calcium signaling: from basic to bedside. In: Islam M. Calcium Signaling. Springer. 2020, 1-6. [13] Liu ZW, Khalil RA. Evolving mechanisms of vascular smooth muscle contraction highlight key targets in vascular disease. Biochem Pharmacol, 2018; 153, 91−122. doi: 10.1016/j.bcp.2018.02.012 [14] Cioffi DL. Redox regulation of endothelial canonical transient receptor potential channels. Antioxid Redox Signal, 2011; 15, 1567−82. doi: 10.1089/ars.2010.3740 [15] Görlach A, Bertram K, Hudecova S, et al. Calcium and ROS: a mutual interplay. Redox Biol, 2015; 6, 260−71. doi: 10.1016/j.redox.2015.08.010 [16] Zhang X, Yan SM, Zheng HL, et al. A mechanism underlying hypertensive occurrence in the metabolic syndrome: cooperative effect of oxidative stress and calcium accumulation in vascular smooth muscle cells. Horm Metab Res, 2014; 46, 126−32. [17] Hall AR, Burke N, Dongworth RK, et al. Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease. Br J Pharmacol, 2014; 171, 1890−906. doi: 10.1111/bph.12516 [18] Wu SN, Lu QL, Wang QL, et al. Binding of FUN14 domain containing 1 with inositol 1, 4, 5-trisphosphate receptor in mitochondria-associated endoplasmic reticulum membranes maintains mitochondrial dynamics and function in hearts in Vivo. Circulation, 2017; 136, 2248−66. doi: 10.1161/CIRCULATIONAHA.117.030235 [19] Youle RJ, Van Der Bliek AM. Mitochondrial fission, fusion, and stress. Science, 2012; 337, 1062−5. doi: 10.1126/science.1219855 [20] Yu R, Jin SB, Lendahl U, et al. Human Fis1 regulates mitochondrial dynamics through inhibition of the fusion machinery. EMBO J, 2019; 38, e99748. [21] Zhang R, Ran HH, Cai LL, et al. Simulated microgravity-induced mitochondrial dysfunction in rat cerebral arteries. FASEB J, 2014; 28, 2715−24. doi: 10.1096/fj.13-245654 [22] Zhang R, Ran HH, Peng L, et al. Mitochondrial regulation of NADPH oxidase in hindlimb unweighting rat cerebral arteries. PLoS One, 2014; 9, e95916. doi: 10.1371/journal.pone.0095916 [23] Peng L, Ran HH, Zhang Y, et al. NADPH oxidase accounts for changes in cerebrovascular redox status in hindlimb unweighting rats. Biomed Environ Sci, 2015; 28, 799−807. doi: 10.1016/S0895-3988(15)30110-0 [24] Ren XL, Zhang R, Zhang YY, et al. Nitric oxide synthase activity in the abdominal aorta of rats is decreased after 4 weeks of simulated microgravity. Clin Exp Pharmacol Physiol, 2011; 38, 683−7. doi: 10.1111/j.1440-1681.2011.05565.x [25] Zhang R, Jia GL, Bao JX, et al. Increased vascular cell adhesion molecule-1 was associated with impaired endothelium-dependent relaxation of cerebral and carotid arteries in simulated microgravity rats. J Physiol Sci, 2008; 58, 67−73. doi: 10.2170/physiolsci.RP010707 [26] Zhang R, Jiang M, Zhang JB, et al. Regulation of the cerebrovascular smooth muscle cell phenotype by mitochondrial oxidative injury and endoplasmic reticulum stress in simulated microgravity rats via the PERK-eIF2α-ATF4-CHOP pathway. Biochim Biophys Acta Mol Basis Dis, 2020; 1866, 165799. doi: 10.1016/j.bbadis.2020.165799 [27] Xue JH, Chen LH, Zhao HZ, et al. Differential regulation and recovery of intracellular Ca2+ in cerebral and small mesenteric arterial smooth muscle cells of simulated microgravity rat. PLoS One, 2011; 6, e19775. doi: 10.1371/journal.pone.0019775 [28] Shah VN, Chagot B, Chazin WJ. Calcium-dependent regulation of ion channels. Calcium Bind Proteins, 2006; 1, 203−12. [29] Cheng J, Wen J, Wang N, et al. Ion channels and vascular diseases. Arterioscler Thromb Vasc Biol, 2019; 39, e146−56. [30] Csordás G, Renken C, Várnai P, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol, 2006; 174, 915−21. doi: 10.1083/jcb.200604016 [31] Rowland AA, Voeltz GK. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol, 2012; 13, 607−25. doi: 10.1038/nrm3440 [32] Sterea AM, El Hiani Y. The role of mitochondrial calcium signaling in the pathophysiology of cancer cells. In: Islam M. Calcium Signaling. Springer. 2020, 747-70. [33] De Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature, 2008; 456, 605−10. doi: 10.1038/nature07534 [34] Chernorudskiy AL, Zito E. Regulation of calcium homeostasis by ER redox: a close-up of the er/mitochondria connection. J Mol Biol, 2017; 429, 620−32. doi: 10.1016/j.jmb.2017.01.017 [35] Eid AH, El-Yazbi AF, Zouein F, et al. Inositol 1, 4, 5-trisphosphate receptors in hypertension. Front Physiol, 2018; 9, 1018. doi: 10.3389/fphys.2018.01018 [36] Finkel T, Menazza S, Holmstrom KM, et al. The ins and outs of mitochondrial calcium. Circ Res, 2015; 116, 1810−9. doi: 10.1161/CIRCRESAHA.116.305484 [37] Vianello A, Casolo V, Petrussa E, et al. The mitochondrial permeability transition pore (PTP)-an example of multiple molecular exaptation? Biochim Biophys Acta Bioenerg, 2012; 1817, 2072−86. doi: 10.1016/j.bbabio.2012.06.620 [38] Feno S, Butera G, Reane DV, et al. Crosstalk between calcium and ROS in pathophysiological conditions. Oxid Med Cell Longev, 2019; 2019, 9324018. [39] Kozlov AV, Lancaster Jr JR, Meszaros AT, et al. Mitochondria-meditated pathways of organ failure upon inflammation. Redox Biol, 2017; 13, 170−81. doi: 10.1016/j.redox.2017.05.017 [40] Avila G, De La Rosa JA, Monsalvo-Villegas A, et al. Ca2+ channels mediate bidirectional signaling between sarcolemma and sarcoplasmic reticulum in muscle cells. Cells, 2019; 9, 55. doi: 10.3390/cells9010055 [41] Bartok A, Weaver D, Golenár T, et al. IP3 receptor isoforms differently regulate ER-mitochondrial contacts and local calcium transfer. Nature Communications, 2019; 10, 3726. doi: 10.1038/s41467-019-11646-3 [42] Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell, 2005; 120, 483−95. doi: 10.1016/j.cell.2005.02.001 [43] Archer SL. Mitochondrial dynamics-mitochondrial fission and fusion in human diseases. N Engl J Med, 2013; 369, 2236−51. doi: 10.1056/NEJMra1215233 [44] Cipolat S, De Brito OM, Dal Zilio B, et al. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA, 2004; 101, 15927−32. doi: 10.1073/pnas.0407043101 [45] Guerrero-Hernandez A, Sanchez-Vazquez VH, Martinez-Martinez E, et al. Sarco-endoplasmic reticulum calcium release model based on changes in the luminal calcium content. In: Islam M. Calcium Signaling. Springer. 2020, 337-70. [46] Alirol E, James D, Huber D, et al. The mitochondrial fission protein hFis1 requires the endoplasmic reticulum gateway to induce apoptosis. Mol Biol Cell, 2006; 17, 4593−605. doi: 10.1091/mbc.e06-05-0377 [47] Vallese F, Barazzuol L, Maso L, et al. ER-mitochondria calcium transfer, organelle contacts and neurodegenerative diseases. In: Islam M. Calcium Signaling. Springer. 2020, 719-46. [48] Zhang R, Ran HH, Gao YL, et al. Differential vascular cell adhesion molecule-1 expression and superoxide production in simulated microgravity rat vasculature. EXCLI J, 2010; 9, 195−204. [49] Jiang M, Wang HM, Liu ZF, et al. Endoplasmic reticulum stress-dependent activation of iNOS/NO-NF-κB signaling and NLRP3 inflammasome contributes to endothelial inflammation and apoptosis associated with microgravity. FASEB J, 2020; 34, 10835−49. doi: 10.1096/fj.202000734R -




 下载:
下载:



 Quick Links
Quick Links