-
With advances in science and technology, space flights have become a reality. Meanwhile, the length of space missions has increased. When astronauts travel to space, they suffer from various biological stressors, including microgravity, radiation, loss of the light-dark cycle, and confinement, which may cause adverse psychological and physiological effects[1]. Clarifying how these biological stressors affect the human body may provide new ideas for protecting astronauts. Microgravity is a significant stressor that has several adverse consequences for the human body. Microgravity can cause many physiological changes in the body, such as osteopenia[2], neurovestibular problems, cognitive alterations[3], bacterial or viral infections[4], venous thromboembolism[5], carbohydrate metabolism and lipid metabolism disorder[6,7], dysfunction in gastric movement rhythm, and intestinal mucosal barrier damage[8]. Several studies have shown that immune cells are vulnerable to microgravity. Microgravity influences immune cell proliferation, differentiation, activation, and metabolism of immune cells[9]. Macrophages, the body's first line of defense against foreign particles, have been the focus of research investigating the microgravity-induced effects on immune cells. Microgravity inhibits the differentiation of hematopoietic stem cells into macrophages and impairs their proliferation. In addition, microgravity can restrain the polarization of macrophages stimulated by lipopolysaccharide (LPS) or IL-4 and alter their gene expression profiles of macrophages[1].
Kupffer cells (KCs) are a special type of resident macrophage in the liver that constitute the majority of resident tissue macrophages in the body. KCs, which are located in the liver sinusoids, can devour pathogens from the gastrointestinal tract. Hence, KCs are the first line of defense for the liver and the final component of gut barrier function[10]. Like many other macrophages, KCs are plastic and can change their phenotype (M1/M2) during the development of liver diseases[11]. KCs play an important role in liver homeostasis maintenance.
To date, few studies have explored changes in KCs under microgravity. We found that simulated microgravity can inhibit KC proliferation by downregulating LMO2 and EZH2[12]. In this study, we analyzed the transcriptome sequencing results of KCs exposed to normal gravity (NG) and simulated microgravity (SMG) and found that SMG can promote apoptosis and alter the inflammatory state of KCs and that the M1 polarization of KCs can be a crucial cause of apoptosis.
-
KCs (from BALB/c mice) were purchased from Guangzhou Jennie Biotech Co., Ltd. (Guangzhou, China). The KCs were cultured in RPMI (Hyclone) supplemented with 10% fetal bovine serum (Dakewe) and 1% Penicillin-Streptomycin Solution (Thermo Fisher Scientific). In the SMG model, KCs (4.5 × 106) were mixed with 250 mg Cytodex-3 microcarriers (Cytiva) and placed into a high-aspect rotating vessel. The high-aspect-rotating vessel was connected to a rotary cell culture system (RCCS) (Synthecon) and rotated at 18 rpm. In the NG model, the KCs were cultured in T75 cell culture flasks (Nest) without microcarriers. KCs were collected, and three biological replicates were analyzed for each sample group.
-
The results of transcriptome sequencing of KCs from the NG and SMG groups were obtained from our former study[12]. Transcriptome sequencing was performed by Beijing Tsingke Biotech Co., Ltd. GSEA and heat maps were generated using the R language. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genome Pathway (KEGG) analyses were performed using the DAVID database (https://david.ncifcrf.gov)[13]. Protein-protein interaction (PPI) was conducted by STRING database (http://string-db.org/)[14].
-
Total RNA was extracted from KCs using the FastPure Cell/Tissue Total RNA Isolation Kit V2 (Vazyme) following the manufacturer’s instructions and reverse transcribed into cDNA using the HiScript III 1st Strand cDNA Synthesis Kit (Vazyme). qPCR was performed using the Taq Pro Universal SYBR qPCR Master Mix (Vazyme) on a Light Cycler 480 instrument (Roche). The primers (Tsingke) used for each gene are shown in Table 1. All of the samples were normalized by GAPDH and the fold changes were calculated by relative quantification (2 - ΔΔCt).
Table 1. Primers used in qPCR
Gene name Forward (5'-3') Rorward (5'-3') IL1B TGCCACCTTTTGACAGTGATG TGATGTGCTGCTGCGAGATT TNFA GATCGGTCCCCAAAGGGATG CCACTTGGTGGTTTGTGAGTG CASP3 AGCTTGGAACGGTACGCTAA GAGTCCACTGACTTGCTCCC CASP9 ACCTTCCCAGGTTGCCAATG CCCCGAGCCTCATGAAGTTTT BCL2L11 TTGTTTCCCTTGCCTCCTCG AGGTTGCTTGGCCATTTGGT GAPDH CAGGAGAGTGTTTCCTCGTCC TGAGGTCAATGAAGGGGTCG -
Proteins were extracted using a Protein Extraction Kit (GenePool). An SDS-PAGE Gel Kit (GenePool) was used to configure the stacking and separation gels. Then, 25 μg samples were placed in each well. Electrophoresis conditions: Stacking gel at a constant pressure of 80 V for 20 min, and the separation gel was maintained at a constant pressure of 120 V until bromophenol blue reached the bottom of the gel. After the protein was transferred onto a PVDF membrane, the membrane was incubated in Milk Blocking Buffer (GenePool / BSA Blocking buffer (GenePool) for one hour. The primary antibody was diluted with 1% BSA in 5% milk. The following primary antibodies included: CASP9 antibody (Abcam), CASP3 antibody (Abcam), Cleaved-CASP3 antibody (Abcam), and GAPDH antibody (Abcam). PVDF membrane was incubated in diluted primary antibody at 4 °C overnight. After washed thrice with TBST, the PVDF membrane was incubated with a diluted secondary antibody for 50 min. The PVDF membrane was washed 4 times with TBST. Signals were developed using an ECL Plus Western Blot Kit (GenePool).
-
KC apoptosis was measured using an Apoptosis Kit (4A Biotech) according to the manufacturer’s instructions. Briefly, KCs were washed with 1 mL of 1×Annexin V binding buffer. Then, KCs were resuspended with 100 μL 1×Annexin V binding buffer, adding Annexin V-Alexa Fluid 647 5 μL/tube, and incubated in the dark for 5 min. Finally, 5 μL PI and 400 μL 1×Annexin V binding buffer were added to each tube and incubated in the dark for 5 min. KCs were detected within 1 h using flow cytometry (Beckman). The JC-1 Mitochondrial Membrane Potential Detection Kit (Beyotime) was used to measure mitochondrial membrane potential. Briefly, KCs were incubated with 1 mL JC‐1 staining solution at 37 °C for 20 min. The KCs were then centrifuged and suspended in 1×JC-1 staining buffer. Finally, KCs were examined using flow cytometry (Beckman).
-
Transmission electron microscopy (TEM) images were acquired using an HT7700 instrument. The sample preparation methods are described in a previously published article[15].
-
All values were presented as mean ± SD. Prism 8.0.2 (GraphPad Inc., La Jolla, CA, USA) was used for statistical analyses. Comparisons between the two groups were performed using Student’s t-test. Significant differences are indicated by *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
-
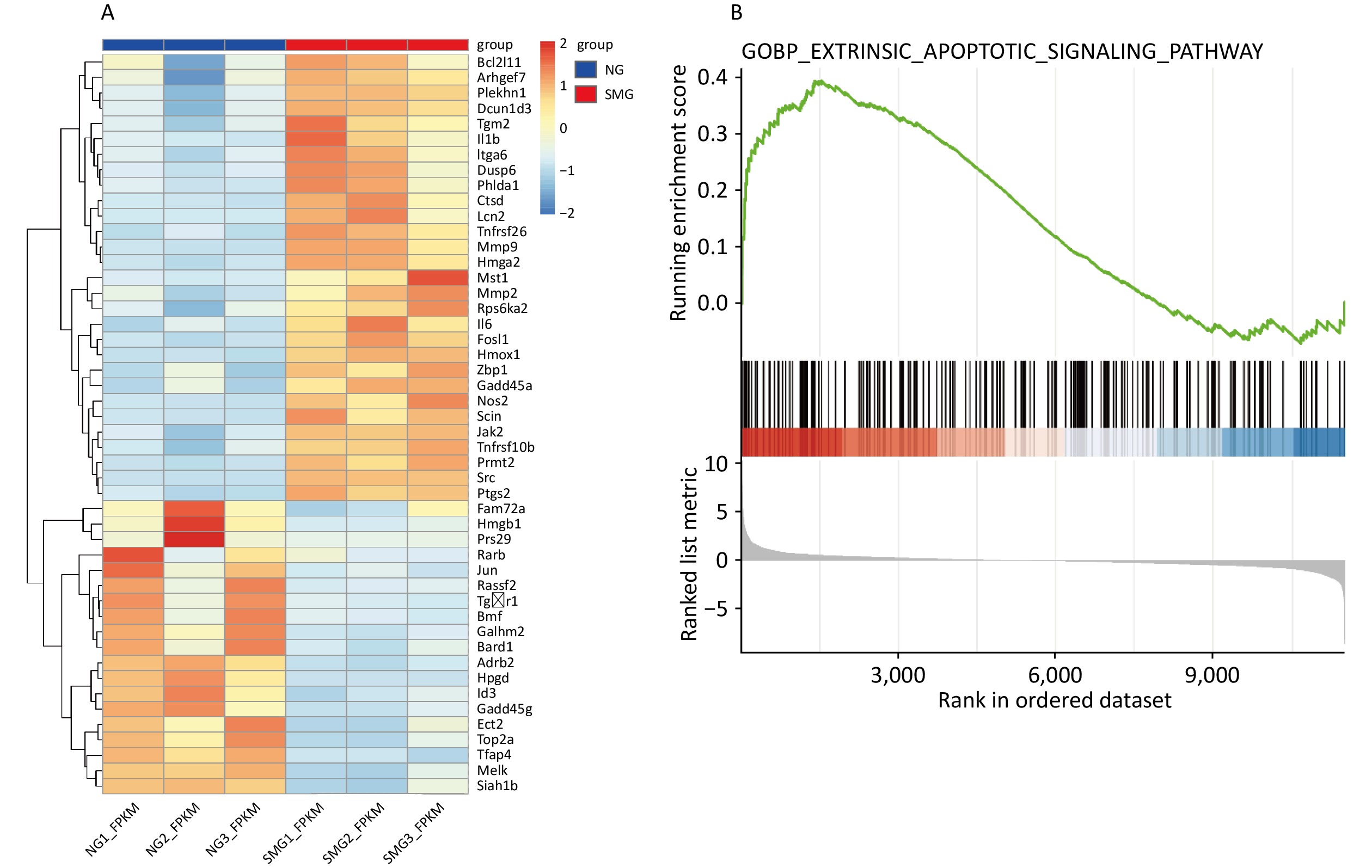
To explore the effects of microgravity on KCs[12], we performed GO and KEGG analyses were performed using DAVID. We performed GO and KEGG analysis on 1,432 differentially expressed genes (DEGs), including 801 downregulated DEGs and 631 upregulated DEGs. 48 DEGs were enriched into “GO(BP): positive regulation of apoptotic process”. In KEGG analysis, 16 DEGs were enriched into “Apoptosis,” and 15 DEGs were enriched into “p53 signaling pathway” (Table 2). To figure out the expression of DEGs relevant to apoptosis, the heatmap of the expression of 48 DEGs enriched into “GO(BP): positive regulation of apoptotic process” was drawn (Figure 1A). Importantly, BCL2L11 expression was significantly upregulated in the SMG group. GSEA also indicated that microgravity promoted KC apoptosis (Figure 1B).
Table 2. DEGs enriched into “positive regulation of apoptotic process (GO)”, “apoptosis (KEGG)”, “p53 signaling pathway (KEGG)”
TERM Differentially expressed genes P-value GO(BP): positive regulation
of apoptotic processTOP2A, HPGD, SRC, DCUN1D3, ADRB2, HMGB1, PTGS2, BCL2L11, SCIN, RASSF2, CALHM2, RPS6KA2, HMOX1, BMF, PLEKHN1, JAK2, ECT2, PHLDA1, CTSD, TGM2, ZBP1, BARD1, JUN, NOS2, PRMT2, GADD45A, MMP2, MST1, HMGA2, TNFRSF10B, SIAH1B, MMP9, DUSP6, TGFBR1, GADD45G, FOSL1, IL6, TFAP4, MELK, RPS29, IL1B, LCN2, RARB, ID3, ITGA6, TNFRSF26, ARHGEF7, FAM72A < 0.001 KEGG: Apoptosis JUN, PARP4, GADD45A, TNFRSF10B, PIK3CD, FOS, LMNB2, CTSS, LMNB1, GADD45G, TUBA1B, BCL2L11, CTSL, CAPN2, BIRC5, CTSD < 0.001 KEGG: p53 signaling pathway RRM2, SIVA1, GADD45A, SERPINE1, TNFRSF10B, IGF1, SIAH1B, GADD45G, CCNB2, CCNB1, CCND2, CCNG2, SESN1, CDK1, GTSE1 < 0.001 Note. GO, Gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genome Pathway; DEGs, differentially expressed genes. 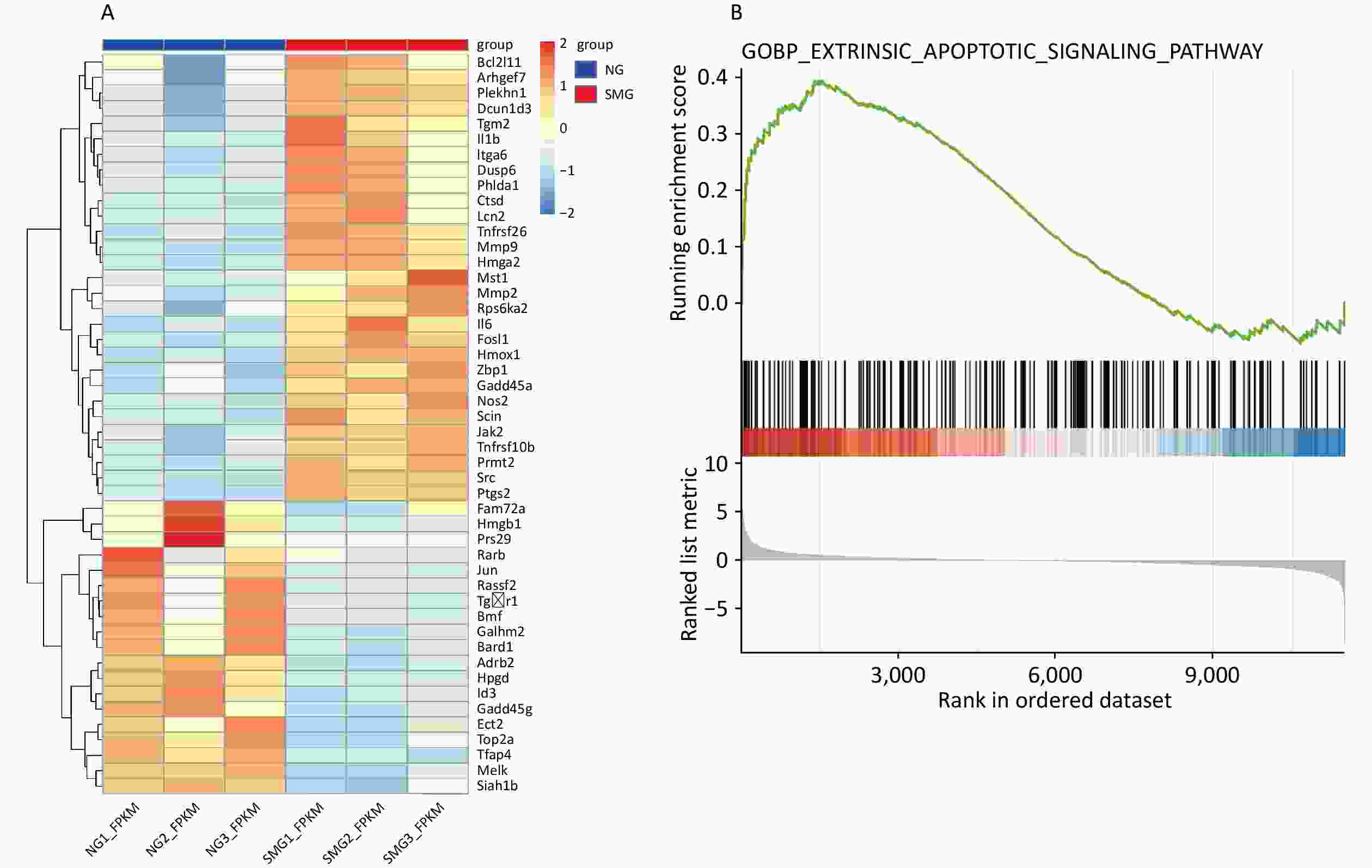
Figure 1. Transcriptome sequencing results showed that SMG can promote apoptosis of KCs. (A) Heatmap of 48 differentially expressed genes (DEGs) enriched into “GO(BP): positive regulation of apoptotic process”. (B) GSEA analysis showed microgravity can influence the apoptosis of Kupffer cells (KCs) (Extrinsic apoptosis signaling pathway, P < 0.001) exposed to simulated microgravity (SMG) for 3 d.
-
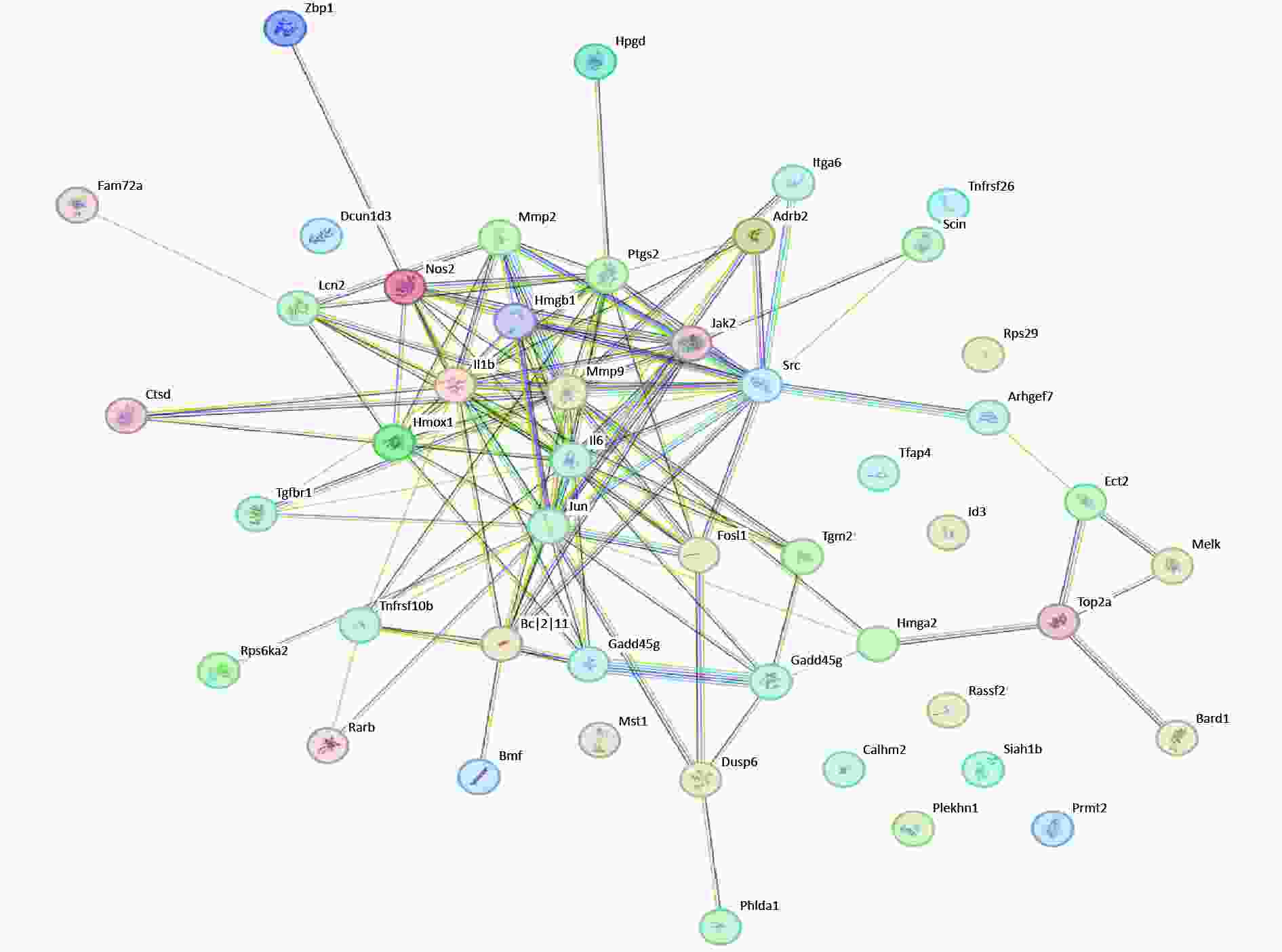
PPI analysis of apoptosis-related DEGs was performed. BCL2L11 and GADD45G regulate apoptosis and interact extensively with other proteins. Moreover, NOS2, IL1B, and IL-6, which are relevant to inflammation, play a central role (Figure 2). These results indicate that inflammation and apoptosis may be inseparable.
-
KCs were stained with annexin V and propidium iodide (PI) to distinguish live, early apoptotic, late apoptotic, and necrotic cells. Exposure to microgravity for three days could cause KC apoptosis, including early and late apoptosis (Figure 3A). A JC-1 Mitochondrial Membrane Potential Detection Kit was used to measure the mitochondrial membrane potential of KCs. When the mitochondrial membrane potential is high, JC-1 aggregates in the mitochondrial matrix to form a polymer. When the mitochondrial membrane potential is low, JC-1 exists as a monomer. We measured the mitochondrial membrane potential of KCs using flow cytometry. SMG reduced the mitochondrial membrane potential of KCs because KCs from the SMG group contained more JC-1 monomers, although this difference was not statistically significant. (Figure 3B). Intracellular vacuolation and autophagy were observed in the KCs from the SMG group (Figure 3C).
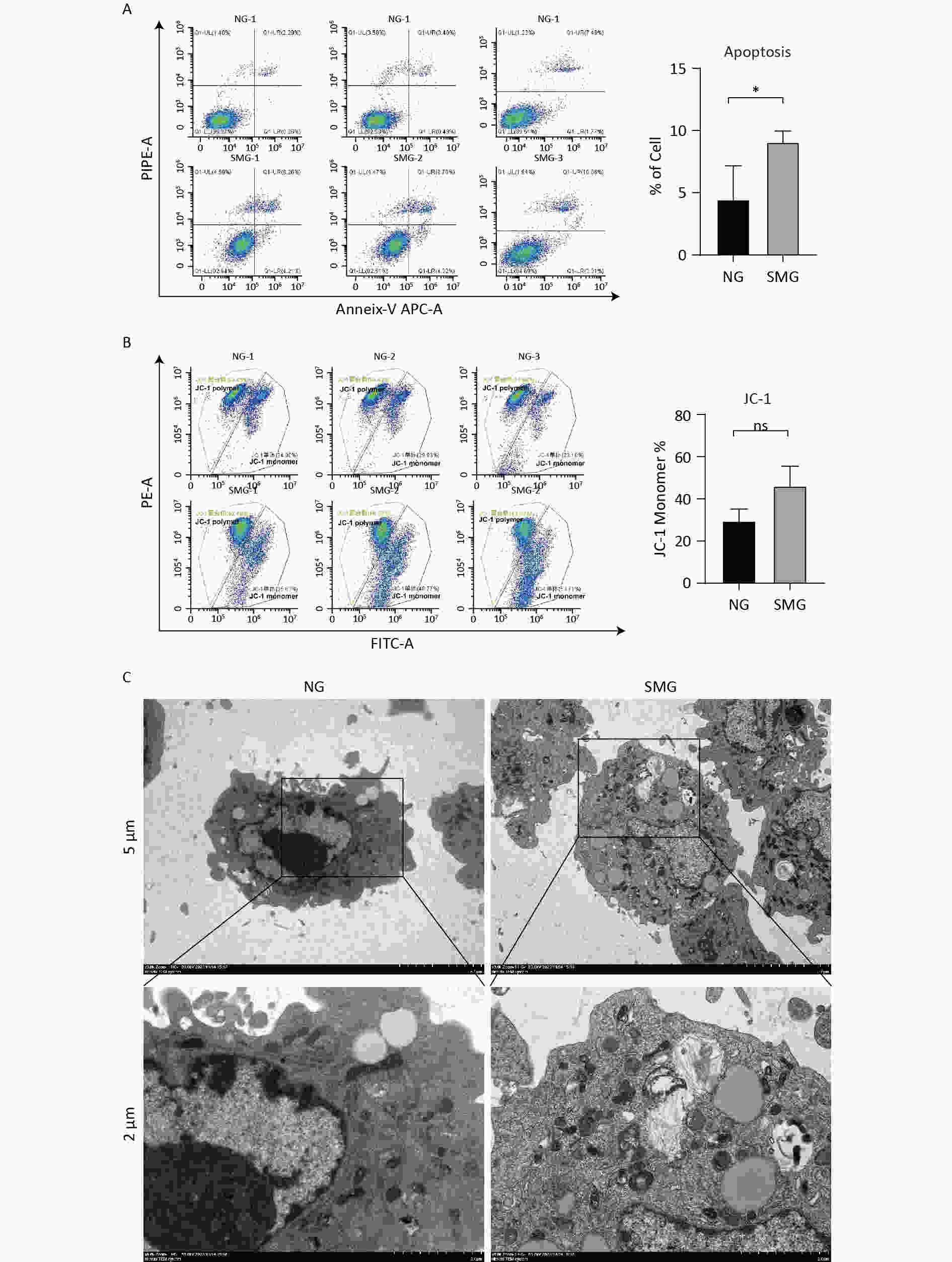
Figure 3. SMG promoted the apoptosis of KCs. (A) Annexin-V/PI assay measuring the percentage of Kupffer cells (SMG = 3 d RCCS at 18 rpm, NG = 3 d in T75). Live cells (annexin-V-/PI-, LL), early apoptotic cells (annexin-V+/PI-, LR), late apoptotic cells (annexin-V+/PI+, UR) and necrotic cells (annexin-V-/PI+, UL). (B) JC-1 Assay detecting the change of mitochondrial membrane potential in Kupffer cells (SMG = 3 d RCCS at 18 rpm, NG = 3 d in T75). (C) Transmission electron microscope detection KCs cultured in T75 (NG) and in RCCS at 18 rpm for 3 d simulated microgravity (SMG).
-
SMG increased the expression of apoptosis-related genes including CASP3 and CASP9 (Figure 4A). BCL2L11 expression was upregulated in the SMG group, promoting apoptosis. As an important protein involved in the mitochondrial apoptotic pathway, CASP9 protein levels were detected using western blotting (Figure 4B). In summary, SMG upregulates the expression of apoptosis-related genes, including CASP3, CASP9, and BCL2L11. More importantly, the CASP9 protein level increased in the SMG group. The CASP3 protein level in KCs exposed to SMG for 3 d was lower than that in KCs in NG-3d, while cleaved-CASP3 increased in KCs cultured in RCCS for 3 d, indicating apoptosis activation (Figure 4C).
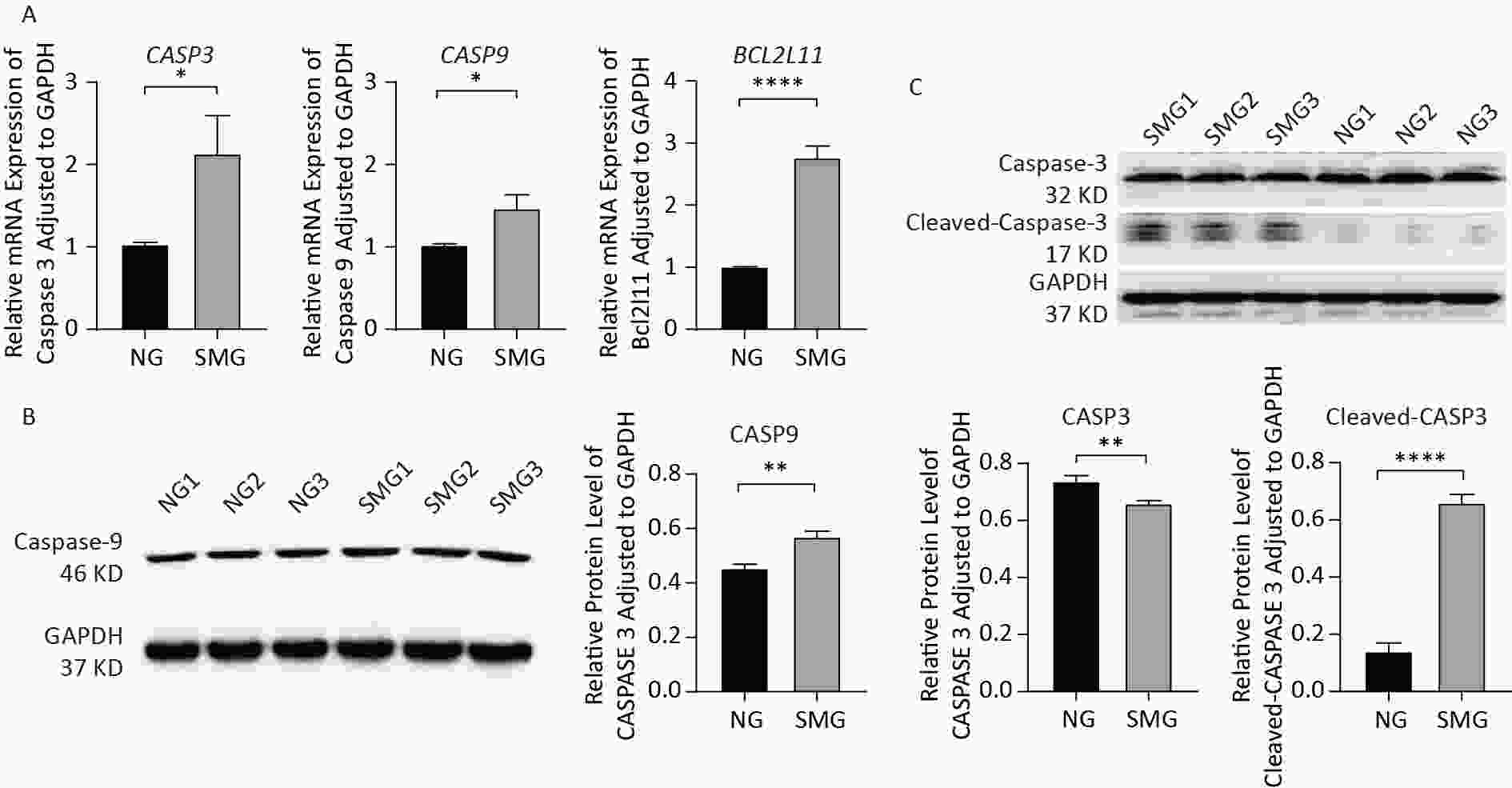
Figure 4. SMG changed expression of apoptosis-related genes and proteins. (A) SMG (in RCCS at 18 rpm for 3 d) could increase the expression of apoptosis-related genes: CASP3, CASP9, and BCL2L11. (B) CASP9 protein level was upregulated in SMG (3 d RCCS at 18 rpm) group. (C) Cleaved-CASP3 protein level was upregulated in the SMG group, while CASP3 protein level was downregulated in the SMG group.
-
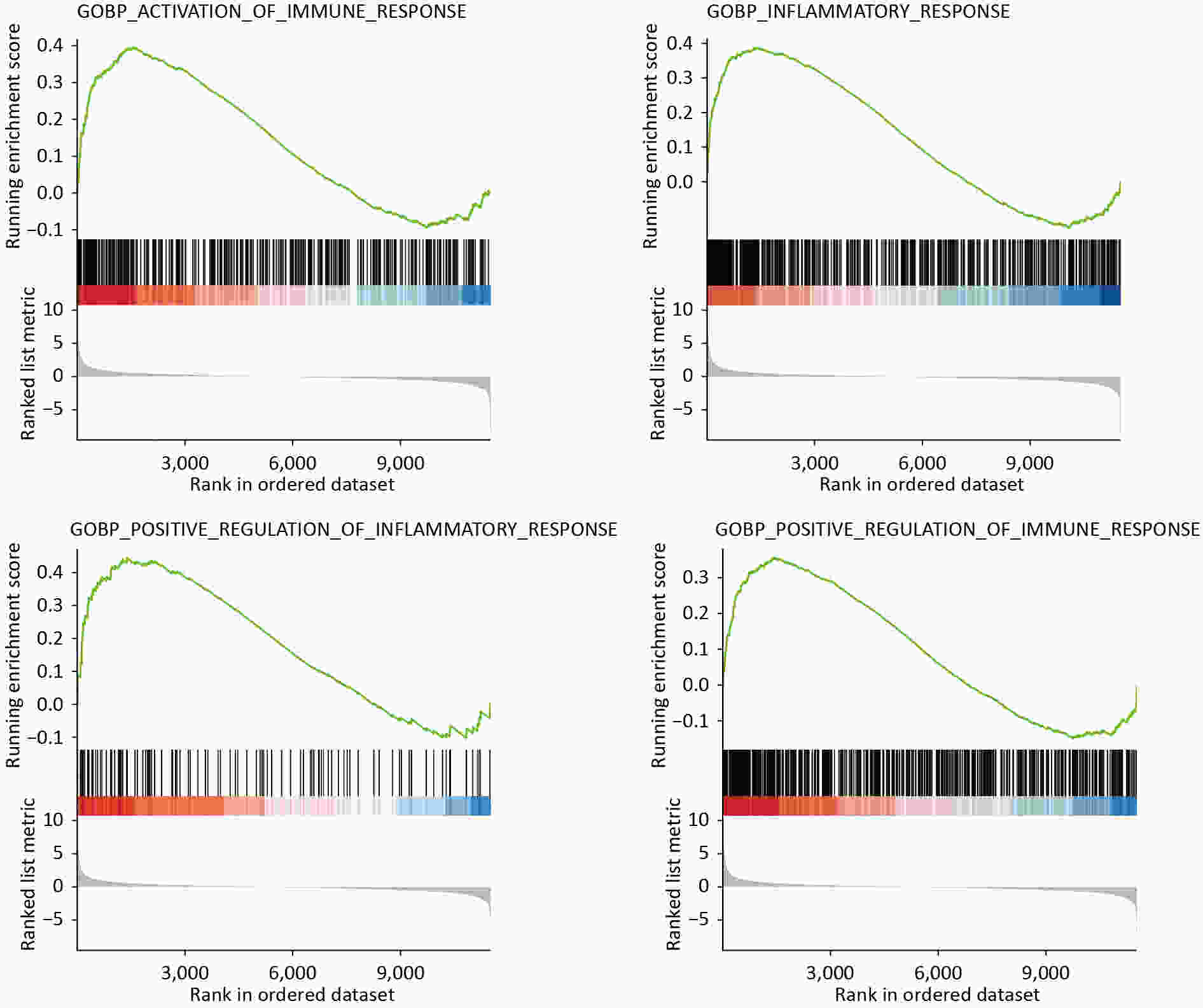
GO analysis showed that SMG altered the inflammatory state of KCs. DEGs were enriched into “inflammatory response (BP)” and “immune response (BP)” (Table 3). GSEA also showed many biological processes, including “activation of immune response” (P < 0.001, “inflammatory response” (P < 0.001), “positive regulation of inflammatory response” (P = 0.003), “positive regulation of immune response” (P < 0.001) (Figure 5). By analyzing transcriptome sequencing results, we found that KCs switched to a pro-inflammatory state.
Table 3. DEGs enriched into “inflammatory response (GO)”, “immune response (GO)”
TERM Differentially expressed gene P-value GO (BP): inflammatory response ECM1, IL1RN, CSF1, HMGB2, PIK3CD, HMGB1, CXCL2, CYSLTR1, C3AR1, OLR1, CCR7, PTGIR, PARP4, IGFBP4, SLC11A1, CYBB, ACOD1, NAIP2, IL1A, IL23A, IL1B, ADAM8, SMPDL3B, CD44, EPHA2, PTGER4, CX3CR1, CFH, PTGER2, CD5L, PTGS2, AIF1, HDAC9, PTGS1, CCL7, CLEC7A, CCL5, CCL4, CCL3, CCL2, ZFP580, CCR1, CCL22, NOS2, TREX1, LY86, IFI202B, CXCL10, IL6, NLRP10, NFKBID < 0.001 GO (BP): inflammatory response CD86, CX3CR1, IL1RN, CSF3, MARCHF1, CFH, CLEC4N, CD80, CXCR4, CST7, CXCL2, CTSS, TNFSF13B, CCL7, CTSL, CCL5, CCL4, CCL3, CCL2, SERPINB9B, CCR7, JAK2, CCR1, H60B, H2-EB1, CCL22, TREX1, OSM, LIF, LY86, IFI44, H2-AA, RAET1D, CXCL10, IL1A, IL6, CLEC4D, RAET1E, IL23A, IL1B, CLEC4E, ULBP1, H2-AB1 0.009 -
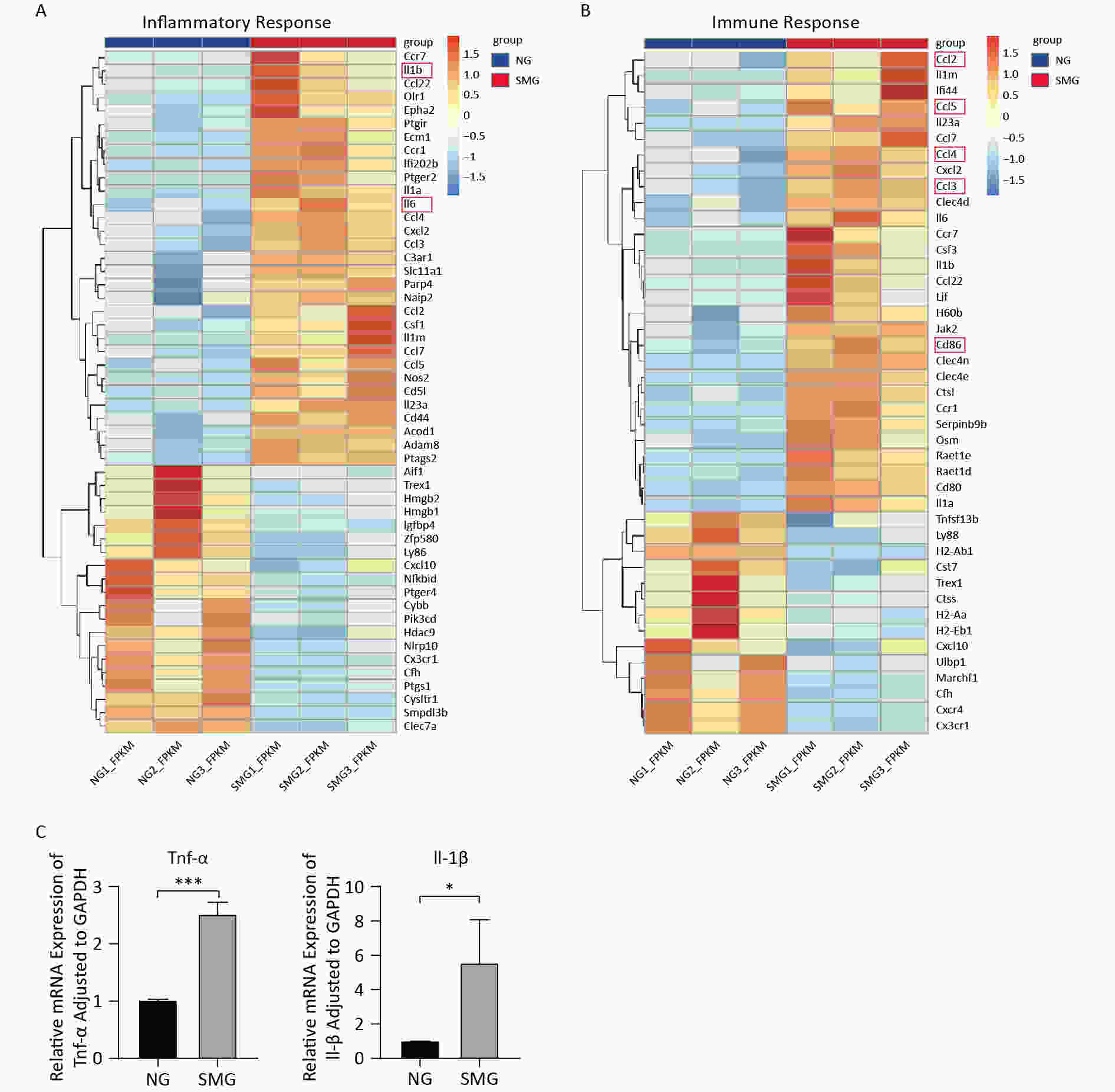
To better understand the expression of DEGs enriched to “inflammatory response (BP)” and “immune response (BP),” heat maps were drawn (Figure 6A, 6B). IL1B and IL-6, two cytokines that are usually released by M1 macrophages, were upregulated. In addition, KCs from the SMG group highly expressed the M1 macrophage cell-surface molecular marker CD86. To verify whether SMG could drive M1 polarization of KCs, we performed qPCR. The expression of TNFA and IL1B increased (Figure 6C), suggesting that SMG promotes M1 polarization of KCs.
-
Astronauts will come across various challenges such as microgravity, ionizing radiation, and claustrophobia, when they conduct space missions, microgravity is the main factor that affects the homeostasis of the human body including the immune system which is susceptible to microgravity[9]. However, bacterial and viral infections can also occur during spaceflights. Half the astronauts on the Apollo mission had bacterial or viral infections[4]. When traveling to space, the virulence of pathogens will increase[4] and it is important to maintain homeostasis of the immune system to counter various virulence-increased pathogens. Therefore, clarifying the effect of microgravity on immune cells and the immune system is vital to ensuring the health of astronauts traveling in space.
The immune system is also susceptible to microgravity. Microgravity inhibits the differentiation of hematopoietic stem/progenitor cells into dendritic cells[16]. Space travel increases the proportion of neutrophils in the blood[17]. However, the effect of microgravity on the number of NK cells remains controversial. SMG did not affect the number of NK cells when a rotary bioreactor system was used to simulate microgravity. Microgravity inhibits the proliferation of NK cells and promotes their apoptosis of NK cells in two-dimensional rotating wall vessels[18]. Shi et al. found that SMG inhibited the proliferation and differentiation of macrophages[1]. In addition to the number of immune cells, microgravity affects immune cell function. For example, microgravity restricts the antigen-presenting ability of dendritic cells[19,20]. Spaceflight impairs the phagocytic ability of monocytes[21]. Therefore, microgravity can affect immune cells in several ways.
KCs, a special type of macrophage residing in the liver, play the role of sentinels in the liver, which is essential for liver homeostasis. KCs phagocytose the products of gastrointestinal microorganisms to maintain a tolerogenic immune environment in the liver. KCs also play an important role in many diseases such as alcoholic liver disease[22], non-alcoholic steatohepatitis[23], hepatic ischemia/reperfusion injury[24], and acute liver injuries[25]. Few studies have focused on the effects of microgravity on KCs. Racine et al. found that the number of KCs decreased in the livers of flight animals[26]. Our previous study showed that SMG inhibited KC proliferation by downregulating LMO2 and EZH2[12].
In this study, further research was conducted to analyze the transcriptome sequencing results of KCs exposed to NG and SMG. The RCCS developed by NASA was used to model the SMG[27]. In normal cells, phosphatidylserine (PS) is located on the inner side of the cell membrane. However, in early apoptotic cells, PS flips from the inner side of the cell membrane to the surface of the cell membrane. Annexin V is a Ca2+-dependent phospholipid-binding protein that can bind with high affinity to PS. Propidine iodide (PI) is a nucleic acid dye that cannot penetrate the complete cell membrane; however, in the late stage of apoptosis and necrotic cells, PI can penetrate the cell membrane and stain the nucleus red. Therefore, by combining annexin V with PI, early and late apoptotic cells can be detected in the cell population. An Annexin-V/ PI assay was performed, and the results showed that KCs from the SMG had higher levels of apoptosis, and transmission electron microscopy detected intracellular vacuolation in KCs from the SMG group. Mitochondrial membrane depolarization, which mainly occurs during the early stages of apoptosis, is an important sign of apoptosis. When the mitochondrial membrane potential is low, JC-1 exists as a monomer. KCs from the SMG group contained more JC-1 monomers. CASP3 is crucial for apoptosis because its spliced form cleaved-CASP3 participates in the occurrence of cell apoptosis. We found that cleaved-CASP3 was notably increased in KCs from the SMG group, whereas the level of CASP3 was reduced in KCs from the SMG group. The decrease in CASP3 is reasonable and may be related to the fact that more CASP3 is sliced into cleaved CASP3. Bioinformatics analysis has shown that SMG can promote the apoptosis of KCs and that the p53 signaling pathway, which can regulate apoptosis, is involved[28]. These results indicate that SMG promotes apoptosis in KCs.
Apoptosis occurs via two main pathways: the extrinsic (death receptor pathway) and intrinsic (mitochondrial) pathways. The mitochondrial pathway arises from a series of stimuli that initiate intracellular signals, such as radiation, toxins, hypoxia, and free radicals[29]. Next, the mitochondrial transmembrane potential is lost, and pro-apoptotic proteins are released into the cytosol, which further promotes the formation of “apoptosome.” Meanwhile, CASP9 activation is an important process in the mitochondrial pathway[29]. Members of the Bcl-2 protein family can manipulate apoptotic mitochondrial events[30]. Simultaneously, p53 regulates the Bcl-2 family of proteins[31]. In this study, we provide sufficient evidence to prove that SMG can promote KCs apoptosis through the mitochondrial pathway: 1) SMG can reduce the mitochondrial membrane potential of KCs; 2) SMG can increase CASP9 expression at the mRNA and protein levels; 3) BCL2L11, which belongs to the Bcl-2 protein family and can induce apoptosis, was upregulated in the SMG group; and 4) KEGG analysis showed that the p53 signaling pathway was involved.
PPI analysis of the apoptosis-related DEGs revealed that some pro-inflammatory cytokines (IL1B and IL-6) were involved in apoptosis. GO and GSEA also indicated that SMG could shift KCs to a pro-inflammatory state. In addition, the expression of IL1B, IL-6, CCL2, CCL3, CCL4, and CCL5 increased in KCs exposed to SMG. Importantly, CD 86, a surface marker of M1 macrophages, was upregulated[32]. To figure out whether M1 polarization occurred in KCs from the SMG group, qPCR was conducted and revealed that the expression of TNFA and IL1B were significantly increased. This suggests that SMG can induce M1 polarization in KCs. Interestingly, in the intrinsic pathway apoptosis, TNF-α/TNFR1 plays an essential role[29,33]. Presumably, M1-polarized KCs promote apoptosis. Macrophages are usually activated by LPS, IFN-γ, and GM-CSF in the process of M1 polarization[34]. Shi et al.[1] found microgravity can inhibit both M1 and M2 polarization of macrophages under IFN-γ and LPS or IL-4 stimulation. These results contradict those of our study. One possible reason for this is that KCs differ from bone marrow-derived macrophages. whether or not, how can SMG promote M1 polarization of KCs worth further exploration.
In this study, we found that SMG promoted KC apoptosis. The results showed that SMG facilitated the apoptosis of KCs through the mitochondrial pathway, meanwhile, SMG activated KCs into M1 polarization, which could secrete TNFA to promote apoptosis.
-
Not applicable
doi: 10.3967/bes2024.141
Simulated Microgravity can Promote the Apoptosis and Change Inflammatory State of Kupffer Cells
-
Abstract:
Objective In this study, we analyzed the transcriptome sequences of Kupffer cells exposed to simulated microgravity for 3 d and conducted biological experiments to determine how microgravity initiates apoptosis in Kupffer cells. Methods Rotary cell culture system was used to construct a simulated microgravity model. GO and KEGG analyses were conducted using the DAVID database. GSEA was performed using the R language. The STRING database was used to conduct PPI analysis. qPCR was used to measure the IL1B, TNFA, CASP3, CASP9, and BCL2L11 mRNA expressions. Western Blotting was performed to detect the level of proteins CASP3 and CASP 9. Flow cytometry was used to detect apoptosis and mitochondrial membrane cells. Transmission electron microscopy was used to detect changes in the ultrastructure of Kupffer cells. Results Transcriptome Sequencing indicated that simulated microgravity affected apoptosis and the inflammatory state of Kupffer cells. Simulated microgravity improved the CASP3, CASP9, and BCL2L11 expressions in Kupffer cells. Annexin-V/ PI and JC-1 assays showed that simulated microgravity promoted apoptosis in Kupffer cells. Simulated microgravity causes M1 polarization in Kupffer cells. Conclusion Our study found that simulated microgravity facilitated the apoptosis of Kupffer cells through the mitochondrial pathway and activated Kupffer cells into M1 polarization, which can secrete TNFA to promote apoptosis. -
Key words:
- Microgravity /
- Apoptosis /
- Kupffer cell /
- Polarization
The authors declare no conflicts of interest regarding the publication of this paper.
Not applicable.
注释:1) AUTHORS’ CONTRIBUTIONS: 2) DECLARATION OF COMPETING INTEREST: 3) ETHICS APPROVAL AND CONSENT TO PARTICIPATE: -
Figure 1. Transcriptome sequencing results showed that SMG can promote apoptosis of KCs. (A) Heatmap of 48 differentially expressed genes (DEGs) enriched into “GO(BP): positive regulation of apoptotic process”. (B) GSEA analysis showed microgravity can influence the apoptosis of Kupffer cells (KCs) (Extrinsic apoptosis signaling pathway, P < 0.001) exposed to simulated microgravity (SMG) for 3 d.
Figure 3. SMG promoted the apoptosis of KCs. (A) Annexin-V/PI assay measuring the percentage of Kupffer cells (SMG = 3 d RCCS at 18 rpm, NG = 3 d in T75). Live cells (annexin-V-/PI-, LL), early apoptotic cells (annexin-V+/PI-, LR), late apoptotic cells (annexin-V+/PI+, UR) and necrotic cells (annexin-V-/PI+, UL). (B) JC-1 Assay detecting the change of mitochondrial membrane potential in Kupffer cells (SMG = 3 d RCCS at 18 rpm, NG = 3 d in T75). (C) Transmission electron microscope detection KCs cultured in T75 (NG) and in RCCS at 18 rpm for 3 d simulated microgravity (SMG).
Figure 4. SMG changed expression of apoptosis-related genes and proteins. (A) SMG (in RCCS at 18 rpm for 3 d) could increase the expression of apoptosis-related genes: CASP3, CASP9, and BCL2L11. (B) CASP9 protein level was upregulated in SMG (3 d RCCS at 18 rpm) group. (C) Cleaved-CASP3 protein level was upregulated in the SMG group, while CASP3 protein level was downregulated in the SMG group.
Table 1. Primers used in qPCR
Gene name Forward (5'-3') Rorward (5'-3') IL1B TGCCACCTTTTGACAGTGATG TGATGTGCTGCTGCGAGATT TNFA GATCGGTCCCCAAAGGGATG CCACTTGGTGGTTTGTGAGTG CASP3 AGCTTGGAACGGTACGCTAA GAGTCCACTGACTTGCTCCC CASP9 ACCTTCCCAGGTTGCCAATG CCCCGAGCCTCATGAAGTTTT BCL2L11 TTGTTTCCCTTGCCTCCTCG AGGTTGCTTGGCCATTTGGT GAPDH CAGGAGAGTGTTTCCTCGTCC TGAGGTCAATGAAGGGGTCG Table 2. DEGs enriched into “positive regulation of apoptotic process (GO)”, “apoptosis (KEGG)”, “p53 signaling pathway (KEGG)”
TERM Differentially expressed genes P-value GO(BP): positive regulation
of apoptotic processTOP2A, HPGD, SRC, DCUN1D3, ADRB2, HMGB1, PTGS2, BCL2L11, SCIN, RASSF2, CALHM2, RPS6KA2, HMOX1, BMF, PLEKHN1, JAK2, ECT2, PHLDA1, CTSD, TGM2, ZBP1, BARD1, JUN, NOS2, PRMT2, GADD45A, MMP2, MST1, HMGA2, TNFRSF10B, SIAH1B, MMP9, DUSP6, TGFBR1, GADD45G, FOSL1, IL6, TFAP4, MELK, RPS29, IL1B, LCN2, RARB, ID3, ITGA6, TNFRSF26, ARHGEF7, FAM72A < 0.001 KEGG: Apoptosis JUN, PARP4, GADD45A, TNFRSF10B, PIK3CD, FOS, LMNB2, CTSS, LMNB1, GADD45G, TUBA1B, BCL2L11, CTSL, CAPN2, BIRC5, CTSD < 0.001 KEGG: p53 signaling pathway RRM2, SIVA1, GADD45A, SERPINE1, TNFRSF10B, IGF1, SIAH1B, GADD45G, CCNB2, CCNB1, CCND2, CCNG2, SESN1, CDK1, GTSE1 < 0.001 Note. GO, Gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genome Pathway; DEGs, differentially expressed genes. Table 3. DEGs enriched into “inflammatory response (GO)”, “immune response (GO)”
TERM Differentially expressed gene P-value GO (BP): inflammatory response ECM1, IL1RN, CSF1, HMGB2, PIK3CD, HMGB1, CXCL2, CYSLTR1, C3AR1, OLR1, CCR7, PTGIR, PARP4, IGFBP4, SLC11A1, CYBB, ACOD1, NAIP2, IL1A, IL23A, IL1B, ADAM8, SMPDL3B, CD44, EPHA2, PTGER4, CX3CR1, CFH, PTGER2, CD5L, PTGS2, AIF1, HDAC9, PTGS1, CCL7, CLEC7A, CCL5, CCL4, CCL3, CCL2, ZFP580, CCR1, CCL22, NOS2, TREX1, LY86, IFI202B, CXCL10, IL6, NLRP10, NFKBID < 0.001 GO (BP): inflammatory response CD86, CX3CR1, IL1RN, CSF3, MARCHF1, CFH, CLEC4N, CD80, CXCR4, CST7, CXCL2, CTSS, TNFSF13B, CCL7, CTSL, CCL5, CCL4, CCL3, CCL2, SERPINB9B, CCR7, JAK2, CCR1, H60B, H2-EB1, CCL22, TREX1, OSM, LIF, LY86, IFI44, H2-AA, RAET1D, CXCL10, IL1A, IL6, CLEC4D, RAET1E, IL23A, IL1B, CLEC4E, ULBP1, H2-AB1 0.009 -
[1] Shi L, Tian HL, Wang P, et al. Spaceflight and simulated microgravity suppresses macrophage development via altered RAS/ERK/NFκB and metabolic pathways. Cell Mol Immunol, 2021; 18, 1489−502. doi: 10.1038/s41423-019-0346-6 [2] Zérath E. Effects of microgravity on bone and calcium homeostasis. Adv Space Res, 1998; 21, 1049−58. doi: 10.1016/S0273-1177(98)00026-X [3] Van Ombergen A, Demertzi A, Tomilovskaya E, et al. The effect of spaceflight and microgravity on the human brain. J Neurol, 2017; 264, 18−22. doi: 10.1007/s00415-017-8427-x [4] Taylor PW, Sommer AP. Towards rational treatment of bacterial infections during extended space travel. Int J Antimicrob Agents, 2005; 26, 183−7. doi: 10.1016/j.ijantimicag.2005.06.002 [5] Kim DS, Vaquer S, Mazzolai L, et al. The effect of microgravity on the human venous system and blood coagulation: a systematic review. Exp Physiol, 2021; 106, 1149−58. doi: 10.1113/EP089409 [6] Vinken M. Hepatology in space: Effects of spaceflight and simulated microgravity on the liver. Liver Int, 2022; 42, 2599−606. doi: 10.1111/liv.15444 [7] Xiong Y, Ma CY, Li Q, et al. Melatonin ameliorates simulated-microgravity-induced mitochondrial dysfunction and lipid metabolism dysregulation in hepatocytes. FASEB J, 2023; 37, e23132. doi: 10.1096/fj.202301137R [8] Yang JQ, Jiang N, Li ZP, et al. The effects of microgravity on the digestive system and the new insights it brings to the life sciences. Life Sci Space Res, 2020; 27, 74−82. doi: 10.1016/j.lssr.2020.07.009 [9] Lv HF, Yang H, Jiang CM, et al. Microgravity and immune cells. J R Soc Interface, 2023; 20, 20220869. doi: 10.1098/rsif.2022.0869 [10] Dixon LJ, Barnes M, Tang H, et al. Kupffer cells in the liver. Compr Physiol, 2013; 3, 785−97. [11] Ge J, Li H, Yang JQ, et al. Autophagy in hepatic macrophages can be regulator and potential therapeutic target of liver diseases: A review. Medicine, 2023; 102, e33698. doi: 10.1097/MD.0000000000033698 [12] Ge J, Yue Y, Nie HY, et al. Simulated microgravity altered the gene expression profiles and inhibited the proliferation of Kupffer cells in the early phase by downregulating LMO2 and EZH2. Life Sci Space Res, 2024; 40, 21−34. doi: 10.1016/j.lssr.2023.11.002 [13] Sherman BT, Hao M, Qiu J, et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res, 2022; 50, W216−21. doi: 10.1093/nar/gkac194 [14] Franceschini A, Szklarczyk D, Frankild S, et al. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res, 2013; 41, D808−15. [15] Tizro P, Choi C, Khanlou N. Sample preparation for transmission electron microscopy. Methods Mol Biol, 2019; 1897, 417−24. [16] Low EK, Brudvik E, Kuhlman B, et al. Microgravity impairs DNA damage repair in human hematopoietic stem/progenitor cells and inhibits their differentiation into dendritic cells. Stem Cells Dev, 2018; 27, 1257−67. doi: 10.1089/scd.2018.0052 [17] Taylor GR, Konstantinova I, Sonnenfeld G, et al. Changes in the immune system during and after spaceflight. Adv Space Biol Med, 1997; 6, 1−32. [18] Liu WL, Zhu X, Zhao L, et al. Effects of simulated weightlessness on biological activity of human NK cells induced by IL-2. Chin J Cell Mol Immunol, 2015; 31, 1297−300,305. (In Chinese) [19] Risso A, Tell G, Vascotto C, et al. Activation of human T lymphocytes under conditions similar to those that occur during exposure to microgravity: a proteomics study. Proteomics, 2005; 5, 1827−37. doi: 10.1002/pmic.200401082 [20] Savary CA, Grazziuti ML, Przepiorka D, et al. Characteristics of human dendritic cells generated in a microgravity analog culture system. In Vitro Cell Dev Biol Anim, 2001; 37, 216−22. doi: 10.1007/BF02577532 [21] Kaur I, Simons ER, Castro VA, et al. Changes in monocyte functions of astronauts. Brain Behav Immun, 2005; 19, 547−54. doi: 10.1016/j.bbi.2004.12.006 [22] Wheeler MD. Endotoxin and Kupffer cell activation in alcoholic liver disease. Alcohol Res Health, 2003; 27, 300−6. [23] Rivera CA, Adegboyega P, Van Rooijen N, et al. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol, 2007; 47, 571−9. [24] Dai QQ, Jiang W, Liu H, et al. Kupffer cell-targeting strategy for the protection of hepatic ischemia/reperfusion injury. Nanotechnology, 2021; 32, 265101. doi: 10.1088/1361-6528/abde02 [25] Tsutsui H, Nishiguchi S. Importance of Kupffer cells in the development of acute liver injuries in mice. Int J Mol Sci, 2014; 15, 7711−30. doi: 10.3390/ijms15057711 [26] Racine RN, Cormier SM. Effect of spaceflight on rat hepatocytes: a morphometric study. J Appl Physiol, 1992; 73, 136S−41S. doi: 10.1152/jappl.1992.73.2.S136 [27] Rembiałkowska N, Baczyńska D, Dubińska-Magiera M, et al. RCCS bioreactor-based modeled microgravity affects gastric cancer cells and improves the chemotherapeutic effect. Membranes, 2022; 12, 448. doi: 10.3390/membranes12050448 [28] Bellamy COC. p53 and apoptosis. Br Med Bull, 1997; 53, 522−38. doi: 10.1093/oxfordjournals.bmb.a011628 [29] Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol, 2007; 35, 495−516. doi: 10.1080/01926230701320337 [30] Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer, 2002; 2, 647−56. doi: 10.1038/nrc883 [31] Schuler M, Green DR. Mechanisms of p53-dependent apoptosis. Biochem Soc Trans, 2001; 29, 684−8. doi: 10.1042/bst0290684 [32] Chen YN, Hu MR, Wang L, et al. Macrophage M1/M2 polarization. Eur J Pharmacol, 2020; 877, 173090. doi: 10.1016/j.ejphar.2020.173090 [33] Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science, 1998; 281, 1305−8. doi: 10.1126/science.281.5381.1305 [34] Wang C, Ma C, Gong LH, et al. Macrophage polarization and its role in liver disease. Front Immunol, 2021; 12, 803037. doi: 10.3389/fimmu.2021.803037 -




 下载:
下载:



 Quick Links
Quick Links