-
An elevated plasma homocysteine (pHcy) concentration is not only an independent risk factor for cardiovascular disease[1-3], but also a risk factor for the development of cognitive impairment and Alzheimer’s disease[4,5]. Nutrition, physiology, pharmacology, lifestyle, disease, and genetics are known factors influencing pHcy concentration[6-8], in which nutrition and genetic factors were deemed to have a greater influence.
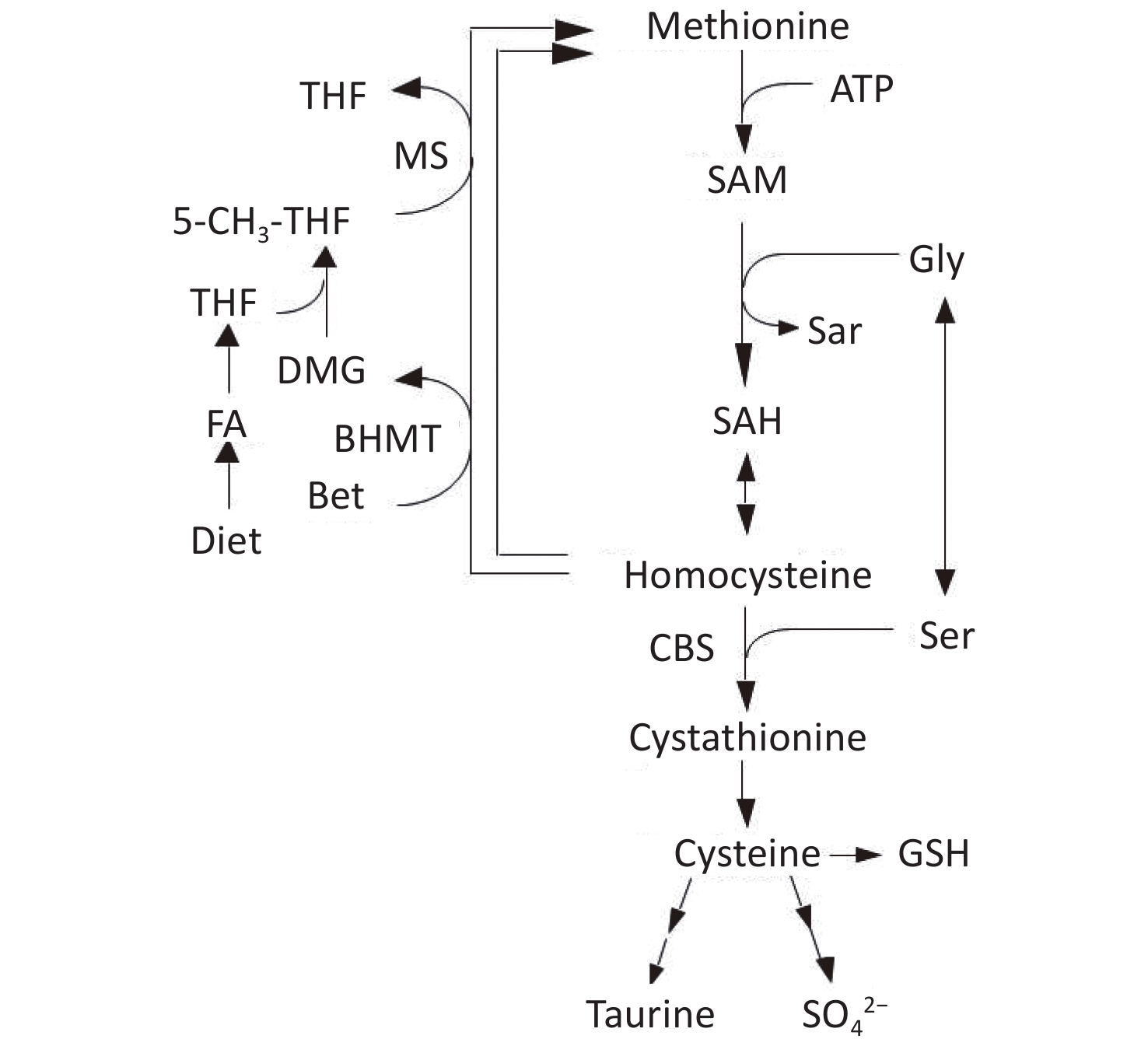
Homocysteine (Hcy) is metabolized by either remethylation or transsulfuration (Figure 1)[9]. In the remethylation process, Hcy is remethylated either by methionine synthase (MS) or betaine-homocysteine S-methyltransferase (BHMT) in which 5-methyltetrahydrofolate (5-MTHF) or betaine act as methyl donors. On the contrary, the transsulfuration pathway of Hcy to cysteine is catalyzed by cystathionine β-synthase (CBS) and cystathionase. Vitamin B12 and folate are cofactors for MS, whereas vitamin B6 is a cofactor for CBS and cystathionase; deficiencies in these micronutrients can cause hyperhomocysteinemia[10,11].
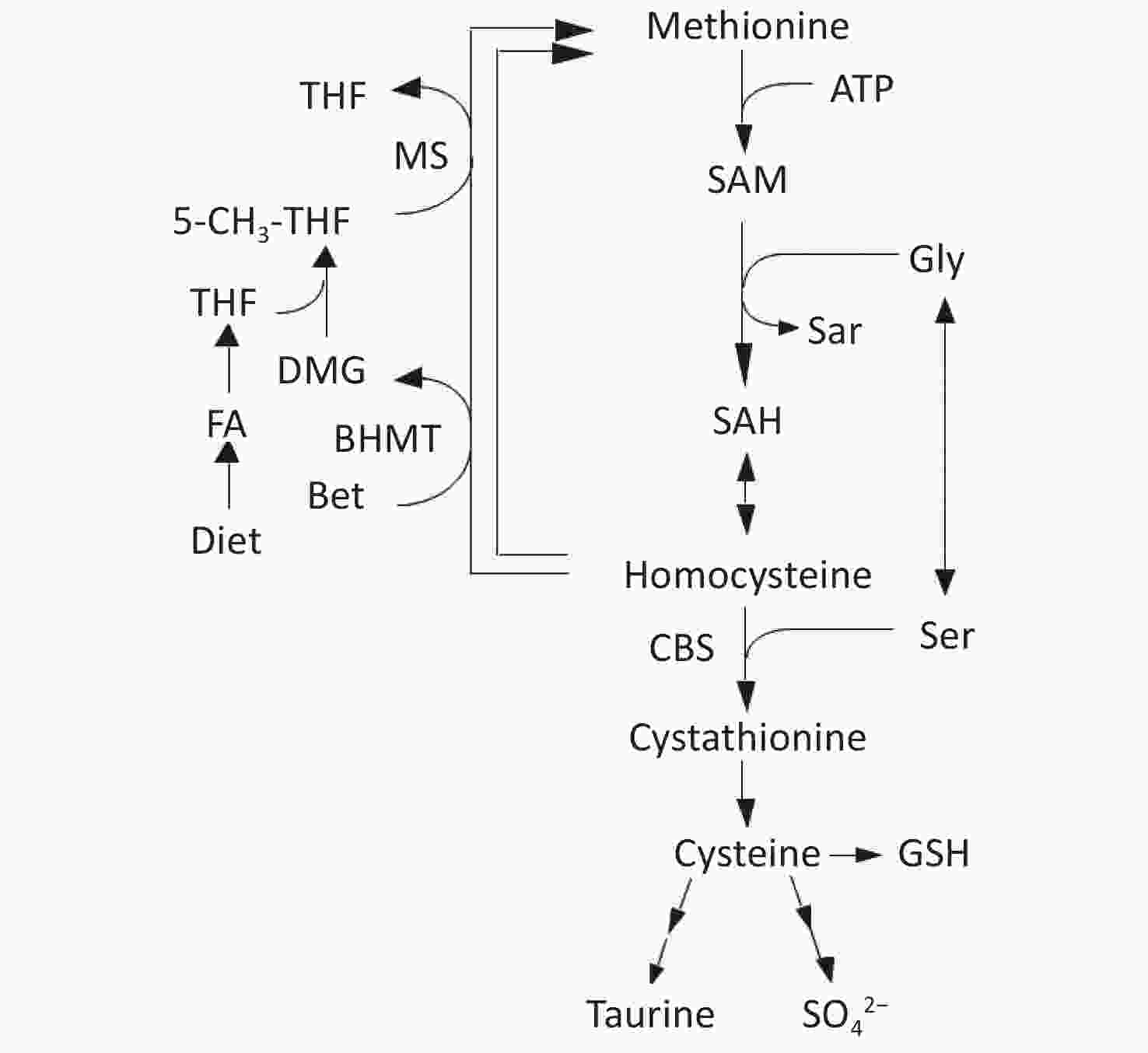
Figure 1. Metabolism of methionine and homocysteine. BHMT, betaine-homocysteine S-methyltransferase (EC 2.1.1.5); CBS, cystathionine β-synthase (EC 4.2.1.22); DMG, N,N-dimethylglycine; FA, folic acid; MS, methionine synthase (EC 2.1.1.13); 5-MTHF, 5-methyltetrahydrofolate; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; THF, tetrahydrofolate.
Folate deficiency-induced hyperhomocysteinemia in rats is one of the representative models of hyperhomocysteinemia[12-14]. Folate deficiency has been thought to induce hyperhomocysteinemia via the following mechanisms[2,15]: 1) disturbed Hcy remethylation due to a decrease in 5-MTHF concentration and 2) decreased transsulfuration due to a decrease in hepatic S-adenosylmethionine (SAM), which is an activator of CBS[16].
Current studies[17-19] have focused on the relationship between the two remethylation pathways of Hcy (i.e., BHMT and MS pathways). Both pathways are separate but closely related, in which several studies have suggested that both are connected via N,N-dimethylglycine (DMG). DMG is a product of the BHMT remethylation pathway, which binds to tetrahydrofolate derived from dietary folic acid to further participate in the MS remethylation pathway. In addition, DMG acts as an inhibitor for the BHMT enzyme[20,21]. As tetrahydrofolate participates in DMG metabolism as a methyl-group acceptor, BHMT pathway was reported to require folate as a cofactor[22]. Therefore, folate deficiency was assumed to decrease tetrahydrofolate availability, which will then reduce DMG consumption. This will result in the impairment of the Hcy remethylation pathways, whereby the BHMT pathway is the focus of this study. In fact, two studies have reported that serum DMG concentration was significantly increased in folate-deficient participants but not in vitamin B12-deficient participants[23,24]. However, limited information is currently available on the compensatory relationship between the BHMT and MS pathways as well as the specific role of DMG in both pathways.
In this study, we aimed to investigate the effect of DMG supplementation on pHcy concentration in folate-sufficient and folate-deficient rats.
-
Folic acid and choline bitartrate were purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals were of analytical grade and purchased from either Wako Pure Chemical (Osaka, Japan) or Sigma-Aldrich. Dietary ingredients such as vitamin-free casein, mineral mixture (AIN-93G), folate-free vitamin mixture (AIN-93), and cellulose powder were purchased from Oriental Yeast (Tokyo), whereas the others were purchased from Wako.
-
Six-week-old male Wistar rats weighing 120–140 g were purchased from Japan SLC (Hamamatsu, Japan). They were individually housed in a temperature (23–25 °C)- and humidity (40%–60%)-controlled animal room with a 12-h light–dark cycle (lights on from 07:00 to 19:00 h). All rats were given free access to water and a 25% casein diet during the 5-day acclimatization period. Thirty-two rats were randomly assigned to one of the four diet groups and fed on different diets respectively: 1) 20% casein (20C), 2) 20C + 0.1% DMG (20C + DMG), (3) folate-deprived 20C (20CFD), and 4) 20CFD + 0.1% DMG (20CFD + DMG); 20C was a folate-sufficient diet, whereas 20CFD was a folate-deficient diet. The composition of 20C was as follows (units are in g/kg): vitamin-free casein (200), α-cornstarch (472.26), sucrose (200), corn oil (50), mineral mixture AIN-93G (35), folate-free vitamin mixture AIN-93 (10), choline bitartrate (2.5), lactose-containing folate (33.3 mg/g at 0.24), susccinylsulfathiazole (10), and cellulose powder (20). Folate levels in folate-sufficient diets (20C and 20C + DMG) were 8 mg/kg, a four-fold increase compared with the folate-free vitamin mixture used in the diet. In folate-deficient diets (20CFD and 20CFD + DMG), folate-free lactose was used instead of lactose-containing folate. In addition to AIN-93 which was folate-free, succinylsulfathiazole was included in the folate-deficient groups to suppress folate synthesis by the rodents’ intestinal bacteria. DMG was added in the diet of the supplementation groups at the expense of cornstarch. Rats were given free access to the experimental diets and water for 28 days. After the experiment, the rats were euthanized between 10:00 and 11:00 h without prior food deprivation, as it was shown that nonfasting pHcy concentration in humans relied on the dietary treatment[25].
This study was approved by the Animal Use Committee of Shizuoka University and adhered to the ‘Guidelines for the Care and Use of Laboratory Animals’ of Shizuoka University.
-
Blood plasma was separated from heparinized whole blood by centrifugation at 2,000 ×g for 15 min at 4 °C and was stored at −30 °C for further analysis. Whole liver was quickly removed, rinsed in ice-cold saline, blotted with filter paper, cut into three portions, weighed, and frozen in liquid nitrogen. Liver samples were stored at −80 °C until further analysis.
One portion of the liver was homogenized in ice-cold 0.3 mol/L trichloroacetic acid solution and then centrifuged at 10,000 ×g for 10 min at 4 °C. The supernatant of the deproteinized liver homogenate was subjected to assays for methionine metabolites, betaine, and serine. Another portion of the liver was homogenized in 10 mmol/L sodium phosphate buffer (pH 7.4) containing 0.15 mol/L KCl and centrifuged at 14,000 ×g for 10 min at 4 °C. The resulting supernatant was subjected to enzyme assays. The third portion of the liver was subjected to mRNA analysis; total mRNA was isolated using a kit purchased from ISOGEN (Nippon Gene, Tokyo), according to manufacturer’s instructions.
-
Hcy and cysteine concentrations in both plasma and liver were measured by high-performance liquid chromatography (HPLC) using the method of Durand et al.[26]. The concentrations of SAM and S-adenosylhomocysteine (SAH) in the liver and that of betaine were measured by HPLC using the methods of Cook et al.[27] and Laryea et al.[28], respectively. 5-MTHF concentration in the plasma and liver were measured by HPLC using the method as mentioned by Shimoda et al.[29]. Hepatic serine concentration was measured by an amino acid autoanalyzer. The BHMT activity in the liver was measured using the method described by Finkelstein et al.[30], whereas DMG concentration was determined by the method mentioned by Laryea et al.[28]. MS, CBS, and cystathione activities in the liver were determined using the methods as described by Huang et al.[31], Mudd et al.[32], and Einarsson et al.[33], respectively. Protein concentration was measured according to the method of Lowry et al.[34] using bovine serum albumin as a standard.
Hcy, Cys, SAM, and SAH concentrations were determined using a chromatographic system with a Shimadzu LC-10AT (VP series) pump equipped with a model RF-10AXL fluorescence detector (Shimadzu Instruments, Japan). Separation was carried out with an analytical reverse phase column (C18 ODS, 150 mm × 4.6 mm, Beckman) protected by a guard column of the same type. The excitation and emission wavelengths were 385 nm and 515 nm, respectively. The analytical column (150 mm × 4.6 mm) used to determine CBS activity was packed with 5 µm ODS-A (YMC Pack), and the wavelength ranged from 290 nm to 356 nm.
The liquid chromatographic system used to determine concentrations of Bet, DMG, and BHMT activity consisted of the following components: Shimadzu HPLC model (VP series) containing LC-10AT (VP series) pump, ultraviolet-visible detector with programmable wavelength (SPD-10AVP) and Rheodyne injector (7725i) with a 20-μL fixed loop (Shimadzu Instruments, Japan). The analytical column (25 cm × 4.6 mm) was packed with 5 µm LC-SCX (SUPELCO, SUPELCOSILTM). The absorption wavelength was 254 nm. The analytical column (25 cm × 4.6 mm) to determine concentrations of 5-MTHF and MS activity was packed with 5 µm ODS-120A (Tskgel, Tosoh Bioscience LLC, King of Prussia, PA). The excitation wavelength was 290 nm, and the emission wavelength was 356 nm.
-
All values were expressed as mean ± SEM. Data were analyzed by one-way analysis of variance, and differences among the experimental groups were analyzed by the Tukey test when the F value was significant. Statistical analysis was performed with Mac Tokei-Kaiseki software (version 1.5; Esumi, Tokyo).
-
No differences were observed in weight gain, food intake, and liver weight of rats assigned to 20C, 20C + DMG, 20CFD, and 20CFD+DMG, indicating that neither folate deprivation nor DMG supplementation affected the aforementioned parameters (Table 1).
Table 1. Effect of dietary supplementation with N,N-Dimethylglycine (0.1%) on body weight gain, food intake and liver weight in rats fed 20% casein diets with or without folate deficiency
Diet Body wt gain
g/28 dFood intake
g/28 dLiver wt % of
body wt20C 101 ± 61 421 ± 21 4.07 ± 0.14 20C + DMG 88 ± 7 439 ± 18 3.99 ± 0.10 20CFD 86 ± 5 402 ± 14 4.14 ± 0.10 20CFD + DMG 100 ± 7 418 ± 14 4.38 ± 0.17 Note. 1Each value is the mean ± SEM, n = 8. Values without a common letter differ, P < 0.05. 20C, 20% casein diet; 20CFD, folate-deprived 20C; different letters are significantly different at P < 0.05. -
pHcy concentration was significantly elevated in rats fed folate-deficient diets (20CFD) compared with those fed a folate-sufficient diet (20C): 28.49 ± 0.50 vs. 14.19 ± 0.39 μmol/L (Figure 2A). When supplemented with DMG, pHcy concentration was significantly decreased (12.23 ± 0.18 μmol/L) in the 20C group but significantly increased (31.56 ± 0.59 μmol/L) in the 20CFD group.
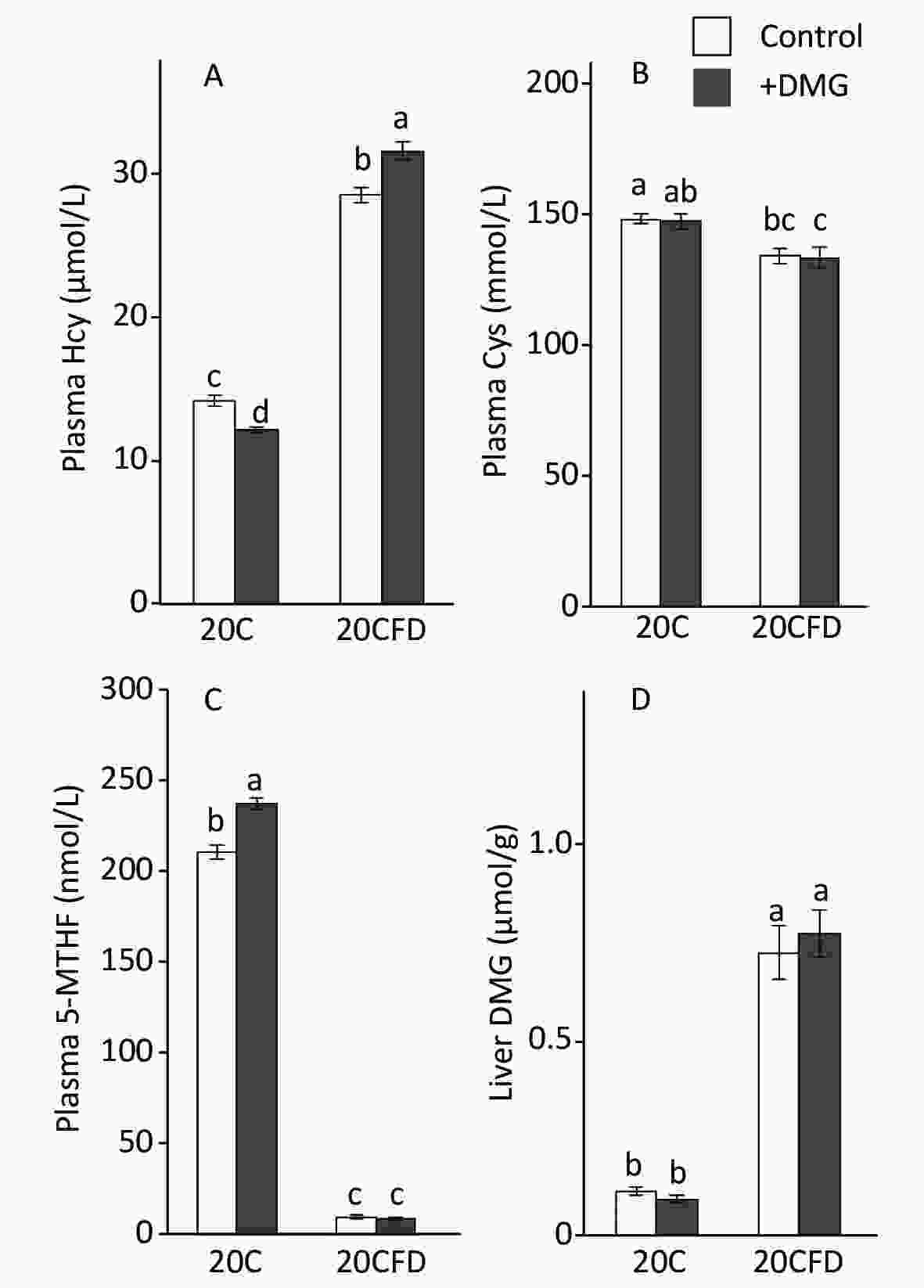
Figure 2. Effects of supplementation with N,N-dimethylglycine on plasma concentrations of homocysteine (A), cysteine (B), 5-methylterahydrofolate (C), and hepatic concentrations of N,N-dimethylglycine (D) in rats fed control and folate-deprived diets. Each value is presented as the mean ± SEM, n = 8. The means in a panel without a common letter are different, P < 0.05. 20C, 20% casein diet; 20CFD, folate-deprived 20C; DMG, N,N-dimethylglycine; 5-MTHF, 5-methyltetrahydrofolate.
In contrast, plasma cysteine and 5-MTHF levels were significantly higher in the 20C group than in the 20CFD group (Figure 2B and C). DMG supplementation did not show an effect on plasma cysteine levels in both 20C and 20CFD groups. However, DMG supplementation significantly increased plasma 5-MTHF concentration, a quantitative index of folate status[35], in the 20C group but not in the 20CFD group (Figure 2, panel C).
The hepatic concentration of DMG was markedly higher in the 20CFD group than in the 20C group and were unaffected by DMG supplementation (Figure 2D).
-
In general, hepatic SAM and SAM/SAH ratio were significantly higher in the fed 20C group, whereas hepatic SAH and Hcy were significantly higher in the 20CFD group (Figure 3). DMG supplementation increased SAM levels in both folate-sufficient and folate-deficient groups, and its effect was significant in the 20C group but not in the 20CFD group (P = 0.05). Moreover, the liver SAM/SAH ratio was significantly higher in the 20CFD+DMG group, but no changes were observed in the 20C+DMG group. DMG supplementation showed no effect on liver Hcy concentration in either folate-deficient or folate- sufficient groups.
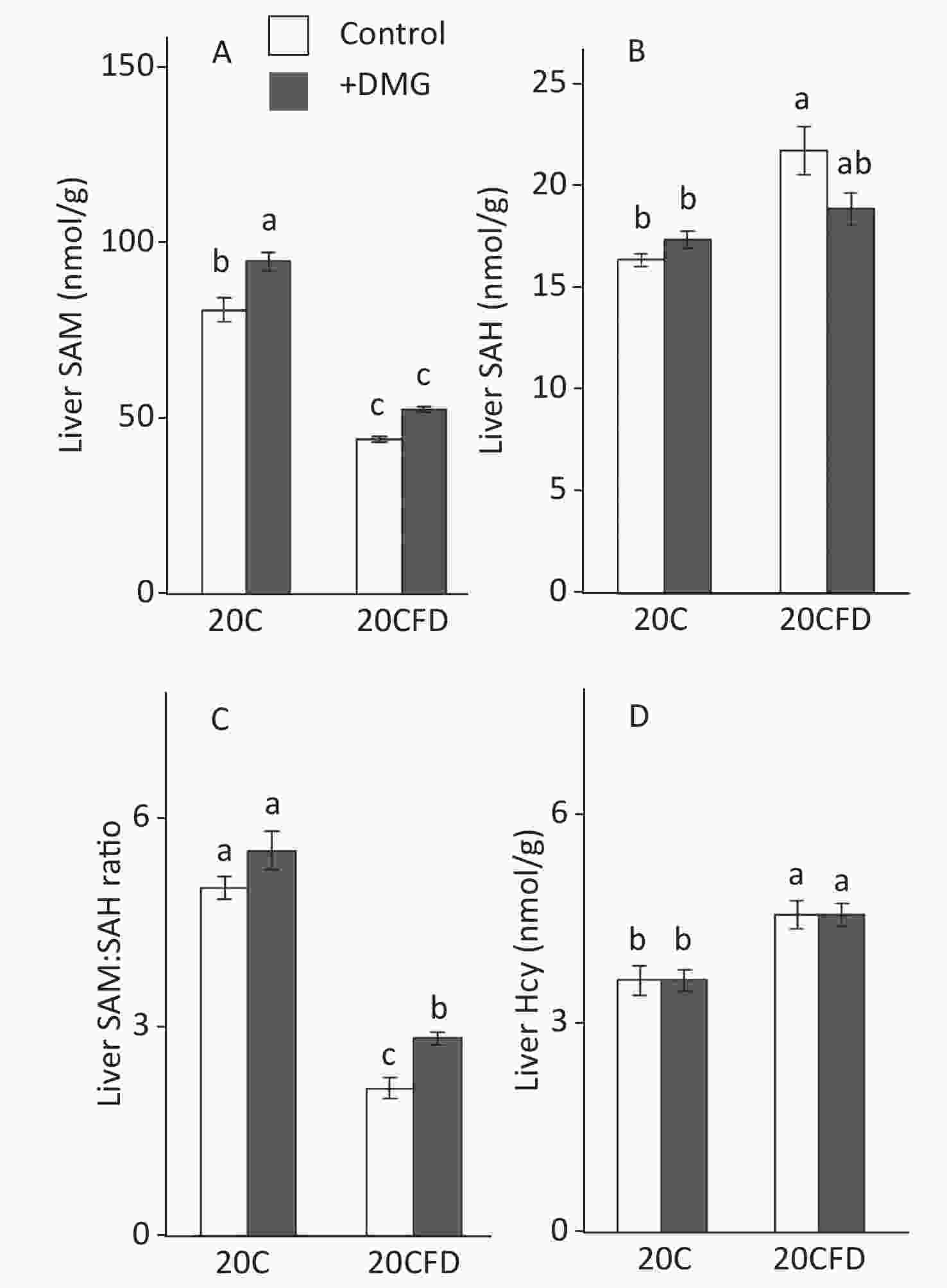
Figure 3. Effects of supplementation with N,N-dimethylglycine on hepatic concentrations of S-adenosylmethionine (A), S-adenosylhomocysteine (B), their ratio (C), and homocysteine (D) in rats. Each value is presented as the mean ± SEM, n = 8. The means in a panel without a common letter are different, P < 0.05. SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine. The means in a panel without a common letter are different, P < 0.05. 20C, 20% casein diet; 20CFD, folate-deprived 20C; DMG, N,N-dimethylglycine; 5-MTHF, 5-methyltetrahydrofolate
-
The BHMT activity in the liver was similar in all groups (20C, 20C+DMG, 20CFD, and 20CFD+DMG). Rats in the 20C group had significantly higher hepatic MS and CBS activity, as well as hepatic betaine and 5-MTHF levels, than those in the 20CFD group. However, hepatic serine levels in the 20C group were significantly lower than those in the 20CFD group (Figure 4).
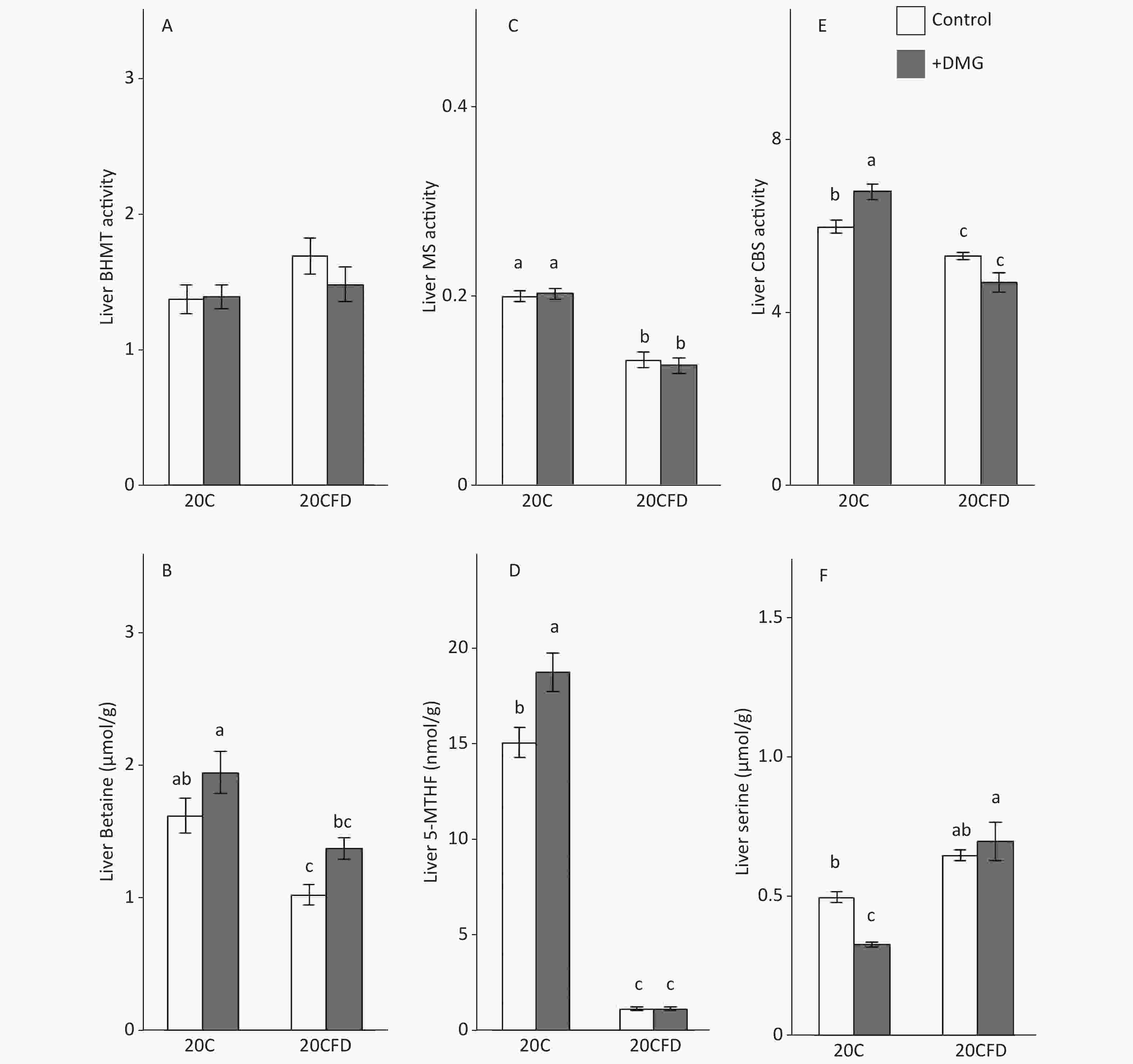
Figure 4. Effects of supplementation with N,N-dimethylglycine on hepatic activities of betaine-homocysteine S-methyltransferase (A), methionine synthase (C), and cystathionine β-synthase (E) and hepatic concentrations of betaine (B), 5-methyltetrahydrofolate (D), and serine (F) in rats. Each value is the presented as the mean ± SEM, n = 8. The means in a panel without a common letter are different, P < 0.05. BHMT, betaine-homocysteine S-methyltransferase; CBS, cystathionine β-synthase; MS, methionine synthase; 5-MTHF, 5-methyltetrahydrofolate. The means in a panel without a common letter are different, P < 0.05. 20C, 20% casein diet; 20CFD, folate-deprived 20C; DMG, N,N-dimethylglycine; 5-MTHF, 5-methyltetrahydrofolate
DMG supplementation had no effect on hepatic MS activity in rats fed either 20C or 20CFD. DMG supplementation elevated hepatic CBS activity in both groups, in which the effect was significant in the 20C group but not in the 20CFD group (Figure 4, panel E). Hepatic betaine was significantly higher in the 20C group than in the fed 20CFD group (1.63 ± 0.12 μmol/g vs. 1.04 ± 0.08 μmol/g). DMG supplementation only slightly increased betaine concentration in both groups (20C+DMG at 1.96 ± 0.16 μmol/g and 20CFD+DMG at 1.39 ± 0.08 μmol/g), but its effects were statistically not significant. DMG supplementation significantly increased hepatic 5-MTHF level and significantly decreased hepatic serine level in the 20C group. On the contrary, no differences were observed in hepatic 5-MTHF and serine levels in the 20CFD+DMG group (Figure 4D and F).
-
This study demonstrated that a relatively low concentration of DMG can significantly reduce pHcy in rats when fed a normal, folate-sufficient diet, whereas concentration significantly increased when rats were fed a folate-deprived diet. It is reasonable to assume that DMG supplementation increased Hcy removal by the MS pathway via an increase in hepatic 5-MTHF concentration, which leads to a reduction in pHcy in rats fed a folate-sufficient diet. This supports the theory that under folate-sufficient conditions, DMG acts as a C1 source. In contrast, during folate deficiency, tetrahydrofolate deficiency may lead to impaired DMG metabolism and impede the role of DMG as a C1 source.
Generally, folate deficiency-induced hyperhomocysteinemia is mainly caused by a decrease in 5-MTHF concentration, in which 5-MTHF acts as a methyl-group donor for MS; a decrease in 5-MTHF will therefore suppress Hcy remethylation[2]. Results of this study coincide with the representative rat models whereby folate deficiency leads to hyperhomocysteinemia[12]. In this study, folate deprivation not only drastically decreased hepatic 5-MTHF concentration but also decreased hepatic MS activity, indicating a great suppression in the 5-MTHF-MS system. Kim et al.[36,37] showed that severe folate deficiency caused secondary depletion of hepatic choline and phosphocholine in rats. The above finding was also true for hepatic betaine concentration, as choline is easily metabolized to betaine, and this study showed that hepatic betaine concentration was significantly decreased by folate deprivation in rats. However, it is unclear whether the decrease in hepatic betaine concentration contributed to hyperhomocysteinemia induced by folate deprivation, as hepatic betaine concentrations (1.04 ± 0.08 µmol/g) in folate-deprived rats was still one-order higher than the reported Km value (120 µmol/L) of BHMT in rats[38]. By contrast, we demonstrated that the hepatic concentration of DMG, a reaction product and an inhibitor of BHMT, was markedly increased by folate deprivation. Although the Ki value of rat BHMT for DMG has not been reported, Finkelstein et al.[20] reported a positive correlation between DMG concentration and inhibition of BHMT reaction in rats; BHMT reactions were inhibited by 19% with 0.02 mol/L DMG, followed by 76% and 90% inhibition with 0.1 mmol/L and 1 mmol/L DMG, respectively. Hence, an increase in hepatic DMG concentration may strongly inhibit BHMT reaction in vivo. In addition to DMG, the betaine/DMG ratio may have some significance in the BHMT reaction similar to the SAM/SAH ratio in SAM-dependent transmethylation reactions, where SAH is known as an inhibitor of various types of methyltransferase. To our knowledge, this study is the first to show that folate deprivation markedly increased hepatic DMG concentration, which supports the concept that folate deprivation impairs not only the 5-MTHF system but also the betaine-BHMT system and subsequently induces hyperhomocysteinemia.
However, it is unknown why DMG supplementation increased pHcy in rats fed folate-deficient diet. In this study, the marked increase in hepatic DMG concentration in rats fed a folate-deprived diet found coincided with the results of Allen et al.[23], who showed that serum DMG concentration was significantly higher in folate-deficient patients than in normal participants. It is presumed that the increase in hepatic DMG concentration by folate deficiency can inhibit endogenous BHMT reaction, which is a contributing factor of hyperhomocysteinemia. Notably, DMG supplementation did not further increase hepatic DMG concentration in rats fed folate-deficient diet. This may be attributed to the relatively low dosage level (0.1%) of DMG used in this study.
-
This study showed that under normal, folate-sufficient conditions, DMG supplementation exhibited hypohomocysteinemic effect, whereas when supplemented in a folate-deficient diet, DMG can have deleterious effect on pHcy concentration. The dosage effect of DMG should be investigated in the future.
-
We are extremely grateful to the participants and stuff who participated in our study.
doi: 10.3967/bes2021.047
Effect of N,N-Dimethylglycine on Homocysteine Metabolism in Rats Fed Folate-Sufficient and Folate-Deficient Diets
-
Abstract:
Objective This study aimed to investigate the effects of N,N-dimethylglycine (DMG) on the concentration and metabolism of plasma homocysteine (pHcy) in folate-sufficient and folate-deficient rats. Methods In this study, 0.1% DMG was supplemented in 20% casein diets that were either folate-sufficient (20C) or folate-deficient (20CFD). Blood and liver of rats were subjected to assays of Hcy and its metabolites. Hcy and its related metabolite concentrations were determined using a liquid chromatographic system. Results Folate deprivation significantly increased pHcy concentration in rats fed 20C diet (from 14.19 ± 0.39 μmol/L to 28.49 ± 0.50 μmol/L; P < 0.05). When supplemented with DMG, pHcy concentration was significantly decreased (12.23 ± 0.18 μmol/L) in rats fed 20C diet but significantly increased (31.56 ± 0.59 μmol/L) in rats fed 20CFD. The hepatic methionine synthase activity in the 20CFD group was significantly lower than that in the 20C group; enzyme activity was unaffected by DMG supplementation regardless of folate sufficiency. The activity of hepatic cystathionine β-synthase (CBS) in the 20CFD group was decreased but not in the 20C group; DMG supplementation enhanced hepatic CBS activity in both groups, in which the effect was significant in the 20C group but not in the other group. Conclusion DMG supplementation exhibited hypohomocysteinemic effects under folate-sufficient conditions. By contrast, the combination of folate deficiency and DMG supplementation has deleterious effect on pHcy concentration. -
Key words:
- Folate deficiency /
- N,N-Dimethylglycine /
- 5-methyltetrahydrofolate /
- Plasma homocysteine
注释: -
Figure 1. Metabolism of methionine and homocysteine. BHMT, betaine-homocysteine S-methyltransferase (EC 2.1.1.5); CBS, cystathionine β-synthase (EC 4.2.1.22); DMG, N,N-dimethylglycine; FA, folic acid; MS, methionine synthase (EC 2.1.1.13); 5-MTHF, 5-methyltetrahydrofolate; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; THF, tetrahydrofolate.
Figure 2. Effects of supplementation with N,N-dimethylglycine on plasma concentrations of homocysteine (A), cysteine (B), 5-methylterahydrofolate (C), and hepatic concentrations of N,N-dimethylglycine (D) in rats fed control and folate-deprived diets. Each value is presented as the mean ± SEM, n = 8. The means in a panel without a common letter are different, P < 0.05. 20C, 20% casein diet; 20CFD, folate-deprived 20C; DMG, N,N-dimethylglycine; 5-MTHF, 5-methyltetrahydrofolate.
Figure 3. Effects of supplementation with N,N-dimethylglycine on hepatic concentrations of S-adenosylmethionine (A), S-adenosylhomocysteine (B), their ratio (C), and homocysteine (D) in rats. Each value is presented as the mean ± SEM, n = 8. The means in a panel without a common letter are different, P < 0.05. SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine. The means in a panel without a common letter are different, P < 0.05. 20C, 20% casein diet; 20CFD, folate-deprived 20C; DMG, N,N-dimethylglycine; 5-MTHF, 5-methyltetrahydrofolate
Figure 4. Effects of supplementation with N,N-dimethylglycine on hepatic activities of betaine-homocysteine S-methyltransferase (A), methionine synthase (C), and cystathionine β-synthase (E) and hepatic concentrations of betaine (B), 5-methyltetrahydrofolate (D), and serine (F) in rats. Each value is the presented as the mean ± SEM, n = 8. The means in a panel without a common letter are different, P < 0.05. BHMT, betaine-homocysteine S-methyltransferase; CBS, cystathionine β-synthase; MS, methionine synthase; 5-MTHF, 5-methyltetrahydrofolate. The means in a panel without a common letter are different, P < 0.05. 20C, 20% casein diet; 20CFD, folate-deprived 20C; DMG, N,N-dimethylglycine; 5-MTHF, 5-methyltetrahydrofolate
Table 1. Effect of dietary supplementation with N,N-Dimethylglycine (0.1%) on body weight gain, food intake and liver weight in rats fed 20% casein diets with or without folate deficiency
Diet Body wt gain
g/28 dFood intake
g/28 dLiver wt % of
body wt20C 101 ± 61 421 ± 21 4.07 ± 0.14 20C + DMG 88 ± 7 439 ± 18 3.99 ± 0.10 20CFD 86 ± 5 402 ± 14 4.14 ± 0.10 20CFD + DMG 100 ± 7 418 ± 14 4.38 ± 0.17 Note. 1Each value is the mean ± SEM, n = 8. Values without a common letter differ, P < 0.05. 20C, 20% casein diet; 20CFD, folate-deprived 20C; different letters are significantly different at P < 0.05. -
[1] Refsum H, Ueland PM, Nygard O, et al. Homocysteine and cardiovascular disease. Annu Rev Med, 1998; 49, 31−62. doi: 10.1146/annurev.med.49.1.31 [2] Selhub J. Homocysteine metabolism. Annu Rev Nutr, 1999; 19, 217−46. doi: 10.1146/annurev.nutr.19.1.217 [3] De Bree A, Verschuren WM, Kromhout D, et al. Homocysteine determinants and the evidence to what extent homocysteine determines the risk of coronary heart disease. Pharmacol Rev, 2002; 54, 599−618. doi: 10.1124/pr.54.4.599 [4] Song F, Poljak A, Smythe GA, et al. Plasma biomarkers for mild cognitive impairment and Alzheimer's disease. Brain Res Rev, 2009; 61, 69−80. doi: 10.1016/j.brainresrev.2009.05.003 [5] Elsherbiny NM, Sharma I, Kira D, et al. Homocysteine induces inflammation in retina and brain. Biomolecules, 2020; 10, 393. doi: 10.3390/biom10030393 [6] Alam SF, Kumar S, Ganguly P. Measurement of homocysteine: a historical perspective. J Clin Biochem Nutr, 2019; 65, 171−7. doi: 10.3164/jcbn.19-49 [7] Kumar A, Palfrey HA, Pathak R, et al. The metabolism and significance of homocysteine in nutrition and health. Nutr Metab (Lond), 2017; 14, 78. doi: 10.1186/s12986-017-0233-z [8] Skovierova H, Vidomanova E, Mahmood S, et al. The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int J Mol Sci, 2016; 17, 1733. doi: 10.3390/ijms17101733 [9] Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr, 2004; 24, 539−77. doi: 10.1146/annurev.nutr.24.012003.132418 [10] Zaric BL, Obradovic M, Bajic V, et al. Homocysteine and hyperhomocysteinaemia. Curr Med Chem, 2019; 26, 2948−61. doi: 10.2174/0929867325666180313105949 [11] Lynn BB, Patrick JS, Daniel JR. Biomarkers of Nutrition for Development- Folate Review. J Nutr, 2015; 145, 1636S−1680S. doi: 10.3945/jn.114.206599 [12] Y LIU, YQ LIU, T Morita, et al. Effect of dietary supplementation with folate on choline deficiency-induced hyperhomocysteinemia in rats. J Nutr Sci Vitaminol, 2012; 58, 20−8. doi: 10.3177/jnsv.58.20 [13] Y LIU, YQ LIU, T Morita, et al. Effects of betaine supplementation and choline deficiency on folate deficiency-induced hyperhomocysteinemia in rats. J Nutr Sci Vitaminol, 2012; 58, 69−77. doi: 10.3177/jnsv.58.69 [14] Cui S, Li W, Wang P, Lv X, et al. Folic acid inhibits homocysteineinduced cell apoptosis in human umbilical vein endothelial cells. Mol Cell Biochem, 2018; 444, 77−86. doi: 10.1007/s11010-017-3232-5 [15] Dayal S, Lentz SR. Murine models of hyperhomocysteinemia and their vascular phenotypes. Arterioscler Thromb Vasc Biol, 2008; 28, 1596−605. doi: 10.1161/ATVBAHA.108.166421 [16] Finkelstein JD, Kyle WE, Martin JL, et al. Activation of cystathionine synthase by adenosylmethionine and adenosylethionine. Biochem Biophys Res Commun, 1975; 66, 81−7. doi: 10.1016/S0006-291X(75)80297-X [17] Josiane S, Aline MC, Dirce MM. Genetic Variants Involved in One-Carbon Metabolism: Polymorphism Frequencies and Differences in Homocysteine Concentrations in the Folic Acid Fortification Era. Nutrients, 2017; 9, 539−50. doi: 10.3390/nu9060539 [18] Williams KT, Garrow TA, Schalinske KL. Type I diabetes leads to tissue-specific DNA hypomethylation in male rats. J Nutr, 2008; 138, 2064−9. doi: 10.3945/jn.108.094144 [19] McGregor DO, Dellow WJ, Lever M, et al. Dimethylglycine accumulates in uremia and predicts elevated plasma homocysteine concentrations. Kidney Int, 2001; 59, 2267−72. doi: 10.1046/j.1523-1755.2001.00743.x [20] Finkelstein JD, Harris BJ, Kyle WE. Methionine metabolism in mammals: kinetic study of betaine-homocysteine methyltransferase. Arch Biochem Biophys, 1972; 153, 320−4. doi: 10.1016/0003-9861(72)90451-1 [21] Pajares MA, Perez-Sala D. Betaine homocysteine S-methyltransferase: just a regulator of homocysteine metabolism? Cell Mol Life Sci, 2006; 63, 2792−803. doi: 10.1007/s00018-006-6249-6 [22] Wagner C. Cellular folate binding proteins; function and significance. Annu Rev Nutr, 1982; 2, 229−48. doi: 10.1146/annurev.nu.02.070182.001305 [23] Allen RH, Stabler SB, Lindenbaum J. Serum betaine, N, N-dimethylglycine and N-methylglycine levels in patients with cobalamin and folate deficiency and related inborn errors of metabolism. Metabolism, 1993; 42, 1448−60. doi: 10.1016/0026-0495(93)90198-W [24] Allen RH, Stabler SB, Savage DG, et al. Metabolic abnormalities in cobalamin (vitamin B12) and folate deficiency. FASEB J, 1993; 7, 1344−53. doi: 10.1096/fasebj.7.14.7901104 [25] Verhoef P, van Vliet T, Katan MB. A high-protein diet increases postprandial but not fasting plasma total homocysteine concentrations: a dietary controlled, crossover trial in healthy volunteers. Am J Clin Nutr, 2005; 82, 553−8. doi: 10.1093/ajcn/82.3.553 [26] Durand P, Fortin LJ, Lussier-Cacan S, et al. Hyperhomocysteinemia induced by folic acid deficiency and methionine load–applications of a modified HPLC method. Clin Chim Acta, 1996; 252, 83−93. doi: 10.1016/0009-8981(96)06325-5 [27] Cook RJ, Horne DW, Wagner C. Effect of dietary methyl group deficiency on one-carbon metabolism in rats. J Nutr, 1989; 119, 612−7. doi: 10.1093/jn/119.4.612 [28] Laryea MD, Steinhagen F, Pawliczek S, et al. Simple method for the routine determination of betaine and N, N-dimethylglycine in blood and urine. Clin Chem, 1998; 44, 1937−41. doi: 10.1093/clinchem/44.9.1937 [29] Shimoda M. Simultaneous determination of tetrahydrofolate and N5-methylterahydrofolate in pig plasma by high-performance liquid chromatography with electrochemical detection. J Vet Med Sci, 1992; 54, 249−53. doi: 10.1292/jvms.54.249 [30] Finkelstein JD, Mudd SE. Trans-sulfuration in mammals: the methionine-sparing effect of cystine. J Biol Chem, 1967; 242, 873−80. doi: 10.1016/S0021-9258(18)96205-8 [31] Huang L, Zhang J, Hayakawa T, et al. Assays of methylenetetrahydrofolate reductase and methionine synthase activities by monitoring 5-methyltetrahydrofolate and tetrahydrofolate using high-performance liquid chromatography with fluorescence detection. Anal Biochem, 2001; 299, 253−9. doi: 10.1006/abio.2001.5421 [32] Mudd SH, Finkelstein JD, Irreverre F, et al. Transsulfuration in mammals. Microassay and tissue distributions of three enzymes of the pathway. J Biol Chem, 1965; 240, 4382−92. [33] Einarsson S, Josefsson B, Lagerkvist S. Determination of amino acids with 9-fluorenylmethyl chloroformate and reversed-phase high-performance liquid chromatography. J Chromatogr, 1983; 282, 609−18. doi: 10.1016/S0021-9673(00)91638-8 [34] Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem, 1951; 193, 265−75. doi: 10.1016/S0021-9258(19)52451-6 [35] Most SJ, Lang D, McDowell IFW, et al. Folate, homocysteine, endothelial function and cardiovascular disease. J Nutr Biochem, 2004; 15, 64−79. doi: 10.1016/j.jnutbio.2003.08.010 [36] Kim YI. Role of folate in colon cancer development and progression. J Nutr, 2003; 133, 3731S−3739S. doi: 10.1093/jn/133.11.3731S [37] Kim Y, Miller JW, da Costa K, et al. Severe folate deficiency causes secondary depletion of choline and phosphocholine in rat liver. J Nutr, 1994; 124, 2197−203. doi: 10.1093/jn/124.11.2197 [38] Lee KH, Cava M, Amiri P, et al. Betaine: homocysteine methyltransferase from rat liver: purification and inhibition by a boronic acid substrate analog. Arch Biochem Biophys, 1992; 292, 77−86. doi: 10.1016/0003-9861(92)90053-Y -




 下载:
下载:



 Quick Links
Quick Links