-
Pteropine orthoreovirus (PRV) is an emerging zoonotic respiratory virus with the potential to spill over from fruit bats to humans. PRV belongs to the family Reoviridae, whose name is derived from an abbreviation of “respiratory enteric orphan”. This family of viruses was initially not believed to cause diseases in humans and therefore was named an “orphan virus”. PRV infections in humans are characterized by symptoms ranging from mild influenza-like illness to severe acute respiratory distress [1-4].
Four isolates of PRV had been discovered in Malaysia: PRV2P (Pulau virus), PRV3M (Melaka virus), PRV4K (Kampar virus), and PRV7S (Sikamat virus). The first case of PRV infection in humans was reported in Malaysia. PRV3M (Melaka virus) was isolated from a throat swab from a patient with high fever and acute respiratory disease[1]. Subsequently, two family members in the household of the patient with PRV3M infection developed similar symptoms. PRV4K (Kampar virus) was isolated from a throat swab of a patient with high fever, acute respiratory disease and vomiting[2], and was found to cause respiratory disease in contact cases. PRV7S (Sikamat virus) was isolated from a throat swab from a patient with sudden onset of high fever, sore throat and myalgia[3]. However, the contact cases associated with the PRV7S-infected patient did not show similar illness. Before documentation of PRV-infected human case reports, PRV2P (Pulau virus) was isolated from pooled urine of Pteropus hypomelanus. PRV2P was not associated with any diseases in Malaysia. PRV1N (Nelason Bay virus) was isolated from the from heart blood of Pteropus poliocephalus and had been shown to cause death and paralysis in mice. In the past decade, numerous PRV variants have been discovered in Asia[4] and Africa[5].
Although newly discovered PRV strains from samples collected from fruit bats are increasingly being reported[4,6-8], data on the seroprevalence of PRV variants in human populations are limited. Surveys of hospital patients in Vietnam and in the Early Dengue Infection and Outcome (EDEN) cohort in Singapore have reported that 4.4% (n = 272; ELISA)[9] and 1.4% [n = 856; Luciferase Immunoprecipitation System (LIPS)][10] of patients, respectively, had anti-PRV antibodies. In comparison, a survey of people in Tioman Island, Malaysia, has detected antibodies in 12.8% of residents (n = 109; virus neutralization test), and these values have remained similar (17.8%; n = 157; virus neutralization test) after nearly a decade[1,6]. These serological surveys have suggested that zoonotic PRV infections may be common in Southeast Asia, particularly in areas with high bat-human interaction. Here, we report the seroprevalence rate of PRV in outpatients in a clinic in Rembau district, Peninsular Malaysia.
Sera included in this study were from patients who presented with upper respiratory tract infection (URTI) at a public primary care clinic in Rembau district in the state of Negeri Sembilan, Peninsular Malaysia, between May and September of 2012. The clinic in Rembau district is approximately 48 km from the government outpatient clinic where the first reported human case of PRV3M (Melaka virus) infection in humans was identified[1] and 22 km from the clinic where subsequent human PRV7S (Sikamat virus) infection was reported[3] (Figure 1). Patients 12 years of age and older who presented with an acute onset (< 5 days) of subjective fever, cough or sore throat without any known cause were enrolled in a previous study[7] after patient consent was obtained. Ethical approval (4.10/JCM-54/2012[BMSc]) from the IMU Joint-Committee of the Research and Ethics Committee was obtained for this study. As previously reported, 17% of participants in the study were positive for PRV viral RNA detection[7]. The clinical presentations associated with URTI in patients who were positive for PRV viral RNA has been investigated and described previously[7], and therefore was not analyzed herein. The current study was aimed at determining the seroprevalence of PRV antibodies in outpatients with mild URTI in the Rembau district, an area with frequent interactions between humans and fruit bats (which are often seen flying in the district at nights by local residents)[7].
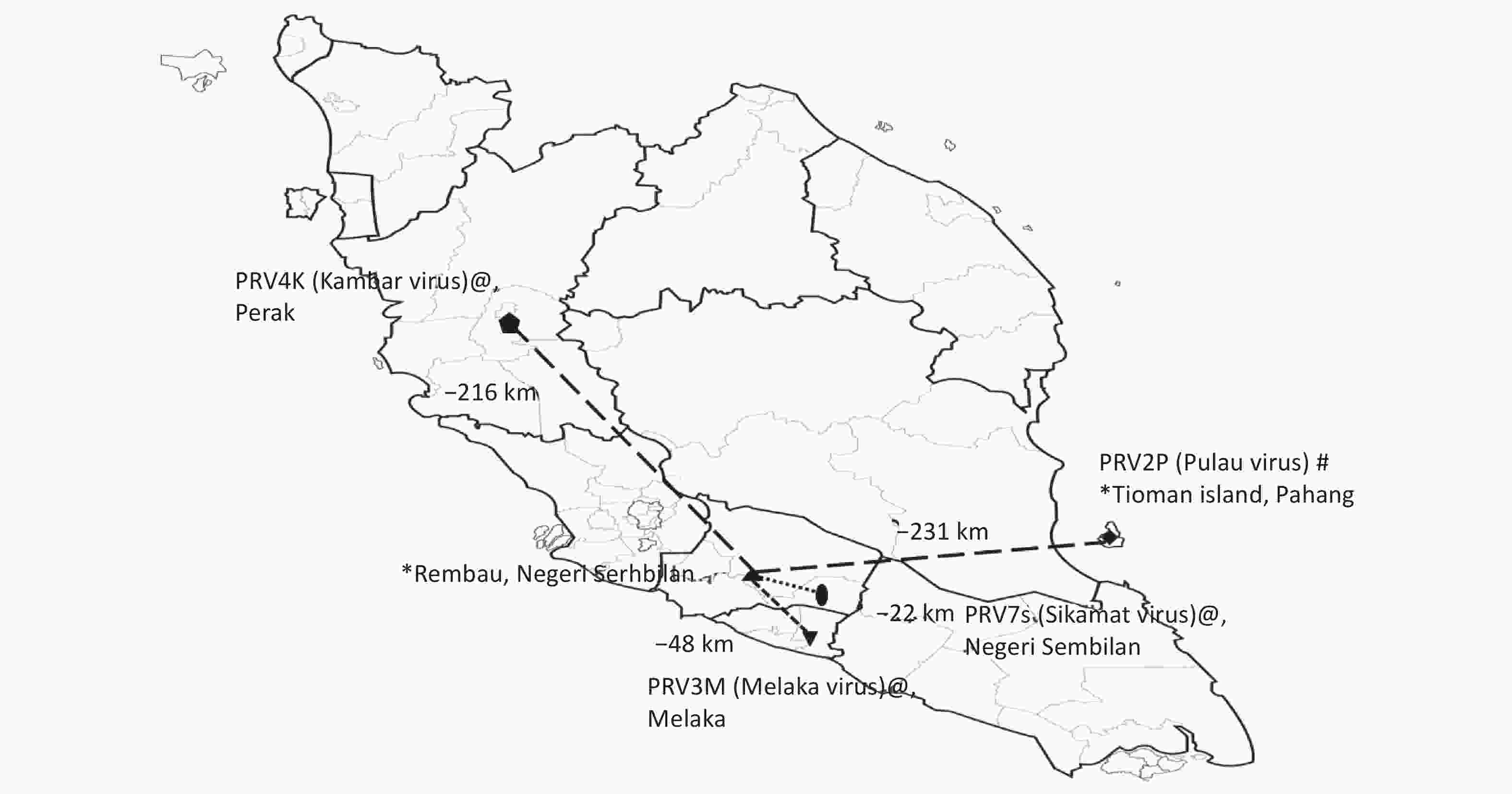
Figure 1. Studies of Pteropine orthoreovirus (PRV) spillover in Peninsular Malaysia. Human serum collection sites [Rembau (this study) and Tioman island (previous study)] are labeled with * on the map. Relative distances (measured by using Google Maps) of human serum collection sites in Rembau with respect to those for other previously reported PRVs in Malaysia are shown on the map. PRV2P (Pulau virus) isolated from pteropodid bat specimens is labeled with #, and PRV3M (Melaka virus, PRV4K (Kampar virus) and PRV7S (Sikamat virus) isolated from human specimens are labeled with @.
Virus neutralization tests were performed in serum samples against PRV2P (Pulau virus, PulV), PRV3M (Melaka virus, MelV), PRV4K (Kampar virus, KamV) and PRV7S (Sikamat virus, SikV), as previously described[1,6]. The sera were also tested against PRV1N (Nelson Bay Virus). In brief, serial 2-fold dilutions from 1:20 to 1:2,560 of control and test sera were prepared in duplicate. An equal volume of each PRV working stock containing 100 TCID50 was added to the diluted sera and incubated for 30 minutes. The preincubated virus/serum mix was added to confluent Vero cell (ATCC CCL81) monolayers. After 1 hour incubation, the inoculum was removed. The cells were washed three times with serum-free DMEM, and subsequently DMEM containing 2% fetal bovine serum was added. All incubations were performed at 37 °C in a humidified 5% CO2 incubator. Vero cell monolayers were observed for cytopathic effects 4 days later. Each virus neutralization test plate included incubation of hyperimmune PRV1NB, PRV2P, PRV3M, PRV4K, and PRV7S rabbit serum with virus as a positive control, and incubation in the presence of only the virus as a negative control. Hyperimmune sera against heterologous virus of PRVs and MRV3 were generated by injection of ultracentrifuged purified viruses into rabbits, as described previously[6]. Hyperimmune PRV1NB, PRV2P, PRV3M, PRV4K, and PRV7S sera show no cross-reactions with MRV[6].
Of the 185 sera samples collected, 20 samples showed the presence of neutralizing antibodies against PRV3M, with a seroprevalence of 10.8% (Table 1). The seropositivity against PRV2P, PRV4K, PRV7S and PRV1N was 5.4% (10/185), 5.9% (11/185), 7.0% (13/185) and 4.3% (8/185), respectively (Table 1). Chi-square tests indicated a significant negative association between seropositivity against PRV3M and the clinical symptoms of headache, poor appetite, hoarseness and tonsillar exudates (Table 2). The P-values were 0.029 for headache (χ2 = 4.80; odds ratio: 0.35, 95% CI: 0.923–0.132), 0.00 for poor appetite (χ2 = 13.40; odds ratio: 0.18, 95% CI: 0.484–0.064), 0.00 for hoarseness (χ2 = 62.00; odds ratio: 0.15, 95% CI: 0.432–0.051) and 0.07 for tonsillar exudates (χ2 = 3.25; odds ratio: 0.19, 95% CI: 1.457–0.024). Because of the limited sample size, no association was observed between seropositivity against PRV3M and contact with bats or the presence of fruit trees near the patients’ homes.
Table 1. Demographic data on seropositivity of specimens and neutralization titers against various PRVs
Specimen Age Gender Industry Fruit tree
near
house?Contact
with
batsPRV1N
(NBV)PRV2P
(PulV)PRV3M
(MelV)PRV4K
(KamV)PRV7S
(SikV)Rembau 7* 47 Female Unemployed and students Yes Yes 1:40 1:80 1:80 1:80 1:40 Rembau 21 19 Male Manufacturing Yes No 1:80 1:160 1:160 1:320 1:80 Rembau 51 17 Female Unemployed and students No No < 1:20 < 1:20 1:20 < 1:20 < 1:20 Rembau 55 68 Male Police and customs No No < 1:20 1:20 1:40 1:20 1:20 Rembau 57 41 Female Education No No < 1:20 1:20 1:20 1:20 1:20 Rembau 58 26 Female Unemployed and students Yes No 1:20 1:40 1:80 1:40 1:40 Rembau 66 61 Male Medical Yes No 1:40 1:40 1:320 1:40 1:80 Rembau 67 22 Male Agricultural Yes Yes < 1:20 < 1:20 1:20 < 1:20 < 1:20 Rembau 68 14 Female Unemployed and students Yes Yes 1:20 1:80 1:80 1:20 1:40 Rembau 82 20 Male Education Yes No < 1:20 < 1:20 1:20 < 1:20 < 1:20 Rembau 87 18 Male Unemployed and students No Yes < 1:20 < 1:20 1:20 < 1:20 < 1:20 Rembau 93 21 Female Unemployed and students Yes No 1:20 1:320 1:80 1:40 1:80 Rembau 95 52 Female Cleaning and Sanitization No No < 1:20 < 1:20 1:20 < 1:20 < 1:20 Rembau 100 54 Male Agricultural Yes No < 1:20 < 1:20 1:20 < 1:20 < 1:20 Rembau 107 72 Female Unemployed and students No No 1:20 1:80 1:80 1:40 1:40 Rembau 108 24 Female Medical No No < 1:20 < 1:20 1:80 1:160 1:40 Rembau 110 20 Female Manufacturing No No < 1:20 < 1:20 1:20 < 1:20 1:20 Rembau 112 24 Female Unemployed and students No No < 1:20 < 1:20 1:40 < 1:20 < 1:20 Rembau 118 35 Female Education Yes Yes < 1:20 < 1:20 1:20 < 1:20 1:20 Rembau 164 17 Female Unemployed and students No No 1:20 1:80 1:160 1:20 1:40 Count 8 10 20 11 13 Percentage (%) 4.3 5.4 10.8 5.9 7.0 Note. *The patient also positive of Pteropine orthoreovirus (PRV) RNA detection. Table 2. Clinical presentation of patients with Pteropine orthoreovirus (PRV) seropositivity
Patient ID No.
days
of
feverCough Phlegm Runny
noseHeadache* Bodyache Diarrhea Fatigue Poor
appetite*Vomiting Sore
throatHoarseness* Cervical
lymph
node
palpableTonsil
erythemaTonsil
injectedTonsil
enlargedTonsil
exudates*Rembau 007 5 Yes Yes Yes No Yes Yes Yes Yes No Yes Yes No No Yes Yes No Rembau 021 2 Yes Yes Yes Yes Yes No No No No Yes Yes No No Yes No No Rembau 051 5 Yes Yes No No No No No No No No No No No No No No Rembau 055 4 Yes Yes Yes No No No No No No No No No No No No No Rembau 057 2 Yes Yes No Yes Yes No Yes Yes No No No No No No No No Rembau 058 4 Yes Yes Yes No No No No No No No No No No Yes No No Rembau 066 3 Yes Yes Yes No No No No No No No No No No Yes No No Rembau 067 2 Yes Yes Yes No No No Yes No No Yes No No No Yes No No Rembau 068 1 No No No Yes No No Yes No No No No No No Yes No No Rembau 082 4 Yes No Yes No No No No No No No No No Yes No No No Rembau 087 3 Yes Yes Yes No Yes No Yes Yes No Yes No No No Yes No No Rembau 093 1 Yes Yes Yes No No No Yes No No No No No No Yes No Yes Rembau 095 2 Yes No Yes No No No No No No Yes No No No No No No Rembau 100 2 Yes Yes Yes Yes Yes No Yes Yes No Yes No No No No No No Rembau 107 2 Yes Yes Yes No Yes No Yes No No Yes No No No Yes No No Rembau 108 2 Yes Yes No Yes Yes Yes Yes No No Yes Yes No No Yes No No Rembau 110 2 Yes Yes Yes No Yes No No No No Yes No No No Yes No No Rembau 112 5 Yes Yes Yes Yes No No No Yes Yes Yes Yes No No Yes No No Rembau 118 1 Yes Yes Yes Yes Yes No Yes Yes No Yes Yes No No Yes No No Rembau 164 5 Yes Yes No No No No No No No No No No No No No No Note. *Headache, poor appetite, hoarseness and tonsil exudate are negatively associated with sero-positivity agaisnt PRV3M (Melaka virus). The P-value for headache is 0.029, ( χ2 = 4.80; Odds ratio 0.35, 95% CI: 0.923–0.132), poor appetite is 0.00 ( χ2 = 13.40; Odds ratio 0.18, 95% CI: 0.484–0.064), hoarseness is 0.00 ( χ2 = 62.00; Odds ratio 0.15, 95% CI: 0.432–0.051), and tonsil exudates is 0.07 ( χ2 = 3.25; Odds ratio 0.19, 95% CI: 1.457–0.024). A total of eight specimens (highlighted in Table 1) showed cross-neutralization against all five PRV variants in this study. Notably, one of the specimens (Rembau 7) was collected from the same patient who had PRV RNA positivity in oropharyngeal swabs in a previous study. The Rembau 7 patient was reported to have cough and sore throat with hyperemic enlarged tonsils, thus closely resembling the signs and symptoms of PRV infection reported previously[1-3]. Although PRV isolation was not attempted in this study, the cumulative findings indicated that the Rembau 7 patient had acute PRV infection at the time of specimen collection. In contrast, although IgM detection was not performed, the seven patients who were not positive for PRV RNA detection but shown positive cross-neutralization antibodies against PRV might indicate that these patients were in the seroconversion phase of PRV infection, with diminished viral RNA shedding.
A total of 16 sera contained neutralizing antibodies that did not cross-neutralize all five PRV variants in this study. Heterogeneity of cross-neutralizing antibodies has also been reported in another study[6,10]. Together, these observations prompt several questions regarding serology surveillance of PRV in human populations: Does the longevity of PRV neutralizing antibodies in infected patients result in loss of cross-neutralization among PRV variants? Do PRV viral particles have different antibody binding sites that may be targeted by different monoclonal antibodies? Is the heterogeneity of cross-neutralizing antibodies associated with different epitopes? On the basis of the current findings, the factors associated with the heterogeneity in cross-neutralizing antibodies were unclear. However, seropositivity against PRV1N was consistently observed to be lower than that against other PRV variants in this study and in previously reported studies[6-7,10]. Notably, PRV1N is less genetically closely related to the four Malaysian PRV isolates[10].
At the time of writing, more than 17 PRV isolates have been discovered, most recently in Africa[5], thus underscoring the high genetic diversity and widespread distribution of PRV. Most index cases of direct PRV spillover have been reported in Malaysia, and imported cases have been reported in Hong Kong, China and Japan in patients who had visited Southeast Asia[4]. This study concluded that PRV spillover was present in the Rembau district, although whether this spillover occurred directly from bats to humans, as suspected for the human cases reported in Malaysia[1-3], or from an intermediate host (or hosts) yet to be identified, remains unclear. The findings from this study and the serological evidence reported in Tioman island indicated higher seroprevalence rates in areas with high interaction between bats and humans. Thus, further research on serological prevalence in areas with high bat-human interaction is warranted.
Acknowledgement We thank Dr. Patricia Lim Kim Chooi from the School of Medicine, International Medical University for critically reviewing and proof-reading the manuscript.
Conflicts of Interest All authors declare no conflicts of interest.
Authors Contributions Conceived and designed the experiments: VOON Kenny, WONG Siew Tung, and TENG Cheong Lieng. Performed the experiments: LEONG Wai Jing and VOON Kenny. Performed data analysis and interpretation: LEONG Wai Jing, VOON Kenny, WONG Siew Tung, and LEONG Pooi Pooi. Contributed reagents and materials: LEONG Pooi Pooi and WANG Linfa. Performed manuscript drafting and review: LEONG Wai Jing, VOON Kenny, WONG Siew Tung, TENG Cheong Lieng, LEONG Pooi Pooi, and WANG Linfa. All authors read and approved the final version of the manuscript.
doi: 10.3967/bes2022.098
Seroprevalence of Pteropine orthoreovirus (PRV) Infection among Outpatients in a Clinic in Rembau, Malaysia
-
&These authors contributed equally to this work.
注释: -
Figure 1. Studies of Pteropine orthoreovirus (PRV) spillover in Peninsular Malaysia. Human serum collection sites [Rembau (this study) and Tioman island (previous study)] are labeled with * on the map. Relative distances (measured by using Google Maps) of human serum collection sites in Rembau with respect to those for other previously reported PRVs in Malaysia are shown on the map. PRV2P (Pulau virus) isolated from pteropodid bat specimens is labeled with #, and PRV3M (Melaka virus, PRV4K (Kampar virus) and PRV7S (Sikamat virus) isolated from human specimens are labeled with @.
Table 1. Demographic data on seropositivity of specimens and neutralization titers against various PRVs
Specimen Age Gender Industry Fruit tree
near
house?Contact
with
batsPRV1N
(NBV)PRV2P
(PulV)PRV3M
(MelV)PRV4K
(KamV)PRV7S
(SikV)Rembau 7* 47 Female Unemployed and students Yes Yes 1:40 1:80 1:80 1:80 1:40 Rembau 21 19 Male Manufacturing Yes No 1:80 1:160 1:160 1:320 1:80 Rembau 51 17 Female Unemployed and students No No < 1:20 < 1:20 1:20 < 1:20 < 1:20 Rembau 55 68 Male Police and customs No No < 1:20 1:20 1:40 1:20 1:20 Rembau 57 41 Female Education No No < 1:20 1:20 1:20 1:20 1:20 Rembau 58 26 Female Unemployed and students Yes No 1:20 1:40 1:80 1:40 1:40 Rembau 66 61 Male Medical Yes No 1:40 1:40 1:320 1:40 1:80 Rembau 67 22 Male Agricultural Yes Yes < 1:20 < 1:20 1:20 < 1:20 < 1:20 Rembau 68 14 Female Unemployed and students Yes Yes 1:20 1:80 1:80 1:20 1:40 Rembau 82 20 Male Education Yes No < 1:20 < 1:20 1:20 < 1:20 < 1:20 Rembau 87 18 Male Unemployed and students No Yes < 1:20 < 1:20 1:20 < 1:20 < 1:20 Rembau 93 21 Female Unemployed and students Yes No 1:20 1:320 1:80 1:40 1:80 Rembau 95 52 Female Cleaning and Sanitization No No < 1:20 < 1:20 1:20 < 1:20 < 1:20 Rembau 100 54 Male Agricultural Yes No < 1:20 < 1:20 1:20 < 1:20 < 1:20 Rembau 107 72 Female Unemployed and students No No 1:20 1:80 1:80 1:40 1:40 Rembau 108 24 Female Medical No No < 1:20 < 1:20 1:80 1:160 1:40 Rembau 110 20 Female Manufacturing No No < 1:20 < 1:20 1:20 < 1:20 1:20 Rembau 112 24 Female Unemployed and students No No < 1:20 < 1:20 1:40 < 1:20 < 1:20 Rembau 118 35 Female Education Yes Yes < 1:20 < 1:20 1:20 < 1:20 1:20 Rembau 164 17 Female Unemployed and students No No 1:20 1:80 1:160 1:20 1:40 Count 8 10 20 11 13 Percentage (%) 4.3 5.4 10.8 5.9 7.0 Note. *The patient also positive of Pteropine orthoreovirus (PRV) RNA detection. Table 2. Clinical presentation of patients with Pteropine orthoreovirus (PRV) seropositivity
Patient ID No.
days
of
feverCough Phlegm Runny
noseHeadache* Bodyache Diarrhea Fatigue Poor
appetite*Vomiting Sore
throatHoarseness* Cervical
lymph
node
palpableTonsil
erythemaTonsil
injectedTonsil
enlargedTonsil
exudates*Rembau 007 5 Yes Yes Yes No Yes Yes Yes Yes No Yes Yes No No Yes Yes No Rembau 021 2 Yes Yes Yes Yes Yes No No No No Yes Yes No No Yes No No Rembau 051 5 Yes Yes No No No No No No No No No No No No No No Rembau 055 4 Yes Yes Yes No No No No No No No No No No No No No Rembau 057 2 Yes Yes No Yes Yes No Yes Yes No No No No No No No No Rembau 058 4 Yes Yes Yes No No No No No No No No No No Yes No No Rembau 066 3 Yes Yes Yes No No No No No No No No No No Yes No No Rembau 067 2 Yes Yes Yes No No No Yes No No Yes No No No Yes No No Rembau 068 1 No No No Yes No No Yes No No No No No No Yes No No Rembau 082 4 Yes No Yes No No No No No No No No No Yes No No No Rembau 087 3 Yes Yes Yes No Yes No Yes Yes No Yes No No No Yes No No Rembau 093 1 Yes Yes Yes No No No Yes No No No No No No Yes No Yes Rembau 095 2 Yes No Yes No No No No No No Yes No No No No No No Rembau 100 2 Yes Yes Yes Yes Yes No Yes Yes No Yes No No No No No No Rembau 107 2 Yes Yes Yes No Yes No Yes No No Yes No No No Yes No No Rembau 108 2 Yes Yes No Yes Yes Yes Yes No No Yes Yes No No Yes No No Rembau 110 2 Yes Yes Yes No Yes No No No No Yes No No No Yes No No Rembau 112 5 Yes Yes Yes Yes No No No Yes Yes Yes Yes No No Yes No No Rembau 118 1 Yes Yes Yes Yes Yes No Yes Yes No Yes Yes No No Yes No No Rembau 164 5 Yes Yes No No No No No No No No No No No No No No Note. *Headache, poor appetite, hoarseness and tonsil exudate are negatively associated with sero-positivity agaisnt PRV3M (Melaka virus). The P-value for headache is 0.029, ( χ2 = 4.80; Odds ratio 0.35, 95% CI: 0.923–0.132), poor appetite is 0.00 ( χ2 = 13.40; Odds ratio 0.18, 95% CI: 0.484–0.064), hoarseness is 0.00 ( χ2 = 62.00; Odds ratio 0.15, 95% CI: 0.432–0.051), and tonsil exudates is 0.07 ( χ2 = 3.25; Odds ratio 0.19, 95% CI: 1.457–0.024). -
[1] Chua KB, Crameri G, Hyatt A, et al. A previously unknown reovirus of bat origin is associated with an acute respiratory disease in humans. Proc Natl Acad Sci USA, 2007; 104, 11424−9. doi: 10.1073/pnas.0701372104 [2] Chua KB, Voon K, Crameri G, et al. Identification and characterization of a new orthoreovirus from patients with acute respiratory infections. PLoS One, 2008; 3, e3803. doi: 10.1371/journal.pone.0003803 [3] Chua KB, Voon K, Yu M, et al. Investigation of a potential zoonotic transmission of orthoreovirus associated with acute influenza-like illness in an adult patient. PLoS One, 2011; 6, e25434. doi: 10.1371/journal.pone.0025434 [4] Tan YF, Teng CL, Chua KB, et al. Pteropine orthoreovirus: an important emerging virus causing infectious disease in the tropics? J Infect Dev Ctries, 2017; 11, 215-9. [5] Bennett AJ, Goldberg TL. Pteropine orthoreovirus in an Angolan soft-furred fruit bat (Lissonycteris angolensis) in Uganda dramatically expands the global distribution of an emerging bat-borne respiratory virus. Viruses, 2020; 12, 740. doi: 10.3390/v12070740 [6] Leong WJ, Quek XF, Tan HY, et al. Seroprevalence of Pteropine orthoreovirus in humans remain similar after nearly two decades (2001-2002 vs. 2017) in Tioman Island, Malaysia. J Med Virol, 2022; 94, 771−5. doi: 10.1002/jmv.27422 [7] Voon K, Tan YF, Leong PP, et al. Pteropine orthoreovirus infection among out-patients with acute upper respiratory tract infection in Malaysia. J Med Virol, 2015; 87, 2149−53. doi: 10.1002/jmv.24304 [8] Voon K, Chua KB, Yu M, et al. Evolutionary relationship of the L- and M-class genome segments of bat-borne fusogenic orthoreoviruses in Malaysia and Australia. J Gen Virol, 2011; 92, 2930−6. doi: 10.1099/vir.0.033498-0 [9] Singh H, Shimojima M, Ngoc TC, et al. Serological evidence of human infection with Pteropine orthoreovirus in Central Vietnam. J Med Virol, 2015; 87, 2145−8. doi: 10.1002/jmv.24274 [10] Uehara A, Tan CW, Mani S, et al. Serological evidence of human infection by bat orthoreovirus in Singapore. J Med Virol, 2019; 91, 707−10. doi: 10.1002/jmv.25355 -




 下载:
下载:



 Quick Links
Quick Links