-
Neisseria meningitidis (N. meningtitidis) is a gram negative diplococcus. Neisseria meningitidis (meningococcus) was first identified in the cerebrospinal fluid of a patient with meningitis in 1887 by Weichselbaum, and the epidemic was traced back to 1805 [1]. The serogroups A, B, C, W, X, and Y were the most frequent causes of meningococcal diseased [1]. N. meningitidis usually colonizes the human nasopharynx [2]. The common clinical manifestations of invasive N. meningitidis infection are meningococcemia and acute meningitis [2]. Some patients with meningococcal disease may present with pneumonia, arthritis, pericarditis, conjunctivitis, epiglottitis, sinusitis, otitis, urethritis, and proctitis [2]. However, patients rarely present with acute peritonitis initially. Tens of cases of peritonitis caused by N. meningitidis had been reported in the USA, France, and some other countries [3-6]. However, until now, no similar unusual case has been reported in China, while many usual meningitis or septicemia cases were found during the last decades.
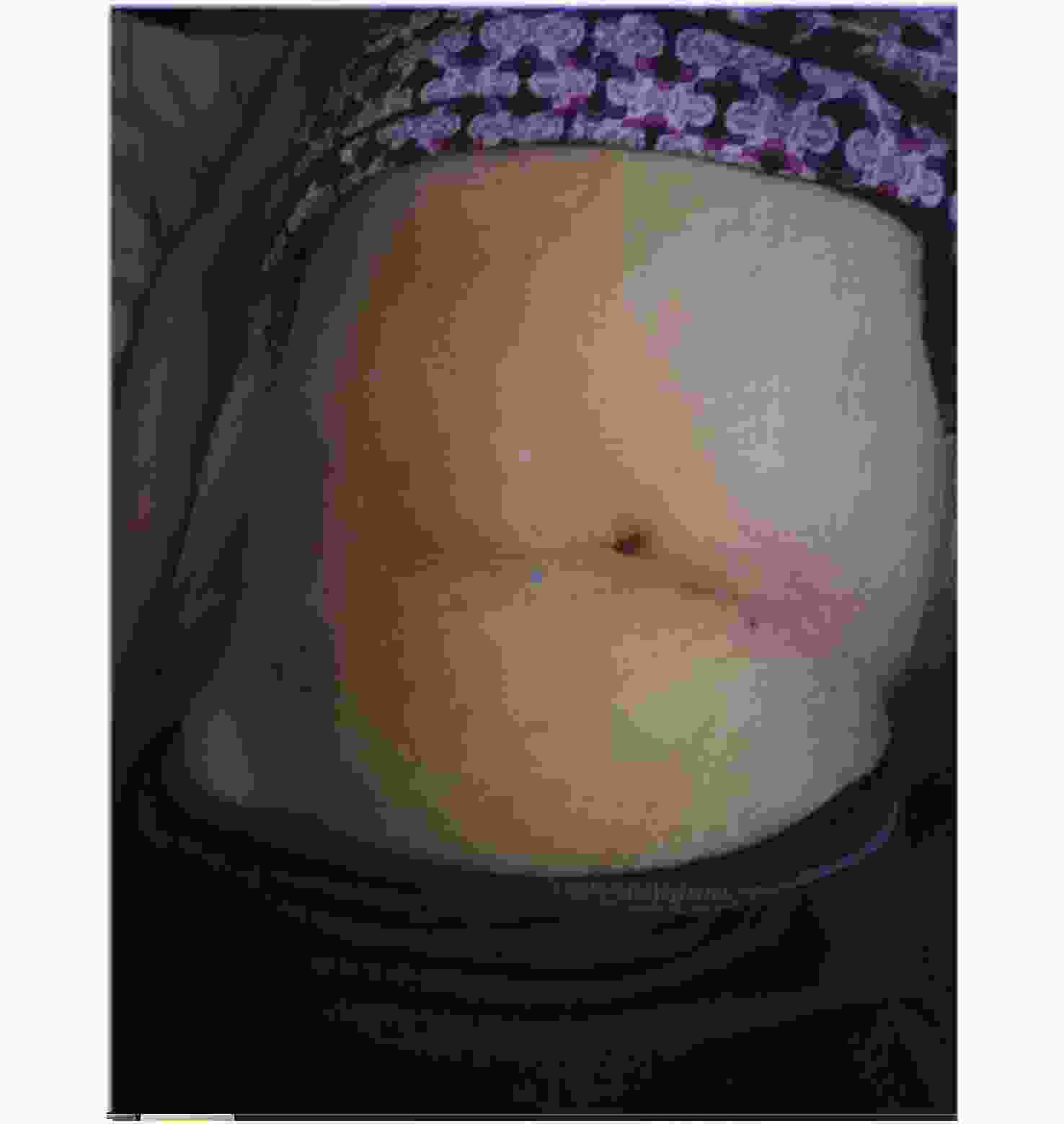
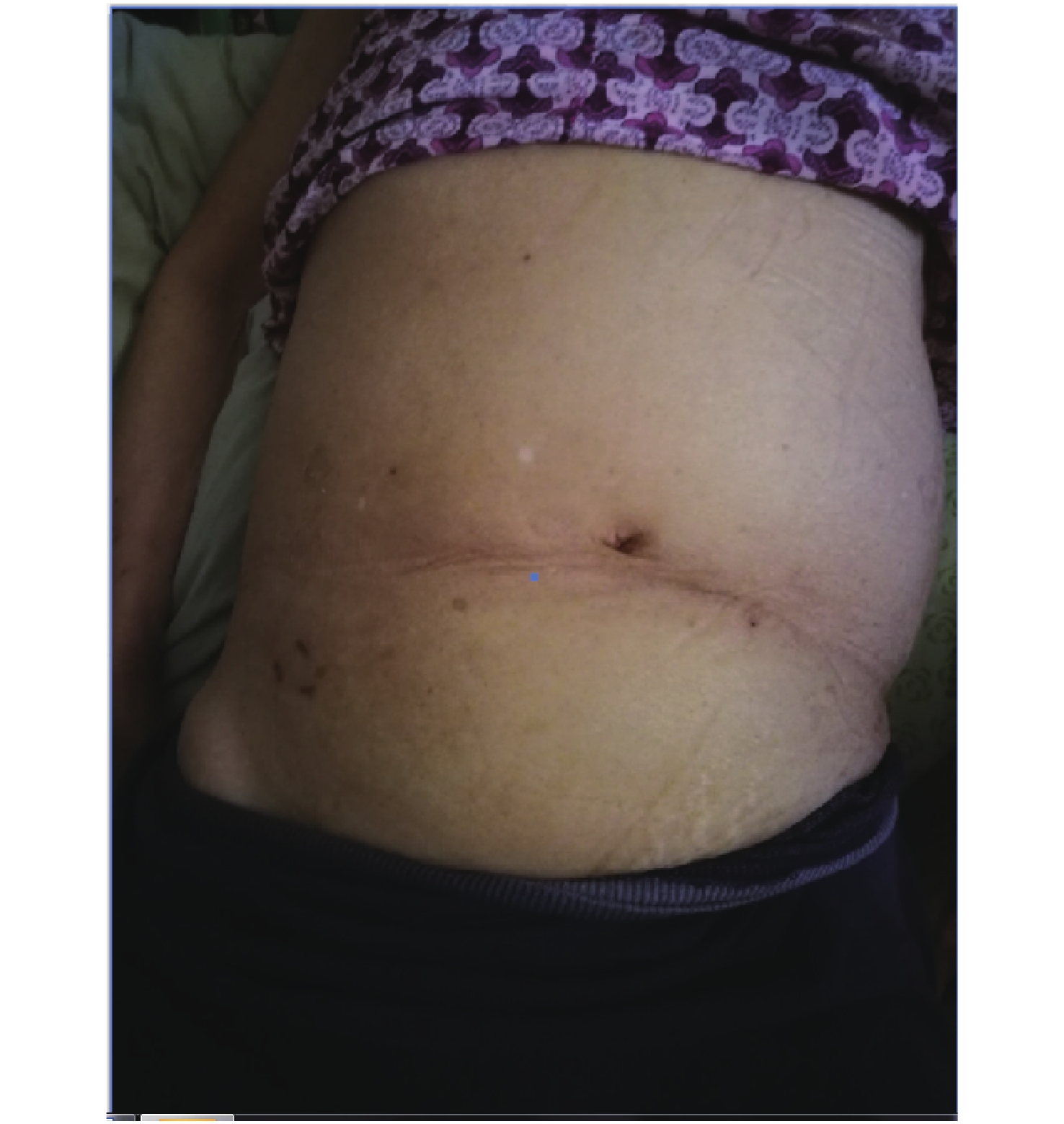
We report a case of acute peritonitis with N. meningitidis blood infection. A 72-year-old woman presented with vomiting during the last two days at the Xiantao First People’s Hospital on May 21, 2019. The patient has a 10-year history of liver cirrhosis with intermittent use of oral medication. On physical examination, her temperature was 37.2 °C and her abdomen was visibly swollen (Figure 1). Color Doppler ultrasound showed splenomegaly, a splenic cyst, and abdominal and pelvic fluid. The abdominal effusion was turbid and cytology of the ascitic fluid aspirate showed a large number of neutrophils, some mesothelial cells, and a few red blood cells and lymphocytes. CT scan revealed bilateral lung infection and a small amount of pleural effusion bilaterally. Laboratory tests showed higher levels of total bilirubin (36.1 μmol/L), direct bilirubin (10.9 μmol/L), indirect bilirubin (25.2 μmol/L), aspartate aminotransferase (47 IU/L), aspartate and glutathione ratio (2.61), chlorine (107.7 mmol/L), and urea (9.29 mmol/L), while values of total protein (47.7 g/L), albumin (30.8 g/L), globulin (16.9 g/L), potassium (2.62 mmol/L), calcium (2.10 mmol/L), phosphorus (0.75 mmol/L), glucose (1.18 mmol/L), and carbon dioxide (20.3 mmol/L) were lower than the normal reference values. Infectious disease screening showed higher liters of antibodies against hepatitis B virus e antigen (15.6 ncu/mL), hepatitis B virus core antigen (183.51 ncu/mL), and hepatitis C virus (30.31 s/co). Based on these results, the patient was diagnosed with chronic hepatitis B, hypersplenism, chronic hepatitis C, liver cirrhosis, and acute peritonitis. During her inpatient stay, the abdominal fluid was drained using peritoneal punctures and catheterization. Ceftazidime, imipenem, and cilastatin were used as treatments. Concomitantly, an antacid was used to protect the stomach, and albumin supplementation and nutritional support were carried out. The patient was discharged on May 30, 2019, and transferred to a community hospital. Upon discharge, the patient had intermittent distension and discomfort in an afebrile context.
Using slide agglutination, a serogroup C N. meningitidis strain was isolated from the patient’s blood on May 21, 2019 (strain HYD) [7]. By further investigating the patient’s close contacts on June 3, 2019, her husband was a N. meningitidis carrier with a N. meningitidis serogroup C strain (strain GQR1) in a throat swab sample. Although her husband was asymptomatic, penicillin was used as prophylaxis. Later, a N. meningitidis serogroup C strain (strain GQR2) was isolated from her husband’s throat swab sample on June 5, 2019, and antibiotics susceptibility tests showed that serotypes HYD, GQR1, and GQR2 were susceptible to penicillin, ceftriaxone, meropenem, azithromycin, and rifampicin, while they were resistant to ciprofloxacin (Table 1). Interestingly, strain GQR2, which was susceptible to penicillin, was isolated from her husband for penicillin treatment for two days. This might be explained by the fact that penicillin could not clear the N. meningitidis in the throat [8].
Table 1. Minimal inhibit concentration to antibiotics of N. meningitidis strains HYD, GQR1, and GQR2
Antibiotics HYD GQR1 GQR2 Penicillin (µg/mL) 0.023 (S) 0.032 (S) 0.032 (S) Ceftriaxone (µg/mL) < 0.016 (S) < 0.016 (S) < 0.016 (S) Meropenem (µg/mL) < 0.016 (S) < 0.016 (S) < 0.016 (S) Azithromycin (µg/mL) 0.75 (S) 0.50 (S) 0.125 (S) Rifampicin (µg/mL) 0.032 (S) 0.016 (S) 0.012 (S) Ciprofloxacin (µg/mL) 0.064 (I) 0.19 (R) 0.094 (I) Note. S, susceptible; I, intermediate; R, resistant. Since 1917, only 33 similar cases have been reported [3-6]. The patients’ ages ranged from 10 months to 91 years, and most were younger than 42 years [3-6], both males and females. Among these 33 patients, nine patients died and 23 recovered [3-6]. Among the seven patients older than 50 years, six died [3-6]. This implies that elderly people with N. meningitidis peritonitis are at a higher risk of death. Patients usually present with fever, nausea, vomiting, and abdominal pain, and some patients presented with exanthema, diarrhea, chills, weight loss, headache, cough, pericarditis, tachycardia, hypotension, and leg edema [3-6]. According to previous reports, fever was the most frequent accompanying symptom of acute peritonitis caused by N. meningitidis [3]. However, in our case, the patient’s temperature was stable and normal throughout the inpatient stay.
Furthermore, our case suffered from cirrhosis of the liver concomitantly with N. meningitidis infection. Although spontaneous bacterial peritonitis (SBP) in patients with advanced cirrhosis is common, N. meningitidis infection is rare [9]. It is worth mentioning that two identical N. meningitidis strains were isolated from the husband’s throat swab, although he developed no abdominal symptom, which suggests that cirrhosis might contribute to the onset of acute peritonitis by N. meningitidis infection from blood. In the literature, three women (60, 72, and 80 years), two men (42 and 65 years), and one 72-year-old person (gender unknown), including our case, are known cirrhosis patients with N. meningitidis infection [3, 4]; four of which died[3, 4]. This shows that cirrhosis might increase the fatality of N. meningitidis peritonitis.
For further studies, the genome of these three strains was sequenced by MGISEQ-2000 and followed by De novo assembly in Wuhan MGI Tech Co., Ltd. These genome sequences were submitted to the PubMLST database, and the accession numbers were 101275 (HYD), 101276 (GQR1), and 101277 (GQR2), respectively. The three strains were all C:ST-4821:P1.7-2,14 according to the genome sequences. All 72 genomes of ST-4821 N. meningitidis isolates available in the PubMLST database, together with the three genomes mentioned above, were used to construct a phylogenetic tree using the Comparator tool in the pubMLST and SplitsTree4 (version 4.14.6). The result indicated that strains HYD, GQR1, and GQR2 were clustered in a single branch and separated from other reported ST-4821 strains (Figure 2). These three stains may be different from the other reported ST-4821 N. meningitidis strains. Compared to other ST-4821 N. meningitidis strains, 25 special genes were identified in the genomes of strain HYD, GQR1, and GQR2 (Supplementary Table S1 available in www.besjournal.com). Among these genes, 16 had substitution mutations (one to 14 amino acids substitutions), one insertion mutation, six synonymous mutation, and two had insertion mutation and substitution mutations (one to three amino acids substitutions). The serogroups A, B, C, and W of N. meningitidis were found to cause acute peritonitis, and most cases were caused by the serogroup C. Serogroup Y was also found in China and other countries. However, there is no reported case of acute peritonitis caused by serogroup Y N. meningitidis strain. These may be due to the difference between serogroup Y and the other serogroups A, B, C, and W. Genome comparison analysis between serogroup Y and serogroups A, B, C, and W may reveal the impact factors. Unfortunately, it is not possible to further predict the potential virulence genes and virulence factors due to the lack of sequence type and genome sequence in previous cases. Here we report the genome sequences and sequence types of our isolates to provide more information for further study in the future.
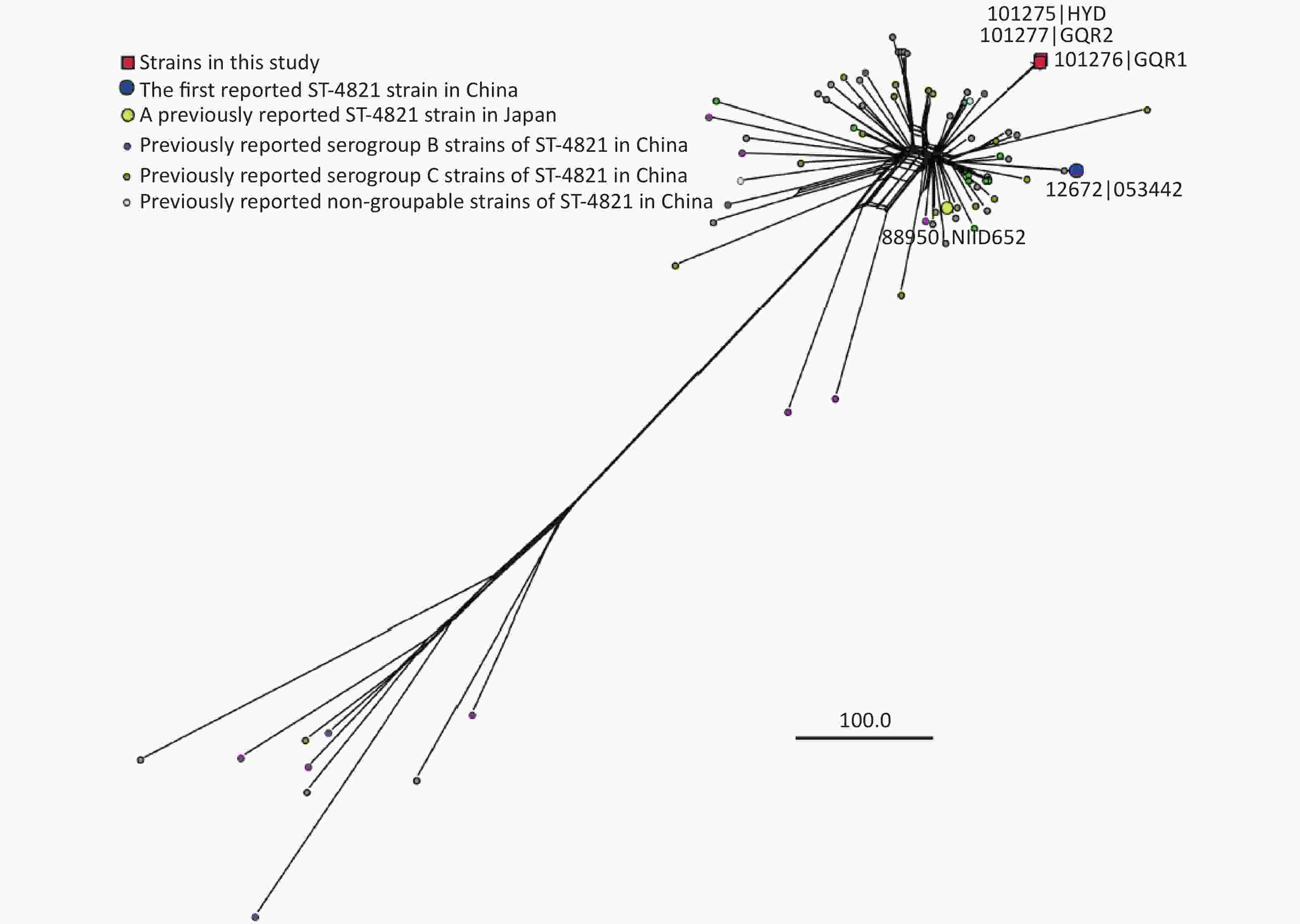
Figure 2. Neighbor-net tree was constructed based on core genes of 75 ST-4821 N. meningitidis genome sequences. The phylogenetic analysis was carried out in the PubMLST database by N. meningitidis cgMLST v1.0 and the parameters of Min identity, Min alignment, BLASTN word size, and core threshold were 50%, 70%, 20, and 90%, respectively. Genomes 101275 (HYD), 101276 (GQR1), and 101277 (GQR2) were sequenced in this study and the others were from the PubMLST database.
In summary, this case was special and showed that (1) no fever, (2) SBP caused by N. meningitidis, and (3) isolates were separated from other reported ST-4821 strains based on phylogenetic analysis. At present, the trigger for N. meningitidis peritonitis and the determinants of invasive disease are still unknown. Some researchers propose that acute peritonitis might occur from insufficient mesenteric perfusion, septic microinfarction in the greater omentum, invasion of the visceral area (through blood, ascending from the genitourinary tract, or swallowing infected saliva), or immune complex deposition [3, 10]. Further study and genome analysis may provide some points. Ignorance of acute peritonitis caused by N. meningitidis may delay the diagnosis. In most cases, surgery is considered if acute peritonitis is suspected, while antibiotic treatment is sufficient [3, 10]. Accordingly, clinicians should pay more attention to peritonitis caused by N. meningitidis. To the best of our knowledge, this is the first report on acute peritonitis caused by N. meningitidis in China and other genomes. This study may provide more information about the clinical diagnosis and study of acute peritonitis and N. meningitidis infection.
No potential conflicts of interest were disclosed.
Table S1. The gene mutations of HYD, GQR1 and GQR2 strains compared to the other ST-4821 strains
Locus Product Types of mutation (Number of amino acid substitution
in HYD, GQR1 and GQR2 strains)NEIS0206 oligopeptidase A Synonymous mutation NEIS0275 putative outer membrane solvent tolerance protein Substitution mutation (1) NEIS0405 hypothetical protein Insertion mutation + Substitution mutations (3) NEIS1031 putative cell-division protein Substitution mutation (1) NEIS1396 fumarate hydratase class II (EC 4.2.1.2) Substitution mutations (5) NEIS1553 Lipid A phosphoethanolamine transferase Synonymous mutation NEIS1635 transcriptional regulator Insertion mutation + Substitution mutation (1) NEIS1924 ClpXP protease specificity-enhancing factor Insertion mutation NEIS1925 pilE expression regulator/putative sspA-like protein Substitution mutation (1) NEIS1930 putative periplasmic protein Substitution mutations (8) NEIS1932 hypothetical protein Substitution mutations (3) NEIS1933 periplasmic/outer membrane protein Substitution mutations (11) NEIS2024 phosphoenolpyruvate-protein phosphotransferase Substitution mutation (1) NEIS2135 putative nicotinamidase Substitution mutation (1) NEIS0819 threonine dehydratase Synonymous mutation NEIS0820 putative sulphate permease ATP-binding protein Substitution mutations (2) NEIS0821 putative sulphate permease inner membrane protein Substitution mutation (1) NEIS0907 dihydrodipicolinate synthase Substitution mutation (1) NEIS1033 gamma-glutamyl kinase Substitution mutations (4) NEIS1034 2-isopropylmalate synthase Synonymous mutation NEIS1036 prolipoprotein diacylglyceryl transferase Substitution mutation (1) NEIS1037 hypothetical protein Synonymous mutations NEIS1250 trigger factor Substitution mutations (3) NEIS1251 ftsK-like cell division/stress response protein Substitution mutations (14) NEIS1721 oxidoreductase Synonymous mutation
doi: 10.3967/bes2022.021
-
&These authors contributed equally to this work.
注释: -
Figure 2. Neighbor-net tree was constructed based on core genes of 75 ST-4821 N. meningitidis genome sequences. The phylogenetic analysis was carried out in the PubMLST database by N. meningitidis cgMLST v1.0 and the parameters of Min identity, Min alignment, BLASTN word size, and core threshold were 50%, 70%, 20, and 90%, respectively. Genomes 101275 (HYD), 101276 (GQR1), and 101277 (GQR2) were sequenced in this study and the others were from the PubMLST database.
Table 1. Minimal inhibit concentration to antibiotics of N. meningitidis strains HYD, GQR1, and GQR2
Antibiotics HYD GQR1 GQR2 Penicillin (µg/mL) 0.023 (S) 0.032 (S) 0.032 (S) Ceftriaxone (µg/mL) < 0.016 (S) < 0.016 (S) < 0.016 (S) Meropenem (µg/mL) < 0.016 (S) < 0.016 (S) < 0.016 (S) Azithromycin (µg/mL) 0.75 (S) 0.50 (S) 0.125 (S) Rifampicin (µg/mL) 0.032 (S) 0.016 (S) 0.012 (S) Ciprofloxacin (µg/mL) 0.064 (I) 0.19 (R) 0.094 (I) Note. S, susceptible; I, intermediate; R, resistant. S1. The gene mutations of HYD, GQR1 and GQR2 strains compared to the other ST-4821 strains
Locus Product Types of mutation (Number of amino acid substitution
in HYD, GQR1 and GQR2 strains)NEIS0206 oligopeptidase A Synonymous mutation NEIS0275 putative outer membrane solvent tolerance protein Substitution mutation (1) NEIS0405 hypothetical protein Insertion mutation + Substitution mutations (3) NEIS1031 putative cell-division protein Substitution mutation (1) NEIS1396 fumarate hydratase class II (EC 4.2.1.2) Substitution mutations (5) NEIS1553 Lipid A phosphoethanolamine transferase Synonymous mutation NEIS1635 transcriptional regulator Insertion mutation + Substitution mutation (1) NEIS1924 ClpXP protease specificity-enhancing factor Insertion mutation NEIS1925 pilE expression regulator/putative sspA-like protein Substitution mutation (1) NEIS1930 putative periplasmic protein Substitution mutations (8) NEIS1932 hypothetical protein Substitution mutations (3) NEIS1933 periplasmic/outer membrane protein Substitution mutations (11) NEIS2024 phosphoenolpyruvate-protein phosphotransferase Substitution mutation (1) NEIS2135 putative nicotinamidase Substitution mutation (1) NEIS0819 threonine dehydratase Synonymous mutation NEIS0820 putative sulphate permease ATP-binding protein Substitution mutations (2) NEIS0821 putative sulphate permease inner membrane protein Substitution mutation (1) NEIS0907 dihydrodipicolinate synthase Substitution mutation (1) NEIS1033 gamma-glutamyl kinase Substitution mutations (4) NEIS1034 2-isopropylmalate synthase Synonymous mutation NEIS1036 prolipoprotein diacylglyceryl transferase Substitution mutation (1) NEIS1037 hypothetical protein Synonymous mutations NEIS1250 trigger factor Substitution mutations (3) NEIS1251 ftsK-like cell division/stress response protein Substitution mutations (14) NEIS1721 oxidoreductase Synonymous mutation -
[1] Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol Biol, 2012; 1−20. [2] Caugant DA, Maiden MCJ. Meningococcal carriage and disease-population biology and evolution. Vaccine, 2009; 27, B64−70. doi: 10.1016/j.vaccine.2009.04.061 [3] Akinosoglou K, Alexopoulos A, Koutsogiannis N, et al. Neisseria meningitidis presenting as acute abdomen and recurrent reactive pericarditis. Braz J Infect Dis, 2016; 20, 641−4. doi: 10.1016/j.bjid.2016.08.005 [4] Kleinpeter MA, Krane NK. Neisseria meningitidis peritonitis in a CAPD patient: first case report and review of the literature. Adv Perit Dial, 1995; 11, 168−71. [5] Contero C, Martín C, Gómez A, et al. Acute abdominal pain as the initial manifestation of meningococcemia in adult patient. Enferm Infecc Microbiol Clin, 2020; 38, 247−8. doi: 10.1016/j.eimc.2019.09.008 [6] de Almeida Gentile JK, Franciss MY, Brasil HR. Neisseria meningitidis peritonitis serotype C: case report. Arq Bras Cir Dig, 2016; 29, 67. [7] Craven DE, Frasch CE, Robbins JB, et al. Serogroup identification of Neisseria meningitidis: comparison of an antiserum agar method with bacterial slide agglutination. J Clin Microbiol, 1978; 7, 410−4. doi: 10.1128/jcm.7.5.410-414.1978 [8] Plotkin SA, Orenstein W, Offit PA, et al. Vaccines E-book. Elsevier Health Sciences. 2017. [9] Bunchorntavakul C, Chamroonkul N, Chavalitdhamrong D. Bacterial infections in cirrhosis: a critical review and practical guidance. World J Hepatol, 2016; 8, 307−21. doi: 10.4254/wjh.v8.i6.307 [10] Sanz Álvarez D, Blázquez Gamero D, Ruiz Contreras J. Abdominal acute pain as initial symptom of invasive meningococcus serogrup A illness. Arch Argent Pediatr, 2011; 109, e39−41. -
 21322Supplementary Materials.pdf
21322Supplementary Materials.pdf

-




 下载:
下载:





 Quick Links
Quick Links