-
Multidrug-resistant tuberculosis (MDR-TB) is caused by mycobacterium tuberculosis strains that are resistant to at least isoniazid and rifampicin; meanwhile, extensively drug-resistant tuberculosis (XDR-TB) is caused by multidrug-resistant strains that have fewer therapeutic drugs[1]. MDR-TB is a serious public health concern in China, as it is one of the 30 countries with a high burden of MDR-TB, with the second-highest number of MDR-TB patients worldwide[2].
Effective anti-TB medications are essential for the treatment of MDR. Bedaquiline is the first new anti-TB drug to be approved since 1971, and the World Health Organization (WHO) has recommended it as a first-priority drug for MDR-TB in 2020 [3] and the most recent Chinese MDR-TB treatment guidelines [4-5]. However, the high price of Bedaquiline restricts its widespread use in many countries, particularly in developing countries such as China. Although Bedaquiline was included as a category B drug in the National Reimbursement Drug List at the beginning of 2020, reimbursement policies vary across provinces, and it is expected that Bedaquiline accessibility will be low to a large proportion of MDR-TB patients. Therefore, conventional regimens (without Bedaquiline and with a longer duration) are still prevalent for lower costs and a more favorable reimbursement policy. More provinces, including Jiangsu and Chongqing, have initiated centralized procurement of anti-TB drugs (including Bedaquiline) to increase MDR-TB patients’ access to treatment [6]. Data on what kinds of drugs and prices are crucial for evidence-informed decision-making.
Based on cost-effectiveness, economic research aids in determining reasonable drug prices. Numerous countries worldwide have conducted economic analyses to inform public health, medical insurance, and health resource investment decisions. The results indicate that Bedaquiline was cost effective in the inspected context [7-15]. In addition, the cost-effectiveness [incremental cost-effectiveness ratio (ICERs)] of Bedaquiline varied based on various national scenarios, such as economic development level, case management, patient characteristics, and the efficacy of the drugs in a real-world setting. Evidence from China is limited and primarily based on local parameters. Although several related studies are focusing on China, the results may be undervalued because they were conducted in a high-income city [14] or lacked the most recent data on the Chinese population [10,13].
This study analyzed the cost-effectiveness of the Bedaquiline regimen for treating MDR-TB patients in China to serve as a guide for policy making, including establishing reasonable drug prices.
-
Using a decision tree and a Markov model, we analyzed the ten-year cost-effectiveness of two regimens for adult MDR-TB patients from the health system’s perspective. Two regimens were used to treat a hypothetical cohort of 1,000 patients with MDR-TB who met the treatment standards[5] according to the technical Guide for Tuberculosis Prevention and Control in China (Table 1). The conventional regimen (CR) contained no Bedaquiline and lasted for 16 months. For the Bedaquiline regimen (BR), depending on the sensitivity of fluoroquinolone and previous usage of the secondary-line drug, three different treatment courses were employed [16]. There were 45% patients receiving a short-course treatment lasting nine months, whereas 30% and 25% of patients receiving a long-course treatment lasting 18 and 20 months, respectively. Once XDR-TB has developed during treatment, the recommended treatment guidelines will be adhered to.
Table 1. Treatment regimens of MDR-TB and XDR-TB patients
Regimen MDR-TB treatment Portion and duration XDR-TB treatment Conventional
regimen6 ZKm(Am)Lfx(Mfx)Cs(PAS,E)Pto
/18ZLfx(Mfx)Cs(PAS,E)Pto100%;
16 months12ZCmMfxPASCs(Pto)Clr(Amx/Clv)/
18ZMfxPASCs(Pto)Clr(Amx/Clv).
30 monthsBedaquiline
regimenshort-course regimen for fluoroquinolone-sensitive
patients: [4-6BdqAmLfx(Mfx)CfzZHhighPtoE/
5Lfx(Mfx)CfzZE];45%;
9 monthsthe course duration is extended to
30 months.long-course regimen for fluoroquinolone-
sensitive patients, [6Lfx(Mfx)BdqLzd(Cs)Cfz/
12Lfx(Mfx)Cfz Lzd(Cs)]30%;
18 monthslong-course regimen for fluoroquinolone
resistance [6Bdq Lzd(Dlm)CfzCs/14Lzd(Dlm)CfzCs]25%;
20 monthsNote. MDR, Multidrug-resistant; TB, Tuberculosis; XDR, Extensively drug-resistant; Z, Pyrazinamide; Km, Kanamycin; Am, Amikacin; Cm, Capreomycin; Lfx, Levofloxacin; Mfx, Moxifloxacin; Cs, Cycloserine; PAS, Para aminosalicylic acid; E, Ethambutol; Pto, Prothionicotinamide; BDQ, Bedaquiline; Cfz, Chlorofazimin; Hhigh, High dose isoniazid; Clr, Clarithromycin; Amx/Clv, Amoxicillin/clavulanic acid; Lzd, Linezolid; Dlm, Delamani. The simulation model computed cumulative quality-adjusted life years (QALY) and direct medical cost per capita. The ICER was computed and compared to the willingness-to-pay (WTP) threshold recommended by WHO [17] (WTP = 1× per capita GDP/QALY, 72,450 yuan/QALY in 2020)[18], although there is no official WTP threshold in China.
-
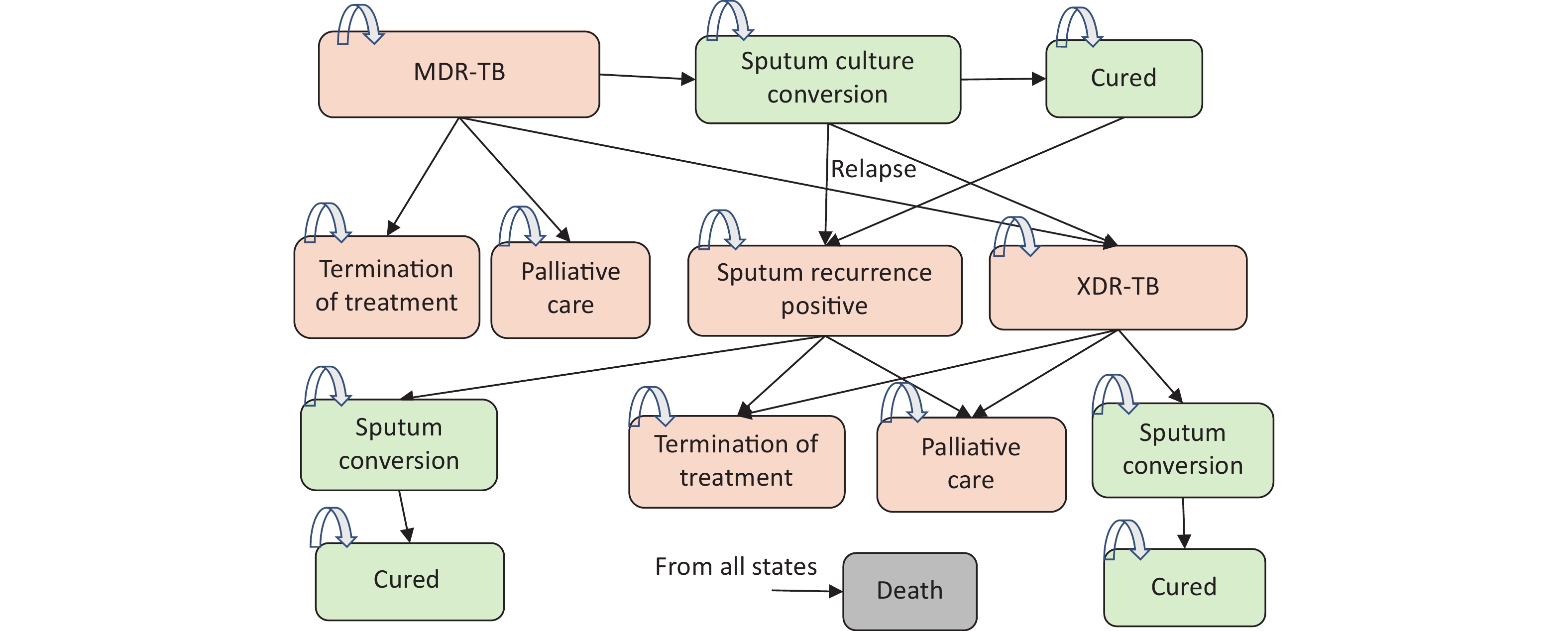
On the basis of published models [7-15], a cohort-based Markov state transition model [19] was constructed to simulate the prognosis of two cohorts of MDR-TB patients treated with two regimens over ten years with a 28-day cycle length. The model’s structure consists of nine fundamental health states (Figure 1). Positive states for sputum culture(SC+) include “MDR” “XDR” “Sputum recurrence positive”“Termination of treatment” and “Palliative care”. Sputum culture negative states(SC-) include “sputum conversion” and “cure”. “Death” is an absorbing state (Figure 1).
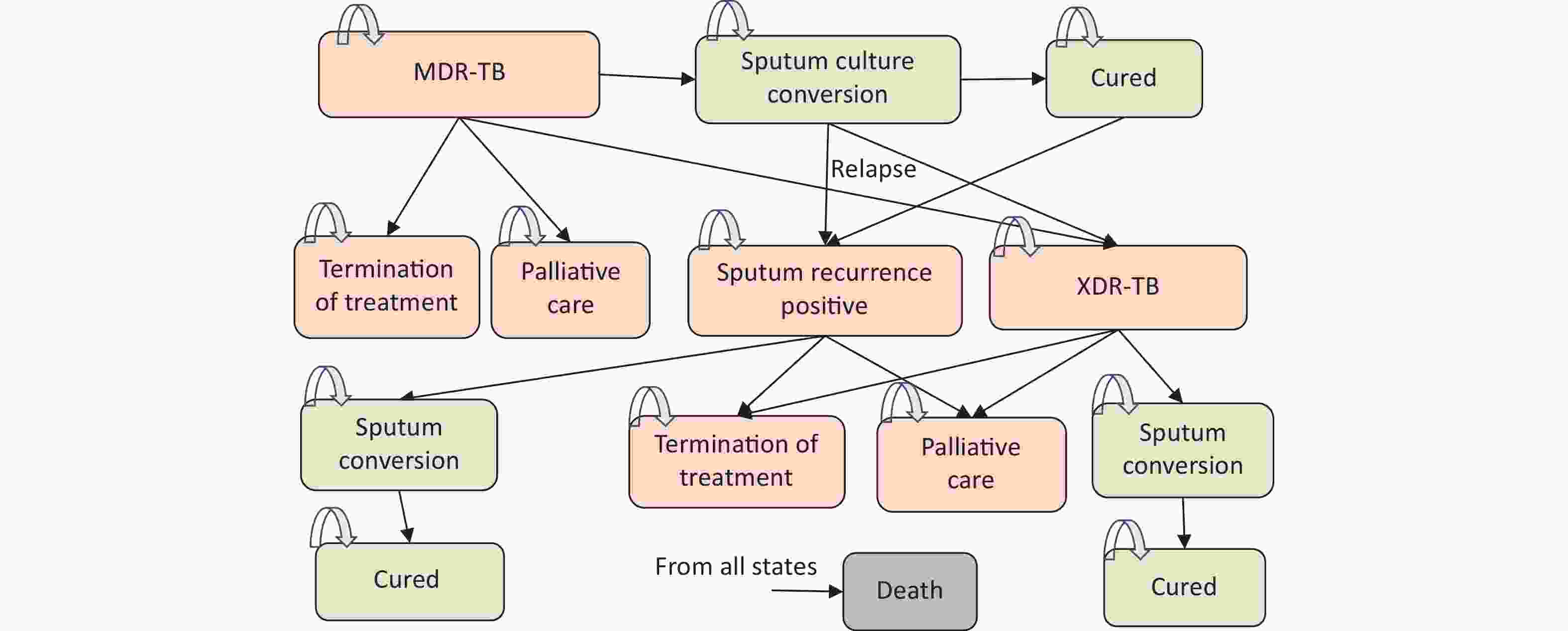
Figure 1. Schematic Diagram of Markov Mode. MDR-TB, Multidrug-resistant tuberculosis; XDR-TB, Extensively drug-resistant tuberculosis.
The cohort initially entered the “MDR” state and received initial treatment; they may remain in the original state, develop into XDR-TB, or convert to SC(-) in each cycle. The remaining “MDR” patients would receive palliative care (identified as “treatment failure”) after the treatment course. The SC(-) patients may be related to SC(+) or XDR, and those who did not relapse until the end of treatment entered the “cure” state. Patients who relapsed to SC(+) continued the prescribed course (secondary treatment), during which they may convert to PC(-) once more, be cured, have their treatment terminated, or enter palliative care after the therapy course. Patients may relapse to PC(+) within two years after the cure, and the same treatment plan is prescribed for relapses. Meanwhile, patients with XDR may experience PC(-) and get cured after treatment or failure of treatment. Death is possible in all states. In the simulation, the objective of drug treatment was to induce sputum culture conversion (transition to sputum culture converted MDR-TB) in the patient cohort and maintain a converted state until treatment completion and assumed cure of MDR-TB.
Once converted to PC(-), patients with a history of relapse will remain PC(-) and be cured upon completion of the entire treatment. MDR and XDR infectiousness fell outside the scope of this model. It was assumed that complications and adverse reactions to treatment would not affect the health utility and cost.
-
Transition Probability For CR, the sputum culture conversion rate in 0–6 months was extracted from actual Global Fund MDR-TB control program data in China[20] (6,115 MDR-TB patients from six provinces were included from 2006 to 2013). Program data from Dalian[21] and Shanghai[22] were used to determine the 12- and 24-month treatment conversion rates. The conversion rate in 0–6 months for BR was derived from the results of the first single-arm multicenter cohort study in the Chinese population in 2021[23]. Due to a lack of relevant data, we employ hazard ratios of conversion rate (BR vs. CR) in the randomized controlled trial (RCT) study to transform the 12-month and 24-month conversion rates of BR[15]. The same approaches were used to calculate secondary, conversion, and cure rates for XDR treatment. Each state’s mortality rate is determined by its spectrum culture statute. All rates were converted to probability in 4 weeks [24] (Table 2).
Table 2. Parameters of transition probability
Parameter Based value Range Source Probability of sputum culture conversion (in 4 weeks) MDR in CR (0–6 months) 0.0873 0.0786–0.0960 MDR-TB control
program[20]MDR in CR (6–12 months) 0.0535 0.0482–0.0589 MDR-TB control
program[21]MDR in CR (12–24 months) 0.0410 0.0367–0.0451 MDR-TB control
program[22]MDR in BR (0–6 months) 0.1230 0.1107–0.1352 literature[23] Hazard Ratio Probability of Sputum conversion for relapse (Secondary vs. primary treatment) 0.540 0.30–0.95 Literature[25] Probability of Sputum conversion for XDR (XDR vs. MDR) 0.398 0.237–0.670 Literature[25] Probability of Sputum conversion for MDR within 6–24 months (BR vs. CR) 2.440 1.57–3.80 Literature[25] Probability of relapse in BR vs. CR 0.320 0.086–1.069 Literature[25] Other probability (in 4 weeks) Relapse from SC(-) 0.010 0.0091–0.0105 Literature[25] Where: relapse to XDR 0.214 0.1712–0.2354 Literature[25] Relapse after cure 0.002 0.0016–0.0022 Literature[25] MDR to XDR 0.002 0.0016–0.0022 Assuming the same
probability of
relapse after cureStopping treatment 0.006 0.0029–0.0091 Literature[26] Mortality (in 4 weeks) Cured state 0.003 0.0025–0.00384 Literature[27] Uncured states (MDR) 0.021 0.0168–0.0252 Literature[27] Uncured states (XDR) 0.027 0.0215–0.0296 Literature[27] Note. MDR, Multidrug-resistant; XDR, Extensively drug-resistant; BR, Bedaquiline regimen; CR, Conventional regimen. Cost Input This study covered direct medical costs, including drug, outpatient, and hospitalization costs (including treatment monitoring and adverse reaction management). The scope of costing was in accordance with a costing program for managing MDR-TB cases by the China CDC (Table 3).
Table 3. Parameters of cost and utility weight
Parameters Based value Range Source Drug cost of CR (RMB, four weeks) MDR (0–6 months) 2,082.93 1,666.35–2,499.52 China CDC drug
price databaseMDR (7–24 months) 2,559.86 2,047.89–3,071.83 China CDC drug
price databaseXDR (0–18 months) 3,270.62 2,616.50–3,924.75 China CDC drug
price databaseXDR (19–32 months) 2,156.33 1,725.06–2,587.60 China CDC drug
price databaseDrug cost of BR (yuan, four weeks) Bedaquiline price (yuan/100 mg) 350.00 280.00–420.00 China CDC drug
price databaseMDR/XDR (0–6 months) Bedaquiline (0–2 weeks, 200 mg/day) 19,600.00 15,680.00–23,520.00 China CDC drug
price databaseBedaquiline (3–24 weeks, 100 mg 3 times a week) 46,200.00 36,960.00–55,440.00 China CDC drug
price databaseBackground Drugs* 4,013.28 3,210.62–4,815.93 China CDC drug
price databaseMDR/XDR (8–20 months) 2,850.229 2,280.18–3,420.28 China CDC drug
price databaseOutpatient and inpatient (yuan, four weeks) Outpatient 364.5 291.6–437.4 TB costing program[16] Hospitalization 755.78 604.62–906.94 TB costing program[16] Utility weight SC(+) state 0.51 0.41–0.61 Literature[28] SC(-) state 0.88 0.70–0.90 Literature[28] Death 0 – Literature[28] Discount rate (annual) 0.05 0.03–0.08 Literature[29] Note. *Drugs excluding Bedaquiline; MDR, Multidrug-resistant; XDR, Extensively drug-resistant; BR, Bedaquiline regimen; CR, Conventional regimen; SC(+), Sputum culture positive; SC(-), Sputum culture negative. The unit drug cost of a standard course for two regimes was calculated based on treatment guidelines [5]. For BR, short- and long-term unit drug costs were integrated [16]. The prices of anti-TB medications were extracted from the China CDC drug price database and through expert consultation. Moreover, the unit cost of outpatient and inpatient services (excluding anti-TB drugs) was removed from a standard costing program in 2021, which was informed by the clinical pathway of MDR-TB case management in China[16]. The outpatient cost includes bacteriological, imaging, and follow-up examination and adjuvant treatment fees, whereas hospitalization fees include hospital examination, diagnosis, and treatment (including treatment of adverse events).
Health Utility and Discount Utility weights of each health status were obtained from a study of quality of life on MDR-TB in Thailand[28], which was also referenced in economic research in Korea [12]. Both cost and health utility were discounted at 0.05 annually[29].
-
Both probabilistic and deterministic sensitivity analyses were conducted following international recommendations[19]. In a one-way sensitivity analysis, model parameters, and assumptions, such as hazard ratios, transition probabilities, utility weights, discount rates, and drug costs, were varied by 20% or between the 95% confidence interval reported in the literature. To explore the price threshold of Bedaquiline, we conducted a multiple-way sensitivity analysis modifying the price of Bedaquiline in conjunction with the combined variation of top-10 parameters identified in a one-way sensitivity analysis.
A probabilistic sensitivity analysis (PSA) was also performed to estimate the joint parametric uncertainty surrounding the ICER of BR versus CR. The probabilities that BR was considered cost effective at various affordability thresholds (WTP thresholds) were calculated.
-
Over a 10-year time horizon, the accumulative sputum culture conversion rate after initial treatment, cure rate, and death rate for BR were 69.88%, 61.00%, and 54.63%, respectively. Compared with CR, the conversion and cure rates for BR increased by 12.09% and 21.8%, respectively, whereas the death rate decreased by 12.8% (Table 4).
Table 4. Health outcomes of the two regimens (10 years)
Regimen Conversion rate after primary treatment (%) Cure rate (%) Death rate (%) CR 57.79 33.45 73.80 BR 69.88 54.63 61.00 Difference 12.09 21.18 −12.80 Note. BR, Bedaquiline regimen; CR, Conventional regimen. The discounted cost for BR was 137,984.25 yuan per capita, an increase of 77,716.05 yuan compared with CR, and the QALYs gained were 3.63 years per capita, an increase of 2.31 QALYs (vs. CR). Meanwhile, the ICER of BR (vs. CR) was 33,700.11 yuan/QALY (Table 5). The BR was deemed cost effective using China’s WTP of 1× per capita GDP in 2020 (WTP = 72,450 yuan/QALY) as a benchmark.
Table 5. Results of cost-effectiveness analysis (10 years)
Regimen Cost per capita (yuan) Incremental cost (yuan) QALY per capita (year) Incremental QALY (year) ICER (yuan/QALY) CR 60,268.20 − 1.32 − − BR 137,984.25 77,716.05 3.63 2.31 33,700.11 Note. BR, Bedaquiline regimen; CR, Conventional regimen. ICER, Incremental cost effectiveness ratio. QALY, Quality-adjusted life years. -
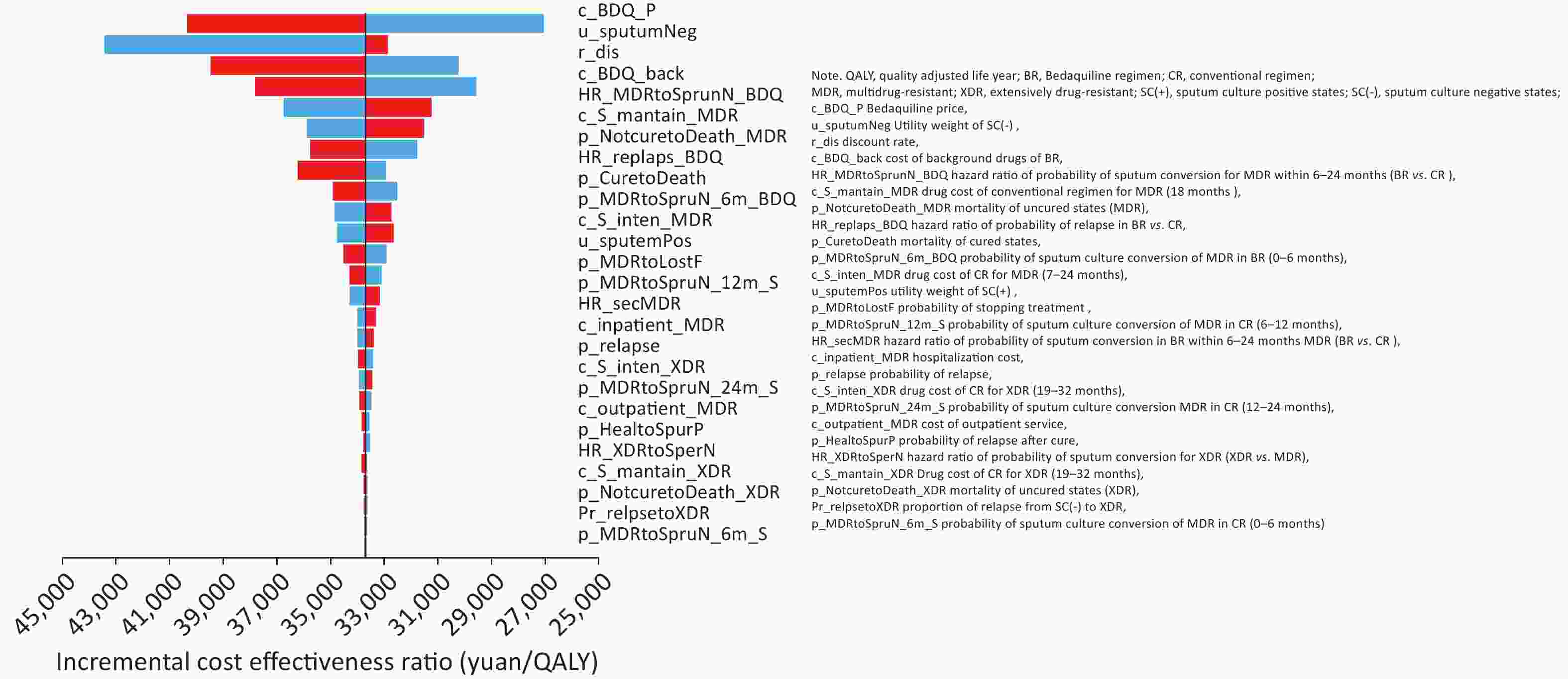
In the one-way deterministic sensitivity analysis, the ICER of BR vs. CR was most sensitive to the cost of Bedaquiline, which ranged from 1,7054.70 to 4,0345.51 yuan/QALY. The other two most sensitive parameters were the health utility weight of the sputum-negative state and the discount rate (Figure 2). Results were generally stable within the variation of parameter values, and the BR’s ICERs were cost effective compared to the WTP threshold (WTP = 72,450 yuan/QALY).
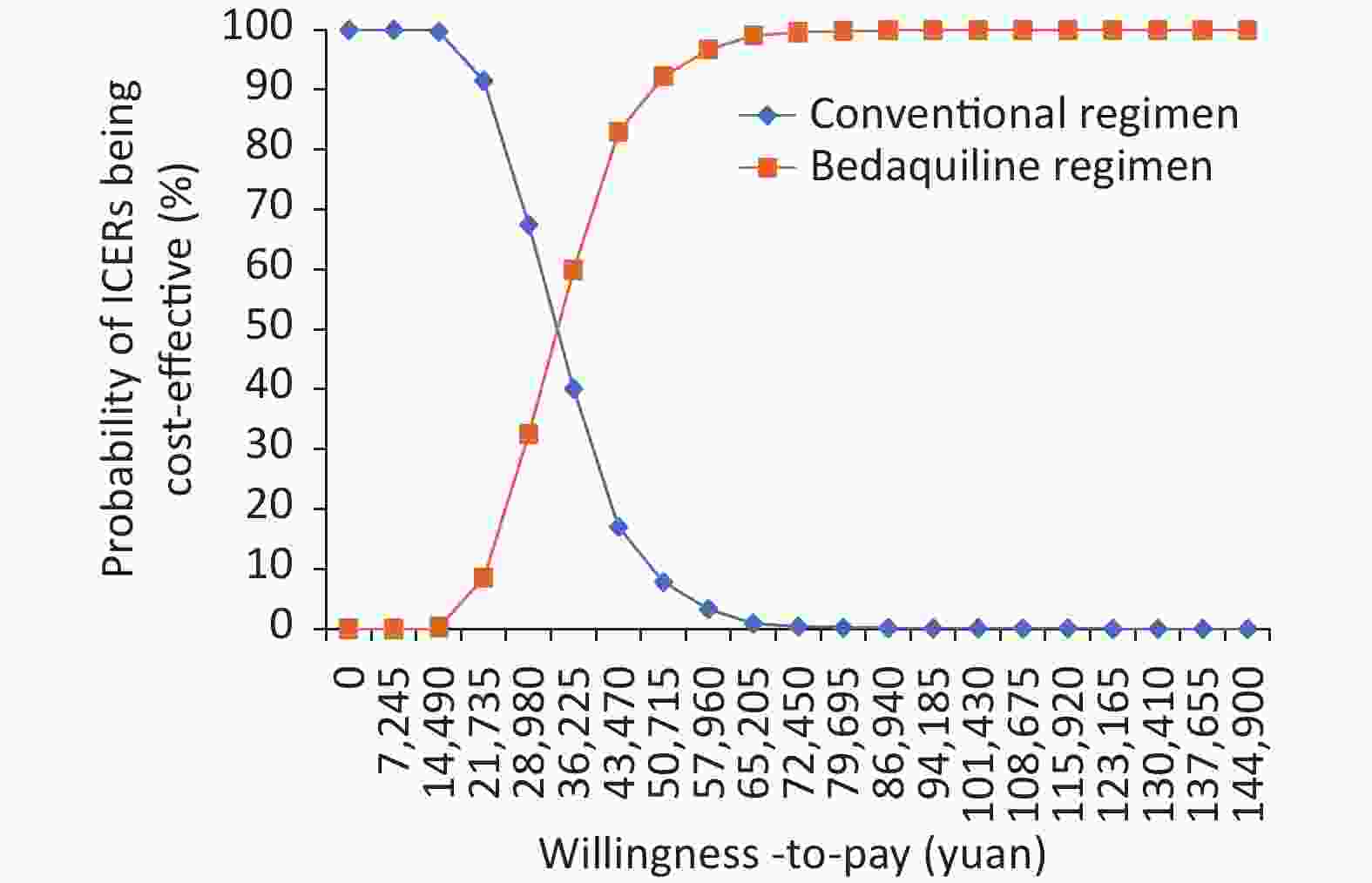
In PSA, most ICER results (green dots in Figure 3) were below the WTP of 72,450 yuan/QALY, indicating that the treatment was cost effective. As WTP rises, so does the likelihood that the BR was cost effective. When WTP reached 72,450 yuan/QALY, there was a 99.6% possibility that the Bedaquiline scheme would be cost-effective (Figure 4).
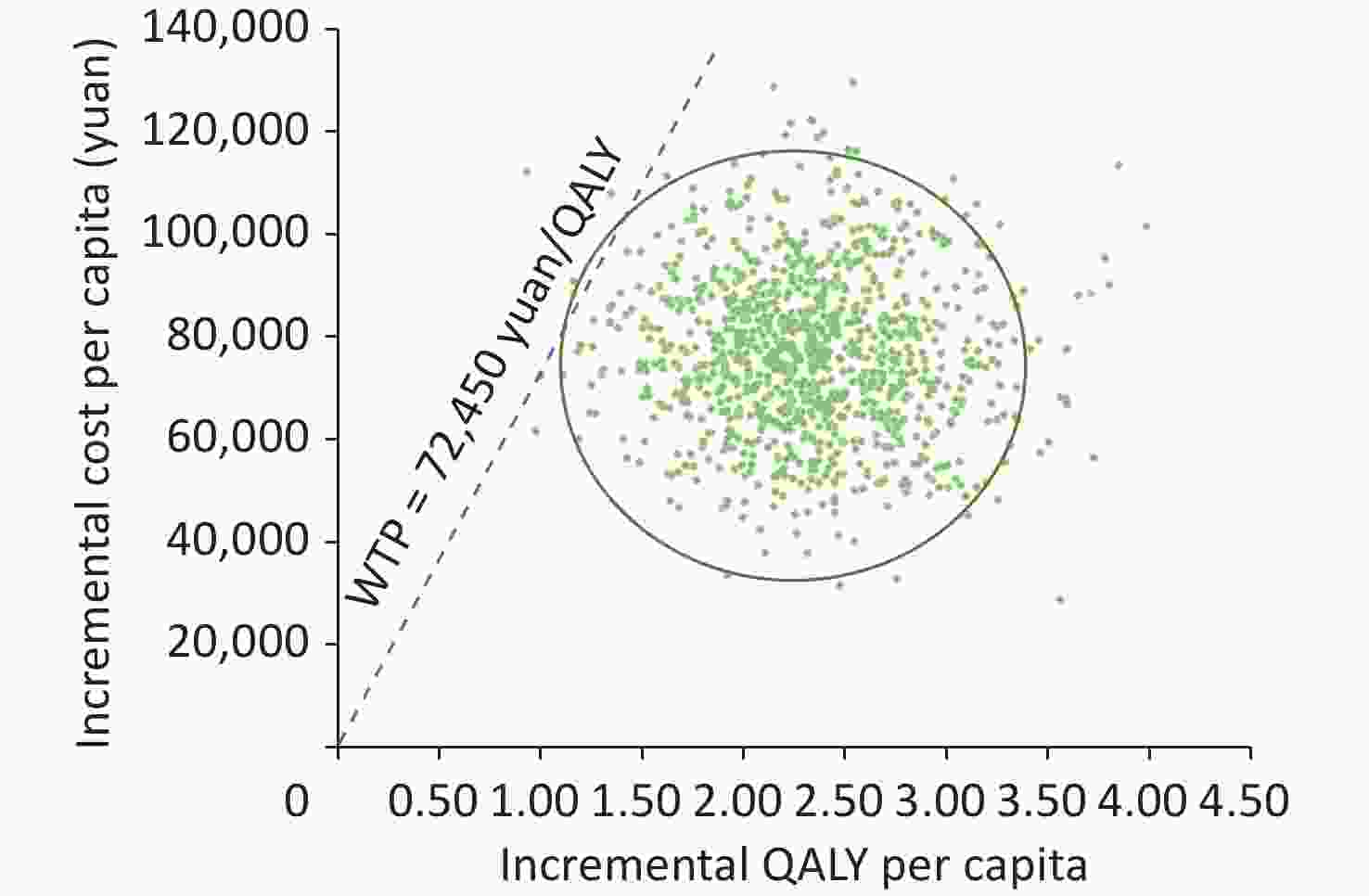
Figure 3. Scatter plot of ICER results of PSA. ICER, incremental cost effectiveness ratio. PSA, probabilistic sensitivity analysis. QALY, quality-adjusted life years.
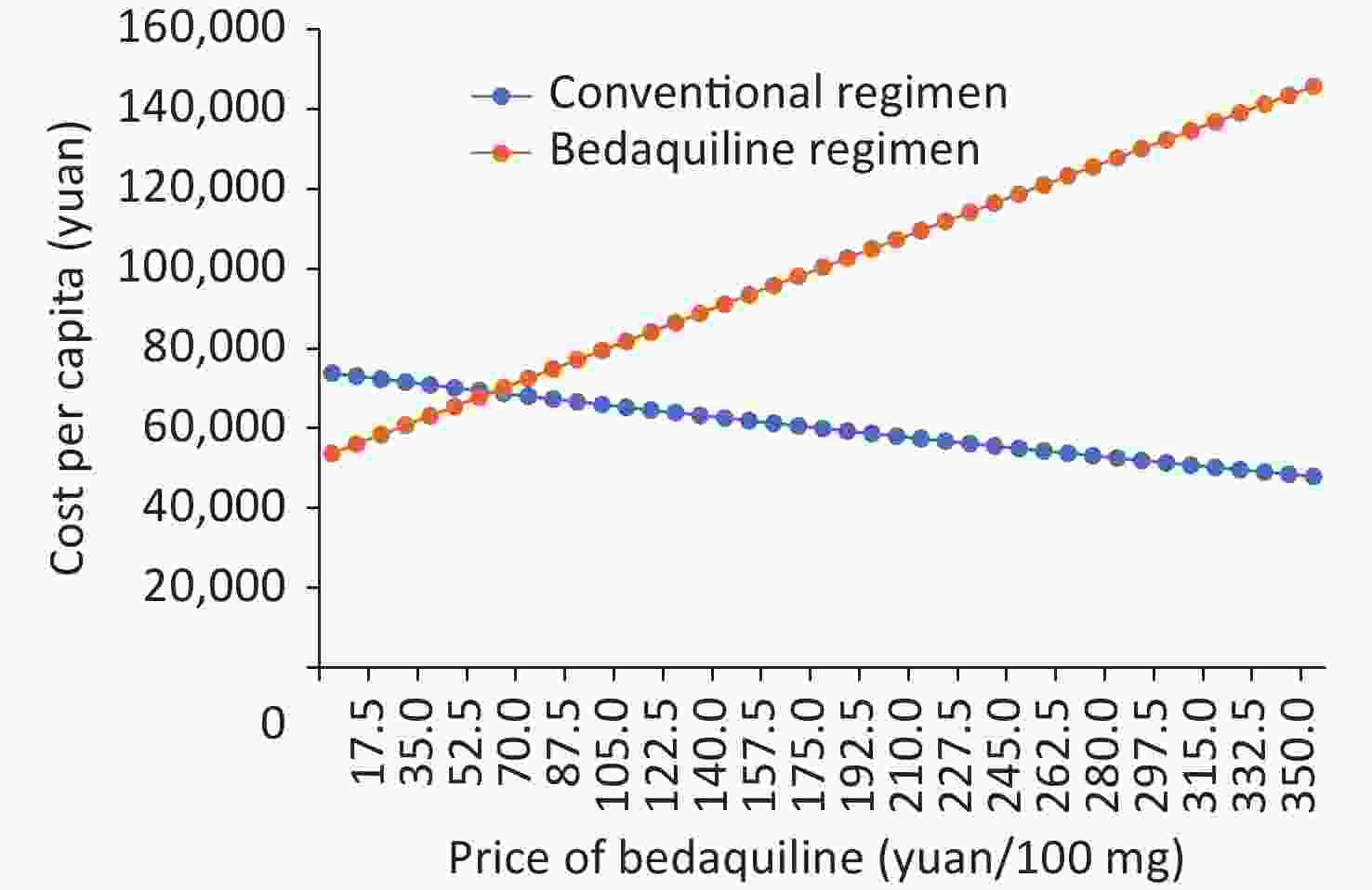
In the threshold analysis, when the price of Bedaquiline fell to or below 57.21 yuan/tablet (100 mg) (Figure 5), the BR became superior to the CR, meaning it gained more QALY with the same or less cost per capita.
-
This study compared the long-term cost-effectiveness of BR and CR and found that BR would produce superior health outcomes and incur higher direct medical costs in China over ten years. The ICER of BR was 33,700 yuan per QALY, making it cost effective at the WHO-recommended WTP threshold. The robustness of the results was shown in the sensitivity analysis.
As directed by a national action plan for combating TB (2019–2022) [30], an increasing number of provinces after Jiangsu and Chongqing conducted centralized procurement of anti-TB drugs (including Bedaquiline) to improve MDR-TB patients’ access to treatment [6]. To the best of our knowledge, this is the first evaluation in mainland China to incorporate the most recent Chinese evidence into the Markov model, including the effectiveness of Bedaquiline and treatment costs. This study also explores the price threshold of Bedaquiline, at which BR predominates over CR. The results may address the lack of evidence in this field and provide valuable information for selecting cost effective drugs. The arguments and implications for policy making are as follows.
BR may provide greater health benefits to patients and cost more direct medical resources to the health system. In MDR patients treated with BR, better health outcomes were achieved, resulting in a higher accumulative conversion rate and cure rate and a lower death rate, which led to an increase in QALYs. The enhanced efficacy and shorter duration of BR versus CR drove favorable health outcomes. Simultaneously, MDR patients treated with BR incurred higher per capita costs. Two factors contribute to the higher price. On the one hand, the cost per treatment course for BR was as high as 138,000 yuan, which was twice that of CR. The high price of Bedaquiline was the primary cost driver. Bedaquiline alone accounts for more than half of the cost of BR (65,800 yuan). On the other hand, the improved health outcomes achieved by BR did not result in avoiding expensive long-term medical costs. Usually, innovative drugs for the treatment of serious chronic diseases, such as hepatitis B and C, can result in the long-term avoidance of costly events (such as liver transplants)[31,32]. Meanwhile, the situation is different for MDR treatment in China, where MDR/XDR patients who have experienced treatment failure are typically swiftly transferred to palliative care requiring few health resources and may die prematurely. Consequently, the long-term medical cost avoided by the BR was not sufficient to offset the additional drug cost of the new regimen (primarily due to Bedaquiline), resulting in a high incremental cost.
Despite high Bedaquiline prices, BR is still shown to be a value for money in China. In the base-case analysis, BR was cost effective in the province with the lowest per capita GDP (Gangsu province = 35,848 yuan per capita in 2022[33]). Further, BR was found cost effective with a high degree of acceptability at a setting WTP threshold in PSA. The results are consistent with previous research conducted in both selected developing and developed countries[9,7,12,13,15]. Simultaneously, the superiority of Bedaquiline becomes more apparent in high-income regions than in middle- and low-income settings due to the avoidance of costly hospitalizations and outpatient medical management. Fan [14] demonstrated that the ICER of Bedaquiline versus background treatment in Hong Kong (a high-income city in China) was USD 12/QALY, which demonstrates a greater cost-effectiveness of Bedaquiline than we found in this study. Moreover, Franke [34] demonstrated that, for MDR-TB patients in high-income countries, adding Bedaquiline to the background regimen is a cost effective alternative to the background regimen alone.
The ICER of Bedaquiline was found to be most sensitive to the unit price of Bedaquiline, and the BR could become the dominant strategy if the unit price of Bedaquiline was reduced from 350 to 57.21 yuan/tablet or less. A health system should accept BR at this price (or below) regardless of the WTP threshold applied, as BR offers greater health benefits at the same or lower cost than CR in this situation. This information may also be useful for negotiating drug prices in China, where there is no official WTP threshold.
The analysis has limitations due to the model’s simplification of the treatment pathway, which was developed on assumptions. In China, there are currently no head-to-head studies comparing the efficacy of Bedaquiline and CR in patients with XDR-TB. In addition, utility weight is absent for the various TB health states that target the Chinese population. In the economic model, the effectiveness of treatment as determined by many studies on the Chinese population and the utility weight of the Thai population were utilized. In cost estimation, we simplified our model and used the overall hospitalization cost per case (including adverse reaction treatment), which was from a costing program of China CDC. Although we explore the uncertainty by sensitivity analysis, any conclusions on ICER of BR vs. CR should be treated with caution due to the weaknesses in available data.
-
Cost-effectiveness analysis demonstrates that the BR is superior to the CR in treating MDR patients. Moreover, despite requiring higher medical costs, it is cost effective. When the price of Bedaquiline falls to or below 57.21 yuan per tablet, the BR becomes dominant and cost effective.
doi: 10.3967/bes2023.061
Cost-Effectiveness Analysis of Combined Chemotherapy Regimen Containing Bedaquiline in the Treatment of Multidrug-Resistant Tuberculosis in China
-
Abstract:
Objective This study aims to estimate the cost-effectiveness of the combined chemotherapy regimen containing Bedaquiline (BR) and the conventional treatment regimen (CR, not containing Bedaquiline) for the treatment of adults with multidrug-resistant tuberculosis (MDR-TB) in China. Methods A combination of a decision tree and a Markov model was developed to estimate the cost and effects of MDR patients in BR and CR within ten years. The model parameter data were synthesized from the literature, the national TB surveillance information system, and consultation with experts. The incremental cost-effectiveness ratio (ICER) of BR vs. CR was determined. Results BR (vs. CR) had a higher sputum culture conversion rate and cure rate and prevented many premature deaths (decreased by 12.8%), thereby obtaining more quality-adjusted life years (QALYs) (increased by 2.31 years). The per capita cost in BR was as high as 138,000 yuan, roughly double that of CR. The ICER for BR was 33,700 yuan/QALY, which was lower than China’s 1× per capita Gross Domestic Product (GDP) in 2020 (72,400 yuan). Conclusion BR is shown to be cost effective. When the unit price of Bedaquiline reaches or falls below 57.21 yuan per unit, BR is expected to be the dominant strategy in China over CR. -
Key words:
- Bedaquiline /
- Cost-effectiveness /
- Multidrug-resistant tuberculosis /
- China
注释: -
Table 1. Treatment regimens of MDR-TB and XDR-TB patients
Regimen MDR-TB treatment Portion and duration XDR-TB treatment Conventional
regimen6 ZKm(Am)Lfx(Mfx)Cs(PAS,E)Pto
/18ZLfx(Mfx)Cs(PAS,E)Pto100%;
16 months12ZCmMfxPASCs(Pto)Clr(Amx/Clv)/
18ZMfxPASCs(Pto)Clr(Amx/Clv).
30 monthsBedaquiline
regimenshort-course regimen for fluoroquinolone-sensitive
patients: [4-6BdqAmLfx(Mfx)CfzZHhighPtoE/
5Lfx(Mfx)CfzZE];45%;
9 monthsthe course duration is extended to
30 months.long-course regimen for fluoroquinolone-
sensitive patients, [6Lfx(Mfx)BdqLzd(Cs)Cfz/
12Lfx(Mfx)Cfz Lzd(Cs)]30%;
18 monthslong-course regimen for fluoroquinolone
resistance [6Bdq Lzd(Dlm)CfzCs/14Lzd(Dlm)CfzCs]25%;
20 monthsNote. MDR, Multidrug-resistant; TB, Tuberculosis; XDR, Extensively drug-resistant; Z, Pyrazinamide; Km, Kanamycin; Am, Amikacin; Cm, Capreomycin; Lfx, Levofloxacin; Mfx, Moxifloxacin; Cs, Cycloserine; PAS, Para aminosalicylic acid; E, Ethambutol; Pto, Prothionicotinamide; BDQ, Bedaquiline; Cfz, Chlorofazimin; Hhigh, High dose isoniazid; Clr, Clarithromycin; Amx/Clv, Amoxicillin/clavulanic acid; Lzd, Linezolid; Dlm, Delamani. Table 2. Parameters of transition probability
Parameter Based value Range Source Probability of sputum culture conversion (in 4 weeks) MDR in CR (0–6 months) 0.0873 0.0786–0.0960 MDR-TB control
program[20]MDR in CR (6–12 months) 0.0535 0.0482–0.0589 MDR-TB control
program[21]MDR in CR (12–24 months) 0.0410 0.0367–0.0451 MDR-TB control
program[22]MDR in BR (0–6 months) 0.1230 0.1107–0.1352 literature[23] Hazard Ratio Probability of Sputum conversion for relapse (Secondary vs. primary treatment) 0.540 0.30–0.95 Literature[25] Probability of Sputum conversion for XDR (XDR vs. MDR) 0.398 0.237–0.670 Literature[25] Probability of Sputum conversion for MDR within 6–24 months (BR vs. CR) 2.440 1.57–3.80 Literature[25] Probability of relapse in BR vs. CR 0.320 0.086–1.069 Literature[25] Other probability (in 4 weeks) Relapse from SC(-) 0.010 0.0091–0.0105 Literature[25] Where: relapse to XDR 0.214 0.1712–0.2354 Literature[25] Relapse after cure 0.002 0.0016–0.0022 Literature[25] MDR to XDR 0.002 0.0016–0.0022 Assuming the same
probability of
relapse after cureStopping treatment 0.006 0.0029–0.0091 Literature[26] Mortality (in 4 weeks) Cured state 0.003 0.0025–0.00384 Literature[27] Uncured states (MDR) 0.021 0.0168–0.0252 Literature[27] Uncured states (XDR) 0.027 0.0215–0.0296 Literature[27] Note. MDR, Multidrug-resistant; XDR, Extensively drug-resistant; BR, Bedaquiline regimen; CR, Conventional regimen. Table 3. Parameters of cost and utility weight
Parameters Based value Range Source Drug cost of CR (RMB, four weeks) MDR (0–6 months) 2,082.93 1,666.35–2,499.52 China CDC drug
price databaseMDR (7–24 months) 2,559.86 2,047.89–3,071.83 China CDC drug
price databaseXDR (0–18 months) 3,270.62 2,616.50–3,924.75 China CDC drug
price databaseXDR (19–32 months) 2,156.33 1,725.06–2,587.60 China CDC drug
price databaseDrug cost of BR (yuan, four weeks) Bedaquiline price (yuan/100 mg) 350.00 280.00–420.00 China CDC drug
price databaseMDR/XDR (0–6 months) Bedaquiline (0–2 weeks, 200 mg/day) 19,600.00 15,680.00–23,520.00 China CDC drug
price databaseBedaquiline (3–24 weeks, 100 mg 3 times a week) 46,200.00 36,960.00–55,440.00 China CDC drug
price databaseBackground Drugs* 4,013.28 3,210.62–4,815.93 China CDC drug
price databaseMDR/XDR (8–20 months) 2,850.229 2,280.18–3,420.28 China CDC drug
price databaseOutpatient and inpatient (yuan, four weeks) Outpatient 364.5 291.6–437.4 TB costing program[16] Hospitalization 755.78 604.62–906.94 TB costing program[16] Utility weight SC(+) state 0.51 0.41–0.61 Literature[28] SC(-) state 0.88 0.70–0.90 Literature[28] Death 0 – Literature[28] Discount rate (annual) 0.05 0.03–0.08 Literature[29] Note. *Drugs excluding Bedaquiline; MDR, Multidrug-resistant; XDR, Extensively drug-resistant; BR, Bedaquiline regimen; CR, Conventional regimen; SC(+), Sputum culture positive; SC(-), Sputum culture negative. Table 4. Health outcomes of the two regimens (10 years)
Regimen Conversion rate after primary treatment (%) Cure rate (%) Death rate (%) CR 57.79 33.45 73.80 BR 69.88 54.63 61.00 Difference 12.09 21.18 −12.80 Note. BR, Bedaquiline regimen; CR, Conventional regimen. Table 5. Results of cost-effectiveness analysis (10 years)
Regimen Cost per capita (yuan) Incremental cost (yuan) QALY per capita (year) Incremental QALY (year) ICER (yuan/QALY) CR 60,268.20 − 1.32 − − BR 137,984.25 77,716.05 3.63 2.31 33,700.11 Note. BR, Bedaquiline regimen; CR, Conventional regimen. ICER, Incremental cost effectiveness ratio. QALY, Quality-adjusted life years. -
[1] WHO. Global tuberculosis report 2021. WHO. 2021. [2] Chinese Center for Disease Control and Prevention. Working plan for the prevention and control of MDR-TB. 2011. [3] World Health Organization. WHO consolidated guidelines on tuberculosis. Module 4: treatment-drug-resistant tuberculosis treatment. World Health Organization. 2020. [4] Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis and Thoracic Tumor Research Institute, Chinese Antituberculosis Association, Editorial Board of Chinese Journal of Antituberculosis. Chinese expert consensus on the all-oral treatment of drug-resistant pulmonary tuberculosis (2021 Edition). Chin J Antituberc, 2021; 43, 859−66. (In Chinese [5] Tuberculosis Prevention and Control Center of China Center for Disease Control and Prevention. Technical Guidelines for tuberculosis prevention and control in China (2021 edition). People's Medical Publishing House. 2022. (In Chinese) [6] Website CGP. Procurement tender announcement of anti-tuberculosis drugs in Jiangsu province 2022. 2022. [7] Wolfson LJ, Walker A, Hettle R, et al. Cost-effectiveness of adding bedaquiline to drug regimens for the treatment of multidrug-resistant tuberculosis in the UK. PLoS One, 2015; 10, e0120763. doi: 10.1371/journal.pone.0120763 [8] Wolfson LJ, Gibbert J, Wirth D, et al. Cost-effectiveness of incorporating bedaquiline into a treatment regimen for MDR/XDR-TB in Germany. Eur Respir J, 2015; 46, 1826−9. doi: 10.1183/13993003.00811-2015 [9] Wirth D, Dass R, Hettle R. Cost-effectiveness of adding novel or group 5 interventions to a background regimen for the treatment of multidrug-resistant tuberculosis in Germany. BMC Health Serv Res, 2017; 17, 182. doi: 10.1186/s12913-017-2118-2 [10] Vassall A. Cost-effectiveness of introducing bedaquiline in MDR-TB regimens: an exploratory analysis. WHO. 2013. [11] Schnippel K, Firnhaber C, Conradie F, et al. Incremental cost effectiveness of bedaquiline for the treatment of rifampicin-resistant tuberculosis in South Africa: model-based analysis. Appl Health Econ Health Policy, 2018; 16, 43−54. doi: 10.1007/s40258-017-0352-8 [12] Park HY, Ku HM, Sohn HS, et al. Cost-effectiveness of bedaquiline for the treatment of multidrug-resistant tuberculosis in the republic of Korea. Clin Ther, 2016; 38, 655−67.e2. doi: 10.1016/j.clinthera.2016.01.023 [13] Lu XY, Smare C, Kambili C, et al. Health outcomes of bedaquiline in the treatment of multidrug-resistant tuberculosis in selected high burden countries. BMC Health Serv Res, 2017; 17, 87. doi: 10.1186/s12913-016-1931-3 [14] Fan QQ, Ming WK, Yip WY, et al. Cost-effectiveness of bedaquiline or delamanid plus background regimen for multidrug-resistant tuberculosis in a high-income intermediate burden city of China. Int J Infect Dis, 2019; 78, 44−9. doi: 10.1016/j.ijid.2018.10.007 [15] Codecasa LR, Toumi M, D’Ausilio A, et al. Cost-effectiveness of bedaquiline in MDR and XDR tuberculosis in Italy. J Mark Access Health Policy, 2017; 5, 1283105. doi: 10.1080/20016689.2017.1283105 [16] Chinese Center for Disease Control and Prevention. Costing of diagnosis and treatment of TB. 2021. [17] Shillcutt SD, Walker DG, Goodman CA, et al. Cost effectiveness in low- and middle-income countries: a review of the debates surrounding decision rules. Pharmacoeconomics, 2009; 27, 903−17. doi: 10.2165/10899580-000000000-00000 [18] National Bureau of Statistics. Statistical communiqué of the People's Republic of China on the 2020 national economic and social development. China Statistics Press. 2021. (In Chinese) [19] Siebert U, Alagoz O, Bayoumi AM, et al. State-transition modeling: a report of the ISPOR-SMDM modeling good research practices task force–3. Med Decis Making, 2012; 32, 690−700. doi: 10.1177/0272989X12455463 [20] Chinese Center for Disease Control and Prevention. The global fund MDR-TB control program in China: experience and accompliment. People’s Medical Publishing House. 2015. (In Chinese) [21] Zhang Y. The evaluation of the mid-term efficacy of Global Fund multi-drug resistant tuberculosis (MDR-TB) Project in Dalian. Dalian Medical University. 2016. (In Chinese) [22] Hao XH, Yao L, Tang SJ, et al. A cohort study on the outcome of multidrug-resistant tuberculosis among newly diagnosed cases. Chin J Infect Dis, 2012; 30, 157−61. [23] Gao M, Gao J, Xie L, et al. Early outcome and safety of bedaquiline-containing regimens for treatment of MDR- and XDR-TB in China: a multicentre study. Clin Microbiol Infect, 2021; 27, 597−602. doi: 10.1016/j.cmi.2020.06.004 [24] Jones E, Epstein D, García-Mochón L. A procedure for deriving formulas to convert transition rates to probabilities for multistate Markov models. Med Decis Making, 2017; 37, 779−89. doi: 10.1177/0272989X17696997 [25] FDA. BRIEFING PACKAGE (NDA 204-384). http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/ucm293600.htm. [2023-3-25]. [26] WHO. Drug-resistant TB treatment. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022/tb-diagnosis-treatment/3-4-drug-resistant-tb-treatment. [2023-3-25]. [27] Liu CH, Li L, Chen Z, et al. Characteristics and treatment outcomes of patients with MDR and XDR tuberculosis in a TB referral hospital in Beijing: a 13-year experience. PLoS One, 2011; 6, e19399. doi: 10.1371/journal.pone.0019399 [28] Kittikraisak W, Kingkaew P, Teerawattananon Y, et al. Health related quality of life among patients with tuberculosis and HIV in Thailand. PLoS One, 2012; 7, e29775. doi: 10.1371/journal.pone.0029775 [29] Liu GE. China guidelines for pharmacoeconomic evaluations 2020. China Market Press. 2020. (In Chinese) [30] National Health Commission. Action Plan for Stopping TB (2019-2022). https://www.gov.cn/gongbao/content/2019/content_5437149. [2023-3-25]. (In Chinese) [31] Wu B, Li T, Chen H, et al. Cost-effectiveness of nucleoside analog therapy for hepatitis B in China: a Markov analysis. Value Health, 2010; 13, 592−600. doi: 10.1111/j.1524-4733.2010.00733.x [32] Dai ZL, Wong IOL, Xie C, et al. Cost-effectiveness analysis of first-line treatment for chronic hepatitis B in China. Clin Microbiol Infect, 2022; 28, 300.e1−8. doi: 10.1016/j.cmi.2021.06.024 [33] National Bureau of Statistics. Gansu Province 2020 annual data. http://www.stats.gov.cn/sj/ndsj/2020/indexch.htm. [2023-3-25]. (In Chinese) [34] Franke MF, Appleton SC, Bayona J, et al. Risk factors and mortality associated with default from multidrug-resistant tuberculosis treatment. Clin Infect Dis, 2008; 46, 1844−51. doi: 10.1086/588292 -




 下载:
下载:



 Quick Links
Quick Links