-
Placental site trophoblastic tumor (PSTT), a rare gestational trophoblastic neoplasm (GTN), exhibits slow growth, late-onset metastasis, and chemotherapy insensitivity[1,2]. The invasive capacity of trophoblasts induces angiogenesis in PSTT, yet the specific mechanism remains unclear[3]. PSTT is typically resistant to chemotherapy and hysterectomy stands as the primary therapeutic choice for patients with an overall cure rate of approximately 75%–80%[4,5]. Therefore, investigating the precise emergence and progression of PSTT is crucial, aiming to identify novel targets for diagnosis and therapy.
WNT5A is a key activator of the non-canonical Wnt pathway, influencing angiogenesis, proliferation, differentiation, and chemoresistance. Additionally, it plays a crucial role in vascular growth during early embryonic development[6]. Previous studies indicated that WNT5A exhibited both angiogenesis-suppressive and angiogenesis-promoting effects in different contexts, leading to a debate on its precise role[7]. The molecular mechanisms of WNT5A signaling in human trophoblast cells remain unknown. MiR154-5p, a 22-nucleotide RNA located on chromosome 14q32.31, has been identified as essential in the tumorigenesis of gastric cancer (GC), colorectal cancer (CRC), and hepatocellular carcinoma (HCC)[8]. A recent study highlighted the significance of interactions between miR154-5p and E2F5 in osteosarcoma[9]. However, the regulatory impact of miR154-5p on angiogenesis and invasion in human trophoblast cells, particularly in the modulation of Wnt signaling, remains unclear. In this study, we demonstrate that miR154-5p regulates angiogenesis and invasion in human trophoblasts through WNT5A. This discovery holds promise for developing novel therapies for PSTT.
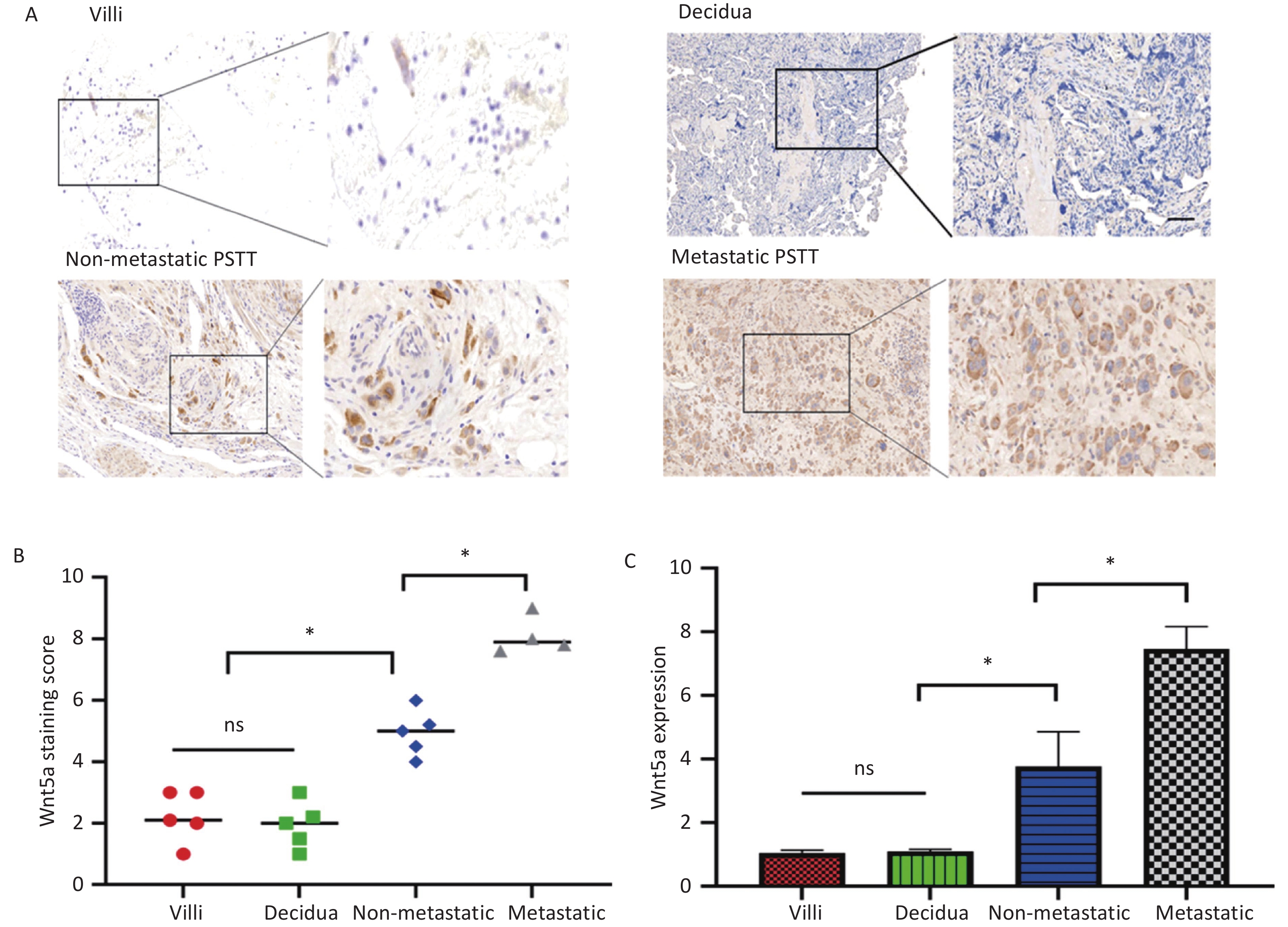
The experimental findings indicated elevated expression of WNT5A in PSTT tissues compared to both normal villi (n = 5) and normal decidua (n = 5). WNT5A was primarily observed in the membrane and cytoplasm of human trophoblast cells, as illustrated in Figure 1A–C. Real-time PCR analysis further demonstrated higher WNT5A expression in metastatic PSTT tissues (n = 4) than in non-metastatic PSTT tissues (n = 5), suggesting a potential correlation between WNT5A expression and PSTT clinical staging (Figure 1C). Clinical information for PSTT patients was detailed in Supplementary Table S1 (available in www.besjournal.com). Previous research highlighted PSTT originated from migratory intermediate trophoblasts, with irregular vaginal bleeding (IVB) as the predominant symptom (67.6%), followed by amenorrhea (24.3%)[1,2]. This prompts speculation that WNT5A may play a role in angiogenesis and the invasion of human trophoblast cells.
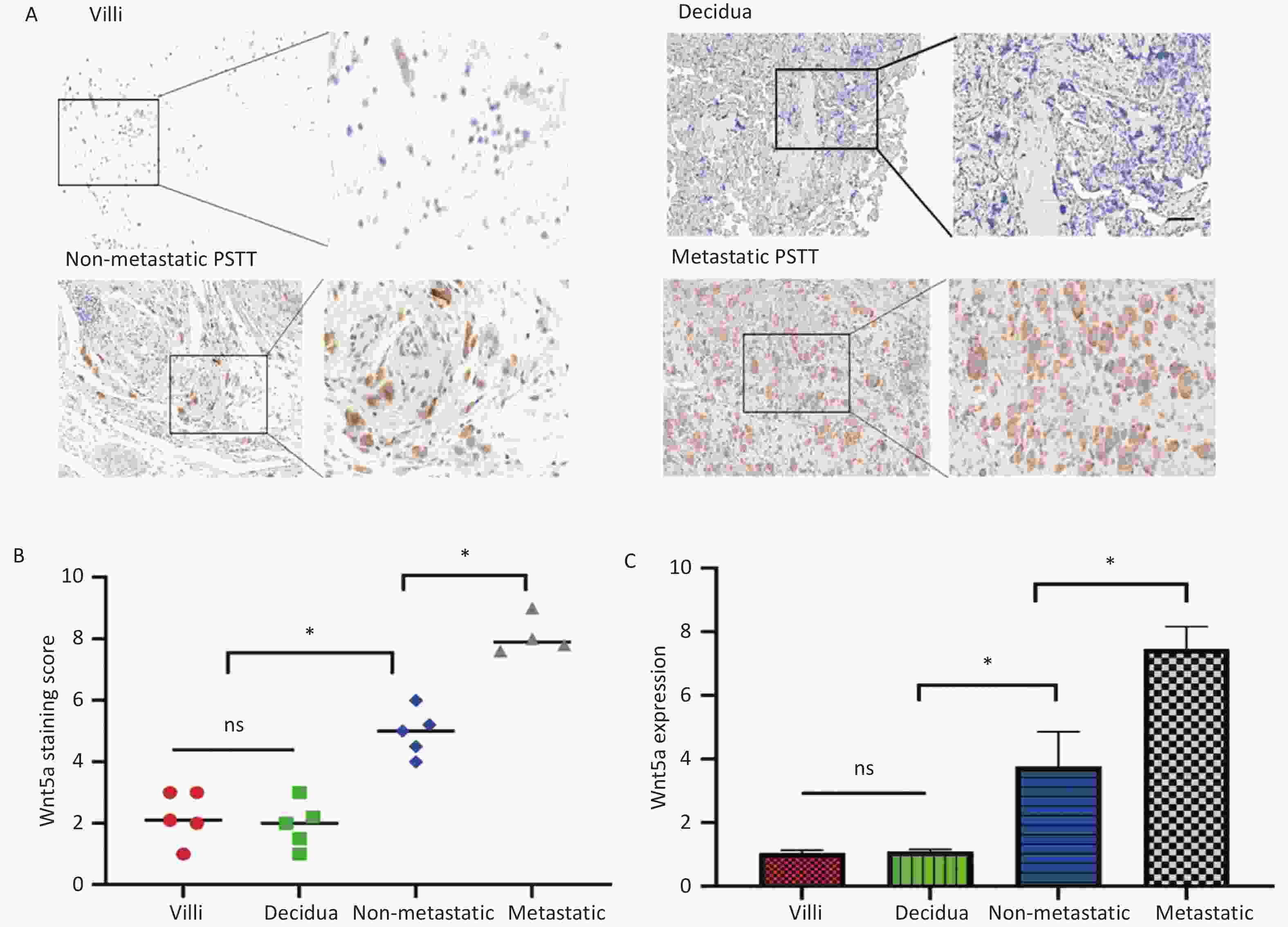
Figure 1. WNT5A is highly expressed in human trophoblast cells and its expression is associated with the clinical staging of PSTT.
The investigation into the upregulation of WNT5A involved the selection of PSTT tissues (n = 4) and normal villus tissues (6–9 weeks, n = 4) for a microarray assay. Results revealed 22 up-regulated and 44 downregulated miRNAs (FC > 2 and P < 0.05), as depicted in Figure 2A. Among the top 10 down-regulated miRNAs, miR154-5p exhibited a 10-base pairing in the 3’UTR of WNT5A, suggesting its potential role as an upstream regulator (Figure 2B). Real-time PCR analysis confirmed significantly lower levels of miR154-5p in PSTT tissues (P < 0.05), hinting at a plausible interaction with WNT5A. To explore the regulatory impact of miR154-5p on WNT5A, miR154-5p inhibitors, and mimics were transfected into HTR-8/SEV8 and JEG3 cells. Real-time PCR and western blot analysis revealed that the miR154-5p mimic reduced WNT5A expression, while the miR154-5p inhibitor enhanced WNT5A expression (Figure 2C–D). This substantiated the notion that miR154-5p regulated WNT5A expression in trophoblast cells at the transcriptional level. Given prior research indicating the significance of the WNT5A-Ror signaling pathway in tissue regeneration, embryonic tissue formation, and reproduction, with WNT5A participating in intercellular signal transduction during embryogenesis[10], it is plausible that downstream molecules in trophoblasts may be regulated by WNT5A. However, additional studies are imperative to validate this hypothesis.
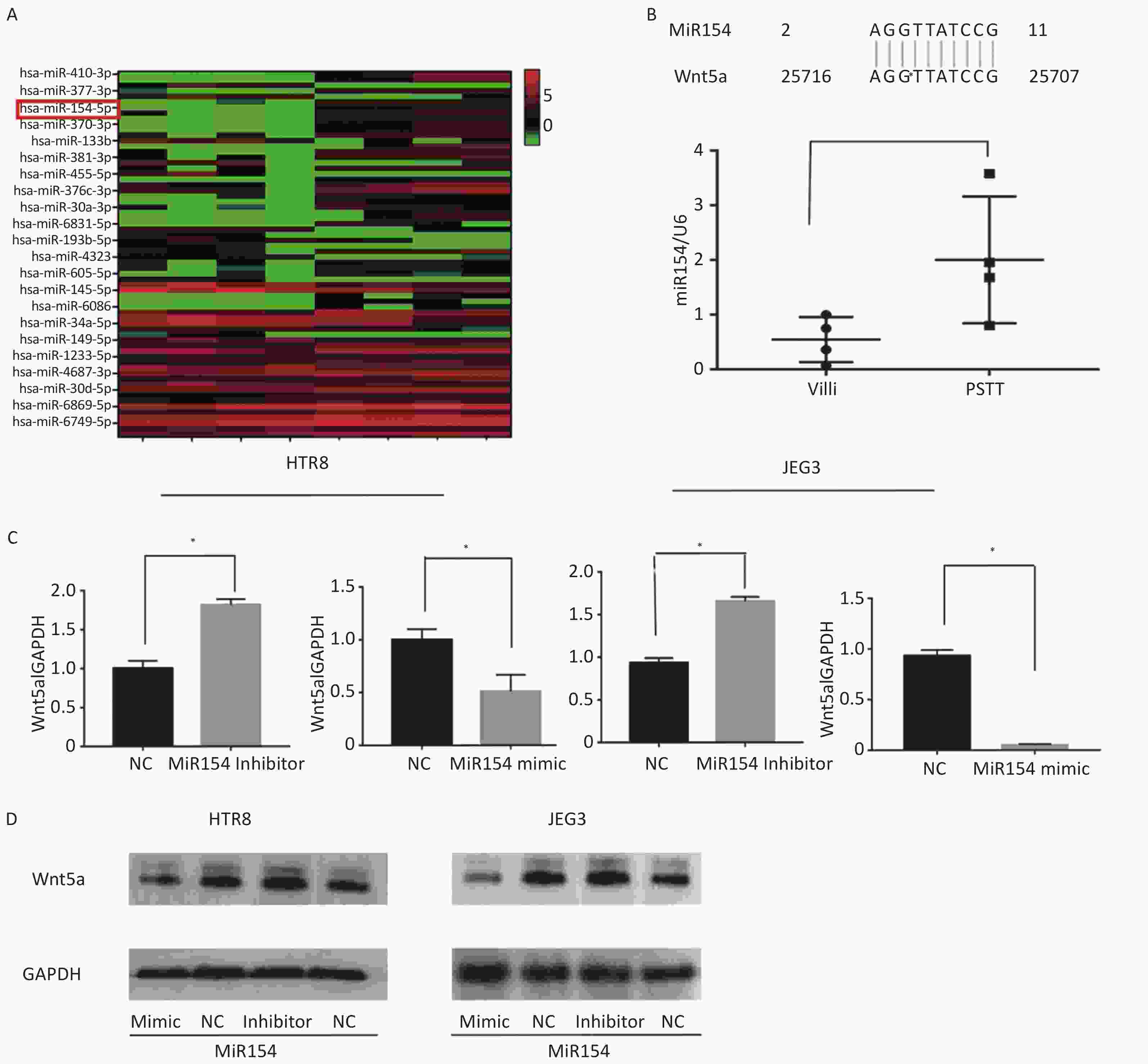
Figure 2. MiR154-5p was upregulated in PSTT tissues and regulated the expression of WNT5A in HTR-8/SEV8 and JEG3.
As angiogenesis was a characteristic feature of PSTT, we hypothesized that miR154-5p regulates angiogenesis in human trophoblasts. To test this, we transfected HTR-8/SEV8 and HUVEC with miR154-5p mimic and inhibitor, respectively. Figure 3A–B illustrated that miR154-5p mimic inhibited tube formation in HTR-8/SEV8 and HUVEC cells compared to the negative control (P < 0.05), while miR154-5p inhibitor significantly enhanced tube formation (P ≤ 0.05). Notably, WNT5A re-expression partially rescued this effect. In summary, these findings indicate that miR154-5p regulates trophoblast angiogenic capacity through WNT5A.
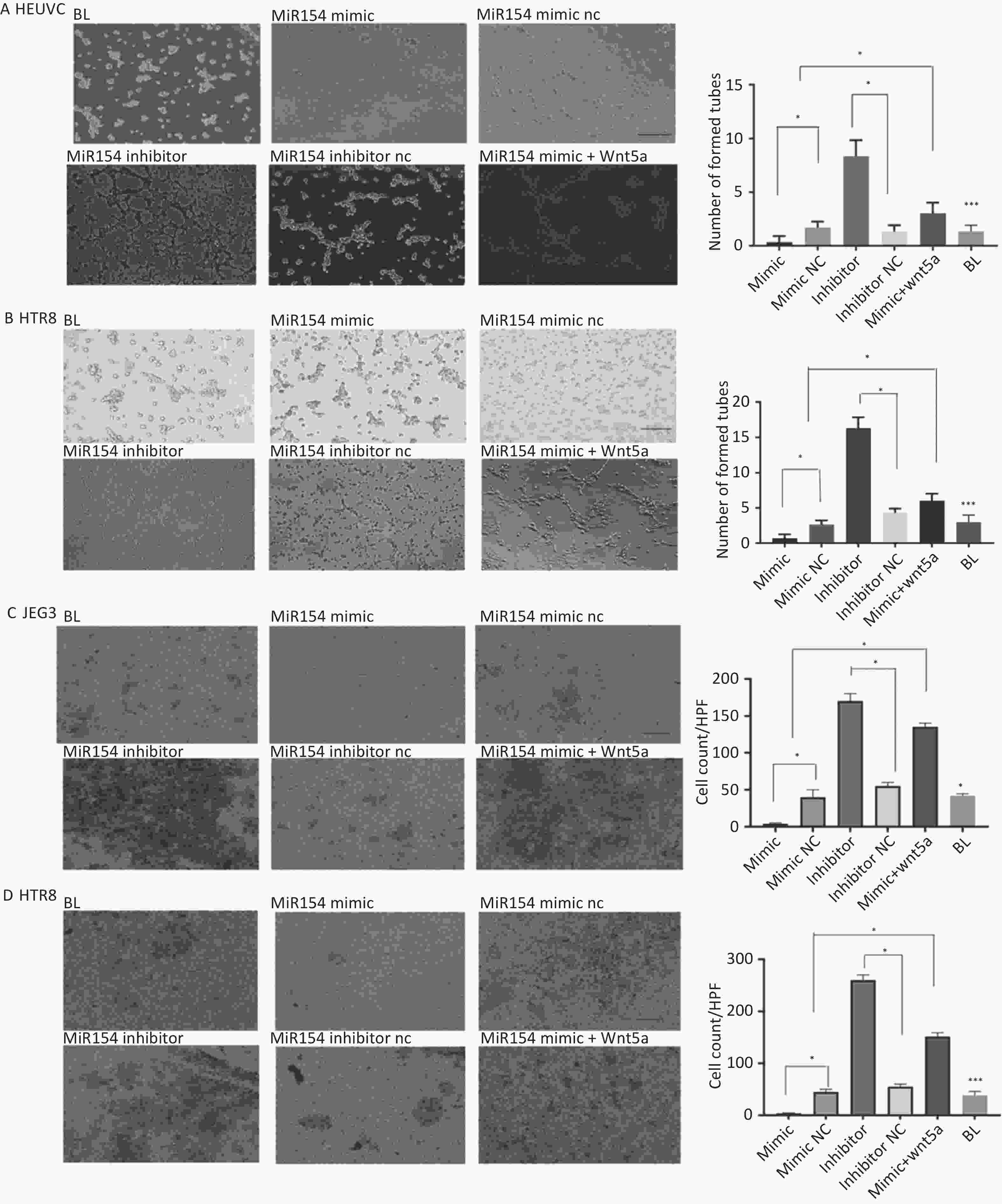
Figure 3. MiR154-5p regulated the tube formation and invasion capacity of HTR-8/SEV8 and HUVEC by targeting WNT5A.
The previous histochemical results indicated a potential correlation between WNT5A expression and the clinical staging of PSTT (Figure 1A–C). Building upon this, our investigation delved into the regulatory role of miR154-5p in trophoblast cell invasion. We transfected HTR-8/SEV8 and JEG3 cells with miR154-5p mimic, miR154-5p inhibitor, and/or WNT5A construct. As depicted in Figure 3C–D, the miR154-5p mimic significantly curtailed the invasion capacity of HTR-8/SEV8 and JEG3 cells when compared to the negative control (P < 0.05). Conversely, transfection with the miR154-5p inhibitor substantially heightened the invasive potential of these cells (P < 0.05). Notably, this effect was reversed by the re-expression of WNT5A. In summary, the downregulation of miR154-5p promoted the invasive capacity, while upregulation inhibited it. WNT5A re-expression counteracted these effects, suggesting that miR154-5p may regulate WNT5A expression, exerting a pivotal influence on the invasive behavior of trophoblast cells.
We hypothesized that miR154-5p served as the upstream regulator of WNT5A, as identified in the microarray assay. This was subsequently confirmed through qRT-PCR and western blotting assays conducted on HTR-8/SEV8 and JEG3 cells. To further substantiate the role of miR154-5p in promoting angiogenesis and trophoblast cell proliferation by modulating WNT5A expression, in vivo experiments were conducted in our subsequent work. Given the rare occurrence of PSTT, the study faced limitations in terms of specimen collection. Future research endeavors will involve a more extensive collection of clinical specimens to validate the correlation between WNT5A expression levels and the clinical staging of PSTT. This will reinforce the association between WNT5A and the angiogenesis and invasion capabilities of trophoblast cells, offering a potential therapeutic target for the clinical diagnosis and treatment of PSTT.
-
Table S1. The clinical information of PSTT patients
Characteristics
clinical tissuesPacient number Age, year GPA Time of antecedent pregnancy, mo FIGO stage Presenting symptoms Outcome Performed assay Follow-up, mo IHC microRNA assay Real-time qPCR Metastatic PSTT 1 25 GAP1A0 9 III IVB NED 30 √ / / 2 29 G1P1A0 13 II IVB NED 60 √ √ √ 3 32 G1P1A1 24 II IVB NED 24 √ √ √ 4 25 G1P1A0 8 III IVB NED 41 √ / / Non-metastatic PSTT 1 28 G1P1A1 9 I IVB NED 36 √ √ √ 2 27 G2P0A2 2 I IVB NED 28 √ / / 3 24 G6P1A5 4 I IVB NED 35 √ / / 4 28 G1P1A0 6 I IVB NED 59 √ √ √ 5 35 G7P1A6 3 I IVB NED 56 √ / / Normal decidua 1 37 G4P1A3 / / / / / √ / / 2 23 G3P1A2 / / / / / √ / / 3 28 G2P0A2 / / / / / √ / / 4 27 G3P1A2 / / / / / √ / / 5 23 G3P0A3 / / / / / √ / / Normal Villi 1 29 G3P2A1 / / / / / √ / / 2 27 G0P0A1 / / / / / √ / / 3 27 G0P0A2 / / / / / √ / / 4 30 G0P0A1 / / / / / √ / / 5 26 G0P0A1 / / / / / √ / / Note. GPA, gravidity, parity, and abortions; IVB, irregular vaginal bleeding; FIGO, International Federation of Gynecology and Obstetrics; NED, no evidence of disease.
doi: 10.3967/bes2024.046
WNT5A Regulated by miR154-5p is Associated with Angiopoiesis and Invasion of Placental Site Trophoblastic Tumor (PSTT)
-
-
Figure 1. WNT5A is highly expressed in human trophoblast cells and its expression is associated with the clinical staging of PSTT.
(A) The representative images from IHC staining analysis of WNT5A expression in normal villi (n = 5), normal decidua (n = 5), non-metastatic PSTT tissues (n = 5), and metastatic PSTT tissues (n = 4) were shown. Scale bar: 100 μm. (B) Statistical analysis of WNT5A IHC staining using composite scores. Red mark, normal villi. Green mark, normal decidua. Blue indicates non-metastatic PSTT tissue. Gray mark, metastatic PSTT tissue. (C) qRT-PCR assay of WNT5A mRNA in normal villi, normal decidua, non-metastatic PSTT tissues, and metastatic PSTT tissues. Data are presented as the mean ± SD, and results were analyzed by two-sided Student’s t-test. *P < 0.05. PSTT, placental site trophoblastic tumor.
Figure 2. MiR154-5p was upregulated in PSTT tissues and regulated the expression of WNT5A in HTR-8/SEV8 and JEG3.
(A) Heat map analysis of the top 22 upregulated and downregulated miRNAs in PSTT and normal villus tissues. The red and blue plots represent up- and downregulated miRNAs with FC ≥ 2.0 and P < 0.05. (B) Bioinformatics analysis of the 3’UTR pairing bases of WNT5A and miR-154. qRT-PCR assay of miR-154 in PSTT tissues and normal villi tissues U6 was used as a loading control. Data are presented as the mean ± SD, and results were analyzed by two-sided Student’s t-test. *P < 0.05. (C) qRT-PCR assay of the influence of the miR-154 mimic or inhibitor on the expression of WNT5A in HTR-8/SEV8 and JEG3 cells Data are presented as the mean ± SD, and results were analyzed by two-sided Student’s t-test. *P < 0.05. (D) Western blotting assay of the role of miR-154 mimic or inhibitor on the expression of WNT5A in HTR-8/SEV8 and JEG3 cells; GAPDH was used as the loading control. NC, negative control; BL, blank control. PSTT, placental site trophoblastic tumor.
Figure 3. MiR154-5p regulated the tube formation and invasion capacity of HTR-8/SEV8 and HUVEC by targeting WNT5A.
(A–B) After being transfection with the miR154 mimic, miR154 NC, miR154 inhibitor, miR154 inhibitor NC, and miR154 mimic+WNT5A, tube formation by HTR-8/SEV8 and JEG3 cells was detected. Representative images (A) and statistical data (B) are presented. Scale bar: 100 μm. *P < 0.05, compared with control cells. HTR-8/SEV8 and JEG3 cells were transfected with miR154 mimic, miR154 NC, miR154 inhibitor, miR154 inhibitor NC, and miR154 mimic+WNT5A, and then detected by transwell assays. Representative images (C) and statistical data (D) are shown. *P < 0.05, compared to control cells. Scale bar: 100 μm. NC, represent negative control; BL, represent blank control.
S1. The clinical information of PSTT patients
Characteristics
clinical tissuesPacient number Age, year GPA Time of antecedent pregnancy, mo FIGO stage Presenting symptoms Outcome Performed assay Follow-up, mo IHC microRNA assay Real-time qPCR Metastatic PSTT 1 25 GAP1A0 9 III IVB NED 30 √ / / 2 29 G1P1A0 13 II IVB NED 60 √ √ √ 3 32 G1P1A1 24 II IVB NED 24 √ √ √ 4 25 G1P1A0 8 III IVB NED 41 √ / / Non-metastatic PSTT 1 28 G1P1A1 9 I IVB NED 36 √ √ √ 2 27 G2P0A2 2 I IVB NED 28 √ / / 3 24 G6P1A5 4 I IVB NED 35 √ / / 4 28 G1P1A0 6 I IVB NED 59 √ √ √ 5 35 G7P1A6 3 I IVB NED 56 √ / / Normal decidua 1 37 G4P1A3 / / / / / √ / / 2 23 G3P1A2 / / / / / √ / / 3 28 G2P0A2 / / / / / √ / / 4 27 G3P1A2 / / / / / √ / / 5 23 G3P0A3 / / / / / √ / / Normal Villi 1 29 G3P2A1 / / / / / √ / / 2 27 G0P0A1 / / / / / √ / / 3 27 G0P0A2 / / / / / √ / / 4 30 G0P0A1 / / / / / √ / / 5 26 G0P0A1 / / / / / √ / / Note. GPA, gravidity, parity, and abortions; IVB, irregular vaginal bleeding; FIGO, International Federation of Gynecology and Obstetrics; NED, no evidence of disease. -
[1] Burkett WC, Soper JT. A review of current management of placental site trophoblastic tumor and epithelioid trophoblastic tumor. Obstet Gynecol Surv, 2022; 77, 101−10. doi: 10.1097/OGX.0000000000000978 [2] Liu W, Zhao W, Huang XF. Outcomes and prognostic factors of placental-site trophoblastic tumor: a retrospective study of 58 cases. Arch Gynecol Obstet, 2022; 306, 1633−41. doi: 10.1007/s00404-022-06502-7 [3] Kaur B. Pathology of gestational trophoblastic disease (GTD). Best Pract Res Clin Obstet Gynaecol, 2021; 74, 3−28. doi: 10.1016/j.bpobgyn.2021.02.005 [4] Froeling FEM, Ramaswami R, Papanastasopoulos P, et al. Intensified therapies improve survival and identification of novel prognostic factors for placental-site and epithelioid trophoblastic tumours. Br J Cancer, 2019; 120, 587−94. doi: 10.1038/s41416-019-0402-0 [5] Zhou Y, Lu H, Yu C, et al. Sonographic characteristics of placental site trophoblastic tumor. Ultrasound Obstet Gynecol, 2013; 41, 679−84. doi: 10.1002/uog.12269 [6] Nakata M, Honda H, Iwama A, et al. WNT5A plays a critical role in anal opening in mice at an early stage of embryonic development. Pediatr Surg Int, 2022; 38, 743−7. doi: 10.1007/s00383-022-05103-4 [7] Bueno MLP, Saad STO, Roversi FM. WNT5A in tumor development and progression: a comprehensive review. Biomed Pharmacother, 2022; 155, 113599. doi: 10.1016/j.biopha.2022.113599 [8] Cao J, Xiao CC, Fong CJTH, et al. Expression and regulatory network analysis of function of small nucleolar RNA host gene 4 in hepatocellular carcinoma. J Clin Transl Hepatol, 2022; 10, 297−307. doi: 10.14218/JCTH.2020.00175 [9] Tian Q, Gu YF, Wang FF, et al. Upregulation of miRNA-154-5p prevents the tumorigenesis of osteosarcoma. Biomed Pharmacother, 2020; 124, 109884. doi: 10.1016/j.biopha.2020.109884 [10] Wang KS, Ma F, Arai S, et al. WNT5A signaling through ROR2 activates the hippo pathway to suppress YAP1 activity and tumor growth. Cancer Res, 2023; 83, 1016−30. -
 23340+Supplementary Materials.pdf
23340+Supplementary Materials.pdf

-




 下载:
下载:





 Quick Links
Quick Links