-
Methicillin-resistant Staphylococcus aureus (MRSA) is a common pathogen causing acquired infections. Antibiotics are routinely employed for bacterial infection treatment. Excessive antibiotic use for bacterial infections heightens resistance, and MRSA resistance is being prevalent[1]. MRSA exhibits substantial resistance to conventional antibiotics, which is associated with its biofilm-forming capability. The bacterial biofilm serves as a barrier, restricting nutrient access and slowing internal metabolism, increasing antibiotic resistance. The bacterial biofilm forms a defensive layer, resisting antibiotic penetration while preventing nutrient entry, inducing a slow bacterial metabolism, and increasing bacterial resistance[2]. Consequently, biofilm formation and the emergence of resistance to conventional antibiotics have become substantial challenges in the field of infectious diseases. The development of agents to remove biofilm barriers is urgently required because biofilms are extremely hazardous.
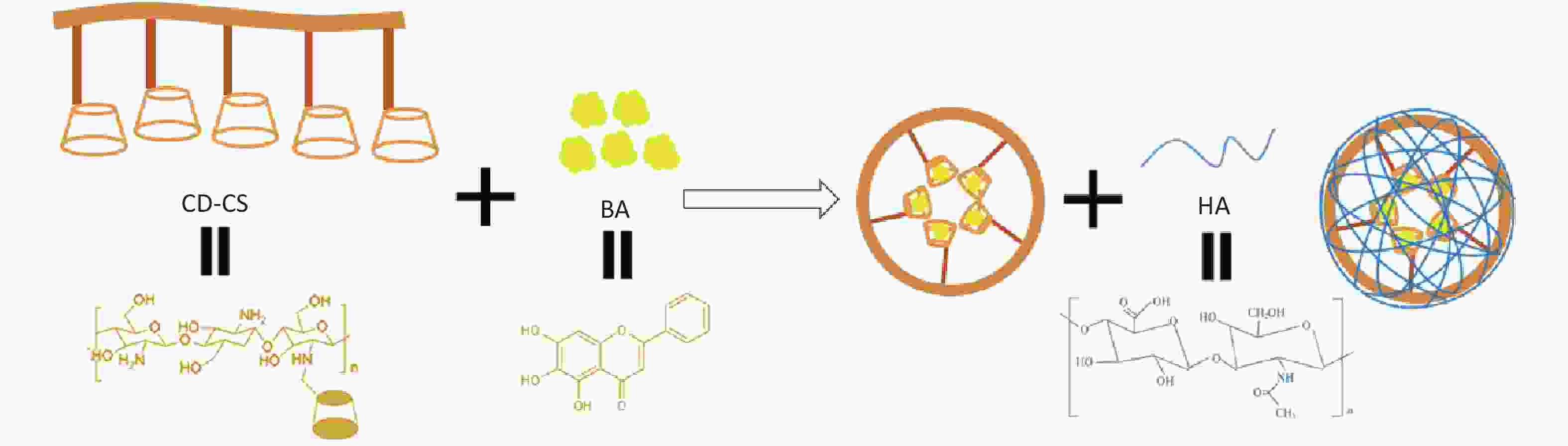
Scutellaria baicalensis’s active constituent, baicalein (BA), exhibits inhibitory activity against MRSA, yet its clinical utility is restricted due to its poor water solubility. Advances in nanotechnology can overcome the shortcomings of traditional drugs. Specifically, CS possesses remarkable antibacterial properties, whereas the dual hydrophilic and lipophilic internal architecture of CD is adept at drug nano-delivery[3]. Furthermore, our previous study demonstrated that CD-CS enhance drug solubility and antibacterial activity. Hyaluronic acid (HA) is a suitable carrier for nanoparticles because it can improve drug aggregation at the site of bacterial infection[4]. Accordingly, we integrated HA into our existing CD-CS nanodelivery platform with the intention to amplify the antibacterial and biofilm-eliminating effects of the medication. Building on the aforementioned groundwork, HA/CD-CS-BA-NPs were characterized and assessed for their in vivo and in vitro biofilm elimination abilities. Finally, the mechanism of action of the nano preparations to enhance drug elimination from the bacterial biofilm was explored by confocal laser scanning microscope (CLSM), flow cytometry (FCM), and Live/Dead staining. This study not only provides a theoretical foundation and experimental basis for the clinical application of HA/CD-CS-BA nanoformulation against MRSA infections but also provides a new idea for the development of anti-bacterial drug-resistant formulations.
HA/CD-CS-BA-NPs were prepared by probe ultrasonication techniques (Supplementary Figure S1, available in www.besjournal.com), subsequently characterized for particle dimension, potential and stability, and payload. The minimum inhibitory concentration (MIC) of HA/CD-CS-BA-NPs against MRSA was determined using the double dilution method. The effectiveness of HA/CD-CS-BA-NPs in eliminating MRSA biofilm was evaluated using the Crystalline Violet staining method. A silicone membrane implantation was employed for in vivo biofilm eradication studies in rats. Finally, the mechanisms were explored by confocal laser scanning microscope (CLSM), flow cytometry (FCM), and Live/Dead staining. Please refer to the attachment for precise experimental procedures.
-
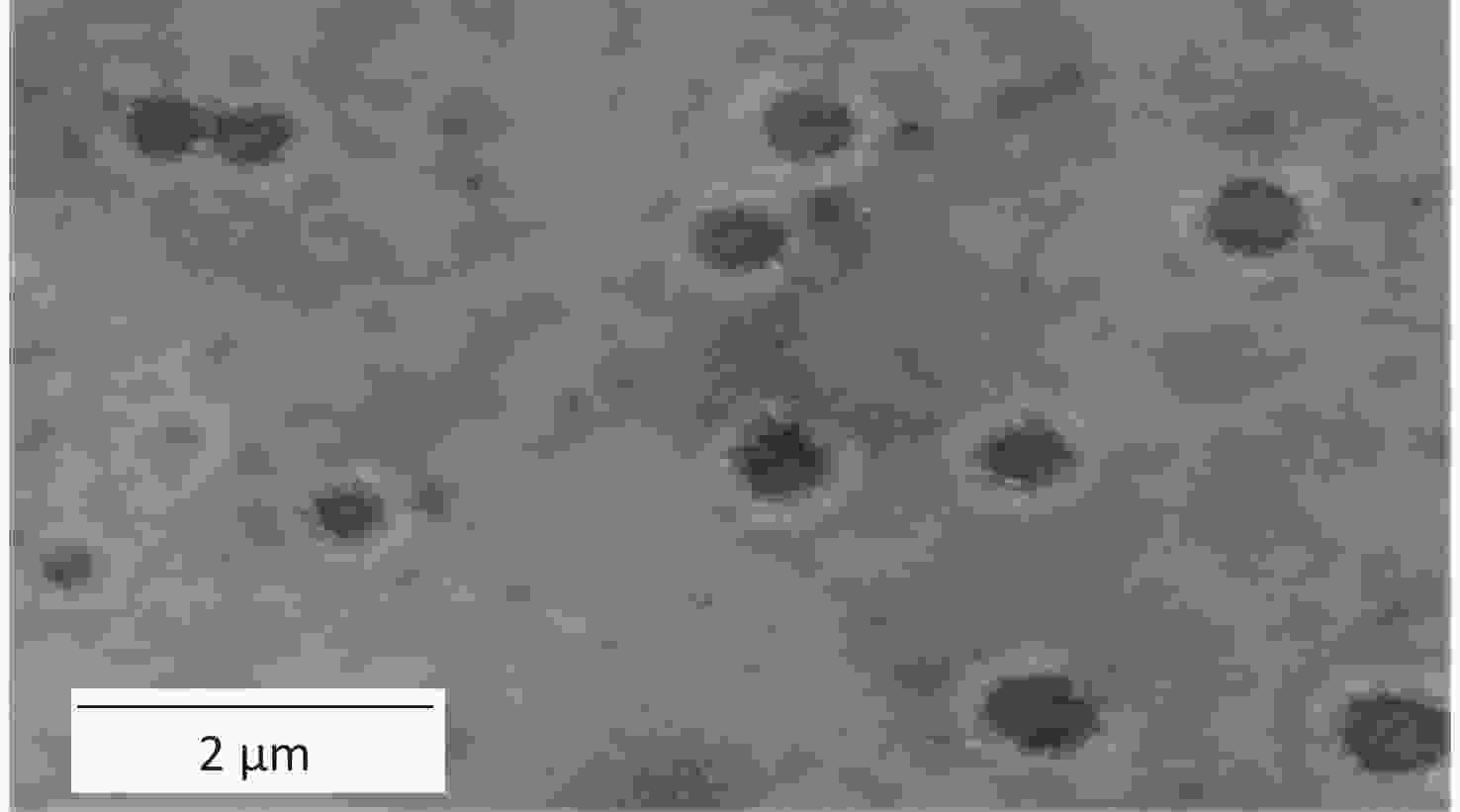
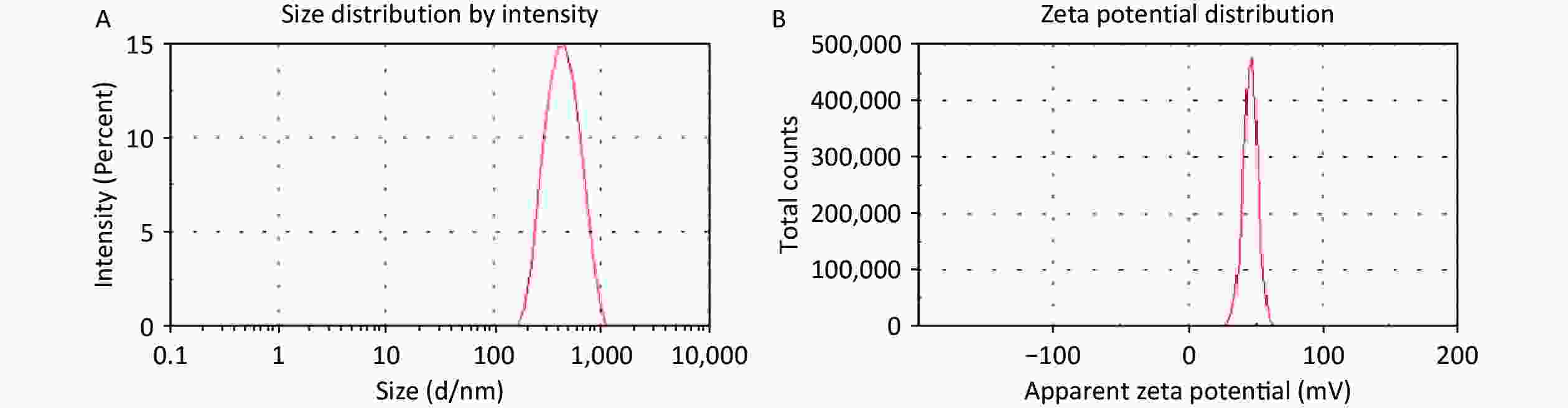
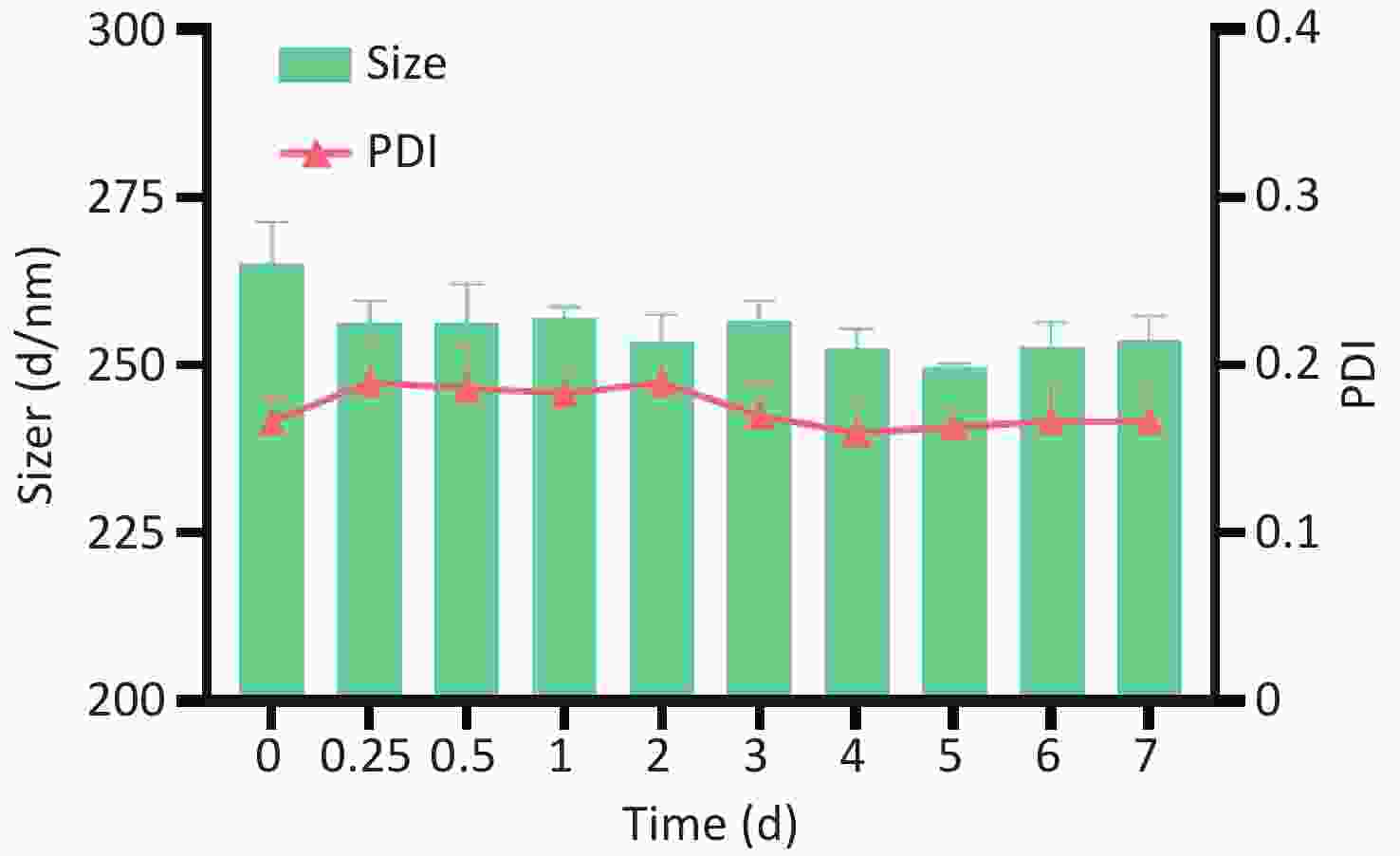
In the present study, the HA/CD-CS-BA-NPs were successfully prepared. As shown in Supplementary Figure S2A and S2B (available in www.besjournal.com), the particle size of the HA/CD-CS-BA-NPs was (263.8 ± 7.65) nm, and the Polydispersity Index (PDI) was 0.178 ± 0.03, which indicates that nanoparticles are evenly dispersed and uniformly distributed. As shown in Supplementary Figure S3 (available in www.besjournal.com), none of the HA/CD-CS-BA-NPs exhibited notable alterations within one week. This also reflects ability of the formulation to remain relatively stable in the short term. The zeta potential has a significant relationship with the stability of the nanoformulation. A formulation with an absolute zeta potential > 30 mV is unlikely to aggregate and demonstrates good stability[5]. The zeta potential of the HA/CD-CS-BA-NPs stands at (32.4 ± 3.04) mV, which favors excellent formulation stability. In this study, the TEM image (Figure 1) of the nanoparticles showed a spherical structure, which coincided with the Malvern instrumental measurements. The drug loading percentage of BA in the HA/CD-CS-NPs was 5.44% ± 0.64%.
-
When assessing the in vitro antibacterial effect (as shown in Supplementary Table S1, available in www.besjournal.com), it was found that the MIC of HA/CD-CS-BA-NPs was 12.5 μg/mL, compared with 25 μg/mL for BA solution alone. These findings indicated that the addition of HA/CD-CS-BA-NPs enhanced the antibacterial efficacy. Similar study has shown that chitosan (CS) disrupts the structure of bacterial cell membranes, leading to antimicrobial effects[6].
Table S1. The determination of MIC
Concentration (μg/mL) 1 2 3 4 5 6 7 8 9 BA solution 200
−100
−50
−25
−12.5
+6.25
+3.13
+1.56
+0.78
+HA/CD-CS-BA-NPs 50
−25
−12.5
−6.25
+3.13
+1.56
+0.78
+0.39
+0.19
+Negative Control 0
+0
+0
+0
+0
+0
+0
+0
+0
+HA/CD-CS-NPs 0
+0
+0
+0
+0
+0
+0
+0
+0
+CD-CS, HA, BA (mixture) 50
−25
−12.5
+6.25
+3.14
+1.56
+0.78
+0.39
+0.19
+Note. “−” indicates that bacterial growth is inhibited and the medium is clear; “+” indicates that there is bacterial growth and the medium is turbid. -
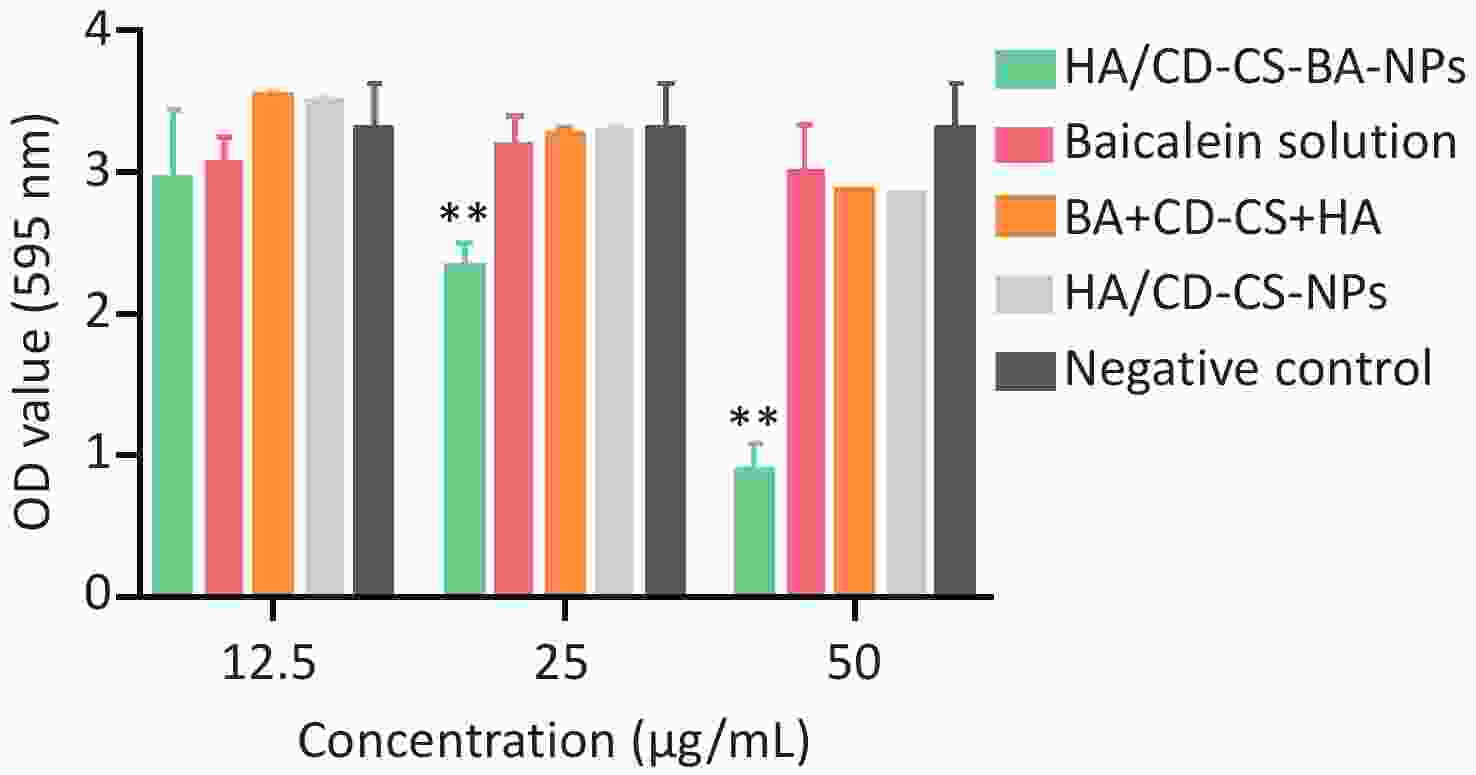
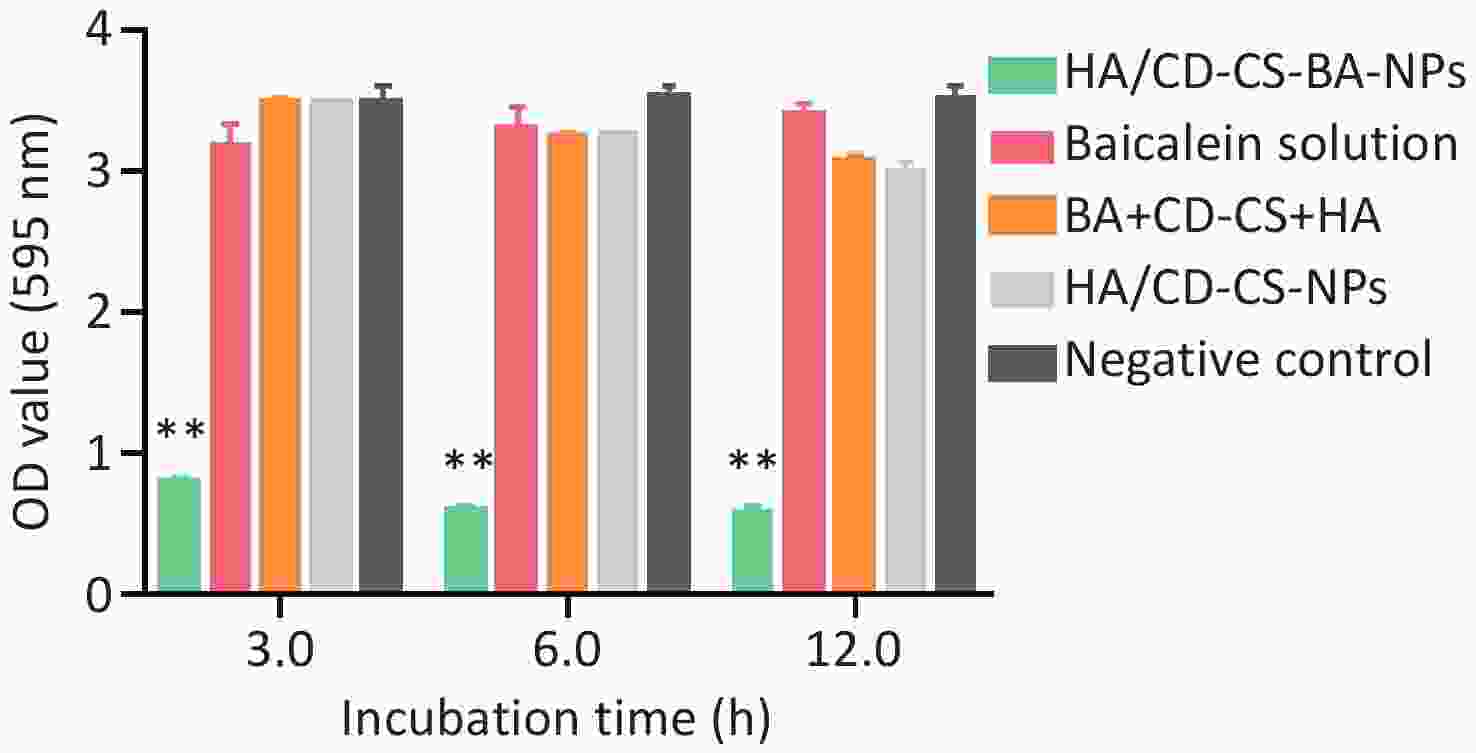
The biofilm elimination results for NPs at different concentrations are depicted in Supplementary Figure S4 (available in www.besjournal.com). All except HA/CD-CS-BA-NPs demonstrated no significant disparity from the negative control, indicating that these groups were not successful in eliminating the biofilm below 50 μg/mL (P > 0.05). However, the OD value of the HA/CD-CS-BA-NPs treatment displayed superior biofilm elimination compared with that of the negative control, and this was enhanced with augmented concentration. Positively charged drug-loaded nanoparticles have been reported to enhance drug elimination of MRSA biofilms by positive surface charge. We postulated that the surface positive charge of the HA/CD-CS-BA-NPs facilitated their adhesion to bacterial negative charge, thereby enhancing their biofilm eradication. In addition, shown in Supplementary Figure S5 (available in www.besjournal.com) that the CD-CS-BA-NPs showed markedly lower OD values than that of others, suggesting its quick effectiveness in eliminating biofilm within 3 h.
-
The skin of the blank group was transparent (Figure 2A), featuring a non-thickened epidermis and organized collagen fibers in the dermis. Numerous hair follicles were noticeable, with no inflammation. The model group (Figure 2B) exhibited an irregular skin structure, with epidermal detachment and dermal exposure, and diminished, thin, and fluid collagen fibers. Inflammatory cell infiltration was apparent. Infiltration of inflammatory cells was also observed. In the BA-treated group (Figure 2C), skin tissue integrity was retained, although collagen fibers within the dermis displayed some irregularity, and minimal inflammatory infiltrate was noted. Compared with the other groups, the skin of the HA/CD-CS-BA NPs-treated group (Figure 2D) exhibited a lucid structure devoid of epidermal thickening, well-aligned, dense collagen fibers within the dermis, and negligible inflammation. The HA/CD-CS-BA NPs treatment group exhibited better antibacterial and anti-inflammatory effects. This may stem from the anti-inflammatory effects of BA and HA. BA counteracts MRSA-induced inflammation, and HA has anti-inflammatory and antibacterial effects.
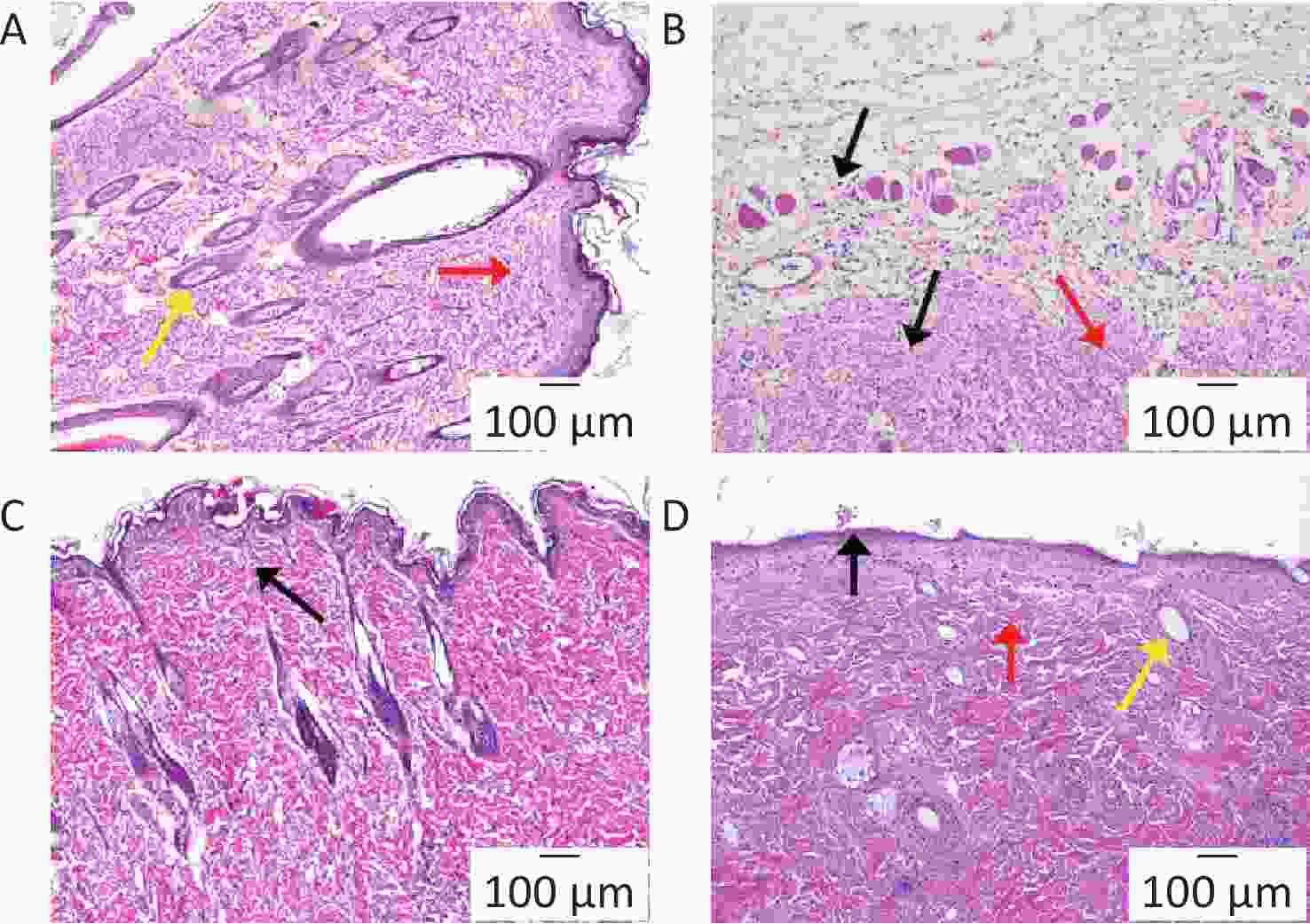
Figure 2. Pathological section analysis of inflammatory tissue around the implantation site from Blank group (A), Model group (B), BA solution-treated group (C), and HA/CD-CS-BA-NPs treated group (D). Figure 2A–D show images at 100×.
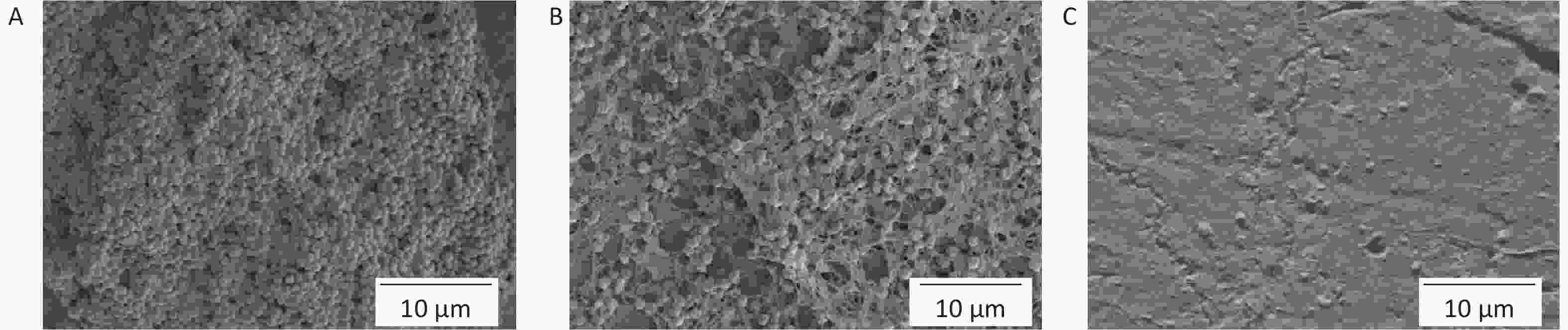
The silicon film was removed for SEM imaging. The model group displayed an intact, dense bacterial biofilm and abundant bacterial adhering (Supplementary Figure S6A, available in www.besjournal.com), with a clear bacterial morphology. Bacterial counts in the BA solution-treated group decreased but remained stuck together (Supplementary Figure S6B). Biofilm disruption on the silica film surface biofilm disruption was observed in the HA/CD-CS-BA-NPs treated group (Supplementary Figure S6C), with substantial bacterial reduction and limited adherence.
-
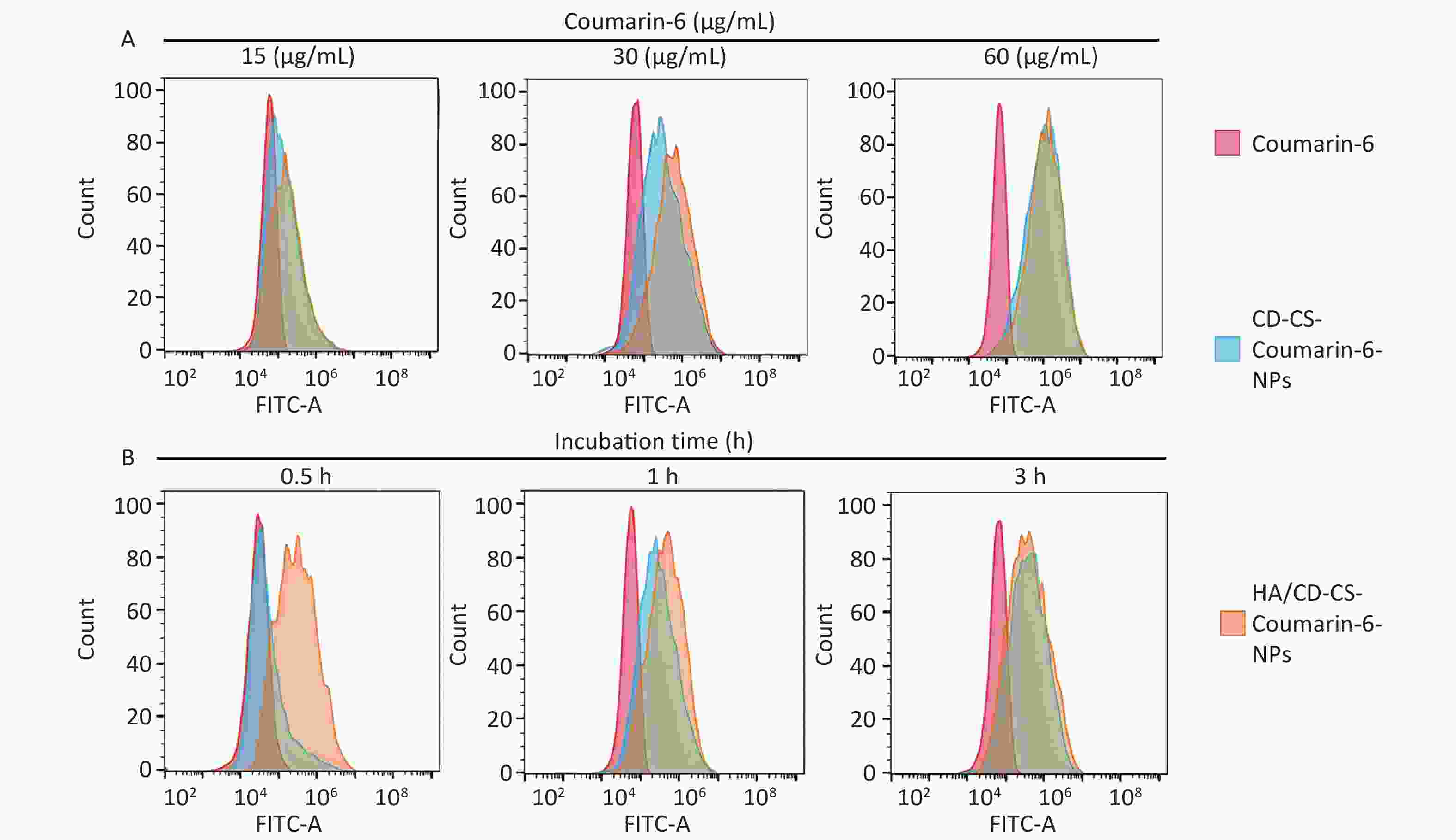
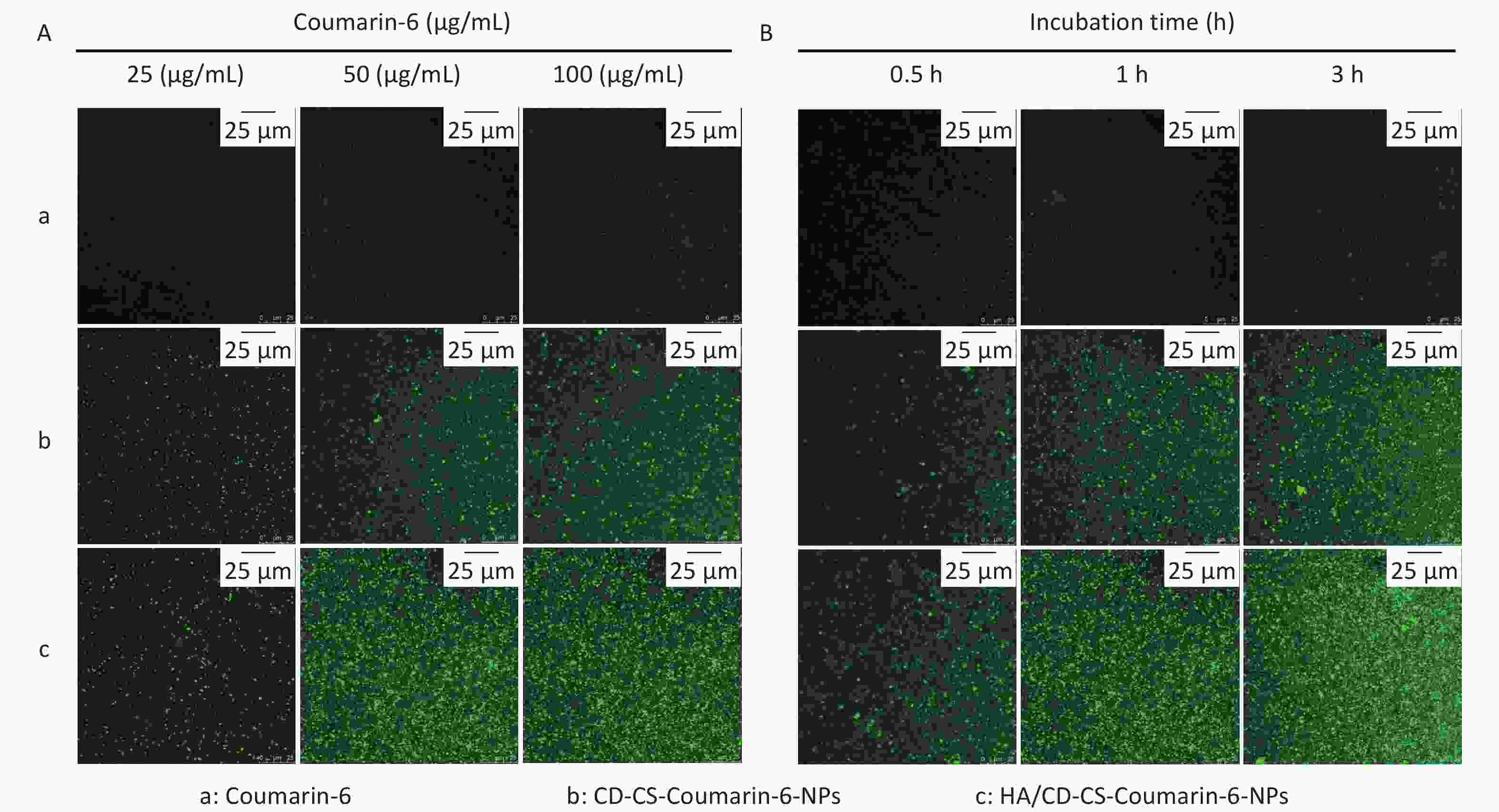
In this study, given that the BA solution itself was not luminescent, fluorescent coumarin-6 was chosen to prepare HA/CD-CS-BA-NPs to probe the drug uptake capacity of MRSA. A higher fluorescence intensity signifies greater drug uptake. A higher fluorescence intensity signifies greater drug uptake. Supplementary Figure S7A and S7B (available in www.besjournal.com) illustrate that the formulation treated group exhibited markedly enhanced fluorescence, compared with that of the other groups. Additionally, bacteria in the HA/CD-CS-BA-NPs treated group ingested substantial amounts of the drug, with an esclating uptake intensity that was positively correlated withto time and concentration. The enhanced in bacterial drug uptake by HA/CD-CS-BA-NPs may have contributed to the reduced MIC and biofilm eradication effects of this nanosystem. This aligns with prior investigations asserting CD-CS potency to enhance bacterial drug uptake, which is potentially linked to the enhanced cell membrane permeation of CD-CS. Moreover, the hydrophobic internal structure and hydrophilic external properties of CD-CS enhance its drug solubility, potentially boosting the bacterial intake of the drug, thereby amplifying its antimicrobial efficacy. HA possesses targeted antibacterial capabilities and HA/CD-CS-Coumarin-6-NPs fabricated with HA exhibited superior penetration power, which is attributed to its potential for adherence to bacterial biofilm, thereby disrupting its structure and resulting in enhanced penetration for enhanced biofilm elimination.
-
To explore the delivery mechanism of HA/CD-CS-BA-NPs, we investigated the drug penetration into MRSA biofilms at varying concentrations and incubation durations using CLSM. As shown in Figure 3A and 3B, the fluorescence intensity of both HA/CD-CS-Coumarin-6-NPs and CD-CS-Coumarin-6-NPs markedly increased with an increase in drug dosage concentration and extended incubation duration. Notably, HA/CD-CS-Coumarin-6-NPs exhibited the most pronounced fluorescence intensity, suggesting greater penetration of the drug into MRSA. These findings can be attributed to the superior permeability of HA/CD-CS for drug delivery. The biofilm, composed primarily of the extracellular polymer EPS, hinders the permeation of different antimicrobial agents and drugs into the bacteria. moreover, it forms a well-segregated protective barrier inside the biofilm, providing an effective defense for the bacteria[7,8]. Biopolymer-coated nanoparticles composed of CS and HA can facilitate drug penetration, retention, and disruption of bacterial biofilms[9]. A previous laboratory investigation determined that co-incubation of CD-CS with Staphylococcus xylosus and E. coli led to an increase in bacterial cell membrane permeability. Several reports have claimed that CD-CS can facilitate drug penetration into the bacterial interior by increasing cell membrane permeability[10]. Based on the aforementioned studies, we concluded that the improved efficiency of HA/CD-CS-BA-NPs in eliminating MRSA biofilms could be attributed to the ability of HA/CD-CS to enhance drug penetration into bacterial biofilms. Consequently, a greater amount of the drug can infiltrate and damage the biofilm, leading to improved biofilm elimination.
-
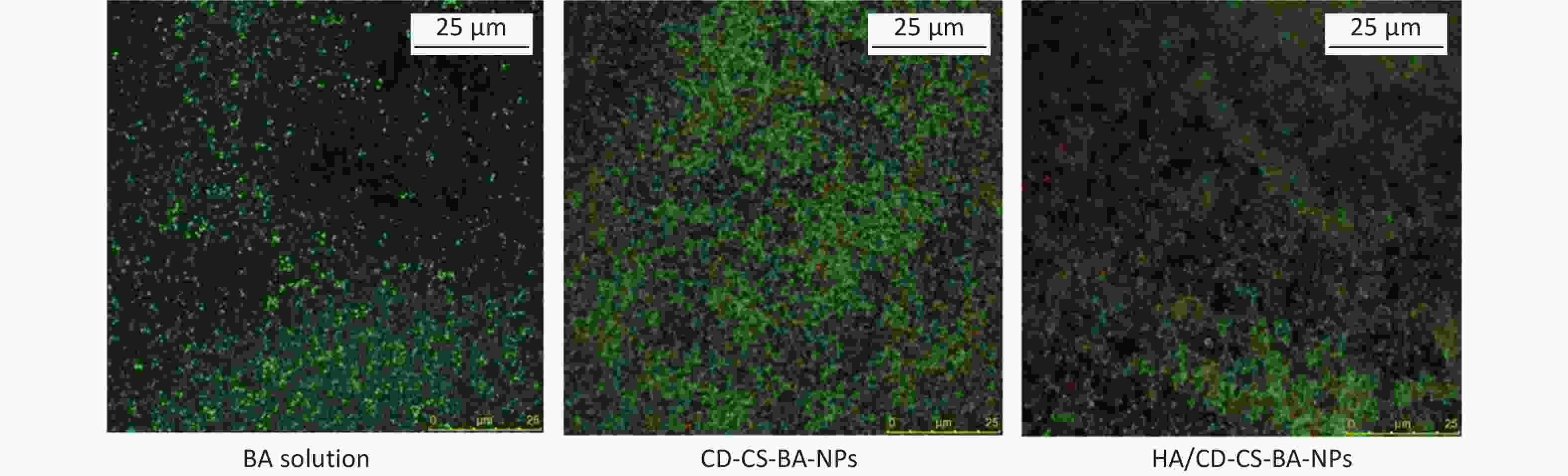
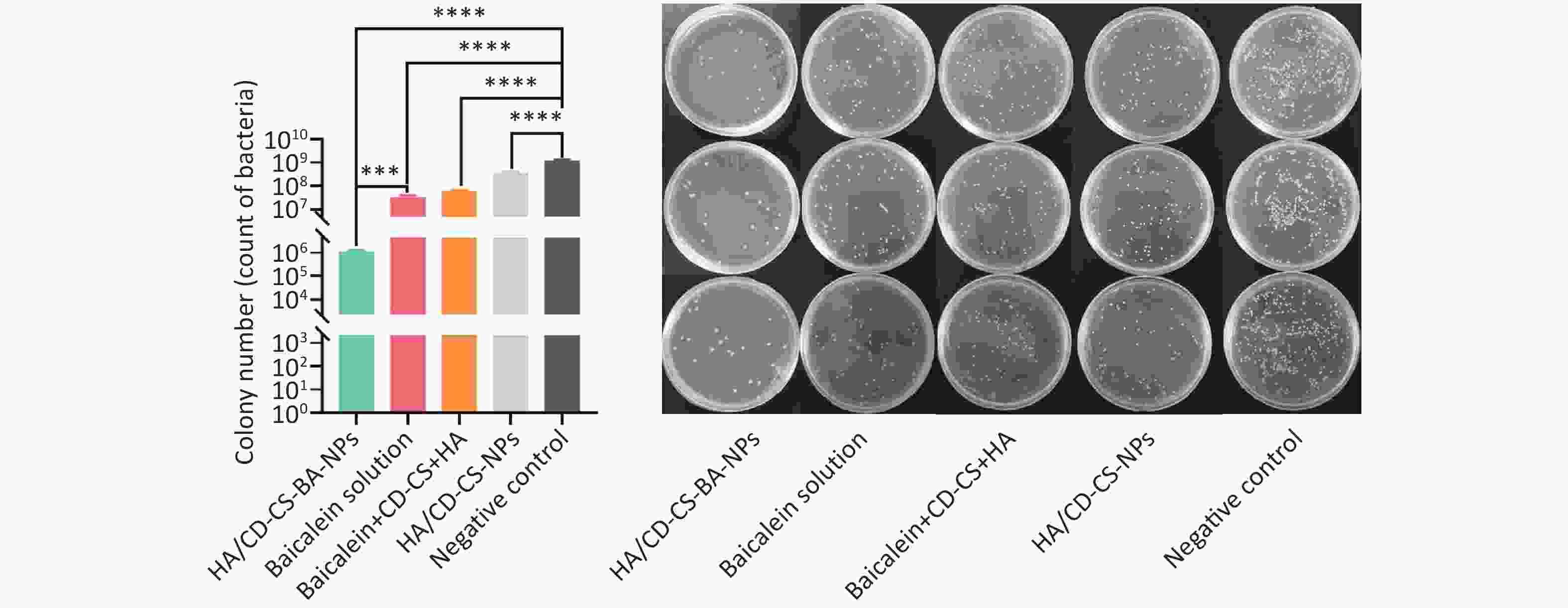
Finally, a Live/Dead kit was used to examine the killing of MRSA biofilms by the NPs. The kit employed Green fluorescence indicated live bacteria and red fluorescence indicated dead bacteria. As shown in Supplementary Figure S8 (available in www.besjournal.com), compared to the baicalein-treated and CD-CS-BA-NPs-treated groups, the HA/CD-CS-BA-NPs showed a significant increase in red fluorescence and a significant decrease in green fluorescence, indicating that the nanoparticles were more capable of killing MRSA which aligns with the results observed in the in vitro biofilm eradication assay. Moreover, colony count analysis (Supplementary Figure S9, available in www.besjournal.com) revealed that the bacteria population in the HA/CD-CS-BA-NPs treated group decreased by approximately 1096-fold, signifying significant reductions compared with that in drug and carrier treated groups, and the mixture group. This is consistent with the MIC evaluation results, suggesting the superior anti-biofilm efficacy of HA/CD-CS-BA-NPs. This may be attributed to the antimicrobial properties of CD-CS, HA-targeted antimicrobial effects, and the encapsulated drugs that enhance drug penetration and absorption. Colony counts were corroborated by bacterial death and survival assessments. The analysis suggested two underlying mechanisms for the effectiveness of HA/CD-CS-BA-NPs against biofilm bacteria: enhanced drug uptake via the nano-delivery system and overcoming the physical barrier of the biofilm to promote drug penetration.
In summary, HA/CD-CS-BA NPs were developed and demonstrated a reduced MIC compared to that of BA alone against MRSA. Importantly, both in vivo and in vitro experiments demonstrated that the HA/CD-CS nano-delivery system was potent in eliminating the MRSA biofilms. Notably, they can act as biofilm eliminators by aiding bacteria in absorbing drugs and enhancing drug penetration. Given the effect of HA/CD-CS-BA-NPs on the elimination of MRSA, they may be used for drug delivery applications against MRSA, and the HA/CD-CS nanocarrier system may be a valuable delivery system for the development of antimicrobial drugs.
-
The animal study was approved by the Ethics Committee of the Experimental Animal Centre of Guangxi University of Chinese Medicine (Nanning, China, No. SYXK-GUI-2019-0001) and carried out in a manner consistent with the Guide for the Care and Use of Laboratory Animals. All guidelines have been strictly followed during the whole study.
doi: 10.3967/bes2024.160
Preparation and Methicillin-Resistant Staphylococcus aureus Biofilm Elimination Effect of Baicalein-Loaded Hyaluronic acid/β-Cyclodextrin grafted Chitosan Nanoparticles
-
Liting Lai: drafted the manuscript, conducted experiments, and analyzed the results. Wenyou Ding: analyzed the experimental data. Guoying Huang, Mengke Wang, and Jinqing Chen: conducted the preparation and characterization of preparations. Liuzhen Lai, Xiuzhen Deng, Ling Tang, Xinyi Yu, and Ya Huang: explored the biofilm elimination effect and mechanism of the preparation. Thi Minh Hien Truong: polished the manuscript. Wenya Ding, and Zhongbin Zhang: obtained experimental materials, provided experimental project, guided the experiment, and revised the manuscript.
None of the authors has any conflicts to declare.
&These authors contributed equally to this work.
注释:1) Author Statement: 2) Conflicts of Interest: -
S1. The determination of MIC
Concentration (μg/mL) 1 2 3 4 5 6 7 8 9 BA solution 200
−100
−50
−25
−12.5
+6.25
+3.13
+1.56
+0.78
+HA/CD-CS-BA-NPs 50
−25
−12.5
−6.25
+3.13
+1.56
+0.78
+0.39
+0.19
+Negative Control 0
+0
+0
+0
+0
+0
+0
+0
+0
+HA/CD-CS-NPs 0
+0
+0
+0
+0
+0
+0
+0
+0
+CD-CS, HA, BA (mixture) 50
−25
−12.5
+6.25
+3.14
+1.56
+0.78
+0.39
+0.19
+Note. “−” indicates that bacterial growth is inhibited and the medium is clear; “+” indicates that there is bacterial growth and the medium is turbid. -
[1] Kasela M, Ossowski M, Dzikoń E, et al. The epidemiology of animal-associated methicillin-resistant Staphylococcus aureus. Antibiotics, 2023; 12, 1079. doi: 10.3390/antibiotics12061079 [2] Sharma D, Misba L, Khan AU. Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob Resist Infect Control, 2019; 8, 76. doi: 10.1186/s13756-019-0533-3 [3] Dong WJ, Ye J, Wang WJ, et al. Self-assembled lecithin/chitosan nanoparticles based on phospholipid complex: a feasible strategy to improve entrapment efficiency and transdermal delivery of poorly lipophilic drug. Int J Nanomedicine, 2020; 15, 5629−43. doi: 10.2147/IJN.S261162 [4] Sahiner N, Suner SS, Ayyala RS. Preparation of hyaluronic acid and copolymeric hyaluronic acid: sucrose particles as tunable antibiotic carriers. J Polym Res, 2020; 27, 192. doi: 10.1007/s10965-020-02168-4 [5] Sun MS, Zhu ZH, Wang HX, et al. Surface density of polyarginine influence the size, zeta potential, cellular uptake and tissue distribution of the nanostructured lipid carrier. Drug Deliv, 2017; 24, 519−26. doi: 10.1080/10717544.2016.1269849 [6] Da Costa D, Exbrayat-Héritier C, Rambaud B, et al. Surface charge modulation of rifampicin-loaded PLA nanoparticles to improve antibiotic delivery in Staphylococcus aureus biofilms. J Nanobiotechnol, 2021; 19, 12. doi: 10.1186/s12951-020-00760-w [7] Pinto RM, Soares FA, Reis S, et al. Innovative strategies toward the disassembly of the EPS matrix in bacterial biofilms. Front Microbiol, 2020; 11, 952. doi: 10.3389/fmicb.2020.00952 [8] Wang YY, Shukla A. Bacteria-responsive biopolymer-coated nanoparticles for biofilm penetration and eradication. Biomater Sci, 2022; 10, 2831−43. doi: 10.1039/D2BM00361A [9] Ding WY, Zheng SD, Qin Y, et al. Chitosan grafted with β-cyclodextrin: synthesis, characterization, antimicrobial activity, and role as absorbefacient and solubilizer. Front Chem, 2019; 6, 657. doi: 10.3389/fchem.2018.00657 [10] Hao Y, Zhang MM, Wang L, et al. Mechanism of antimicrobials immobilized on packaging film inhabiting foodborne pathogens. LWT, 2022; 169, 114037. doi: 10.1016/j.lwt.2022.114037 -
 23386+Supplementary Materials.pdf
23386+Supplementary Materials.pdf

-




 下载:
下载:





 Quick Links
Quick Links