-
Dental caries is a widespread oral disease and is particularly significant during childhood, as it can impact growth and development[1]. Early childhood caries (ECC), defined as the presence of decayed, missing, or filled primary teeth in children under six years old, represents a major global health issue[2]. Untreated ECC can lead to pain, impaired digestion, disruption of permanent tooth development, and can negatively affect both the physical and mental health of children.
The etiology of ECC involves complex interactions among bacteria, host factors, diet, and time. In addition, the condition is impacted by additional factors such as breastfeeding, oral hygiene practices, diet, family history, socioeconomic status, and geographic location. A growing number of studies have investigated the role of the oral microbiota in health and disease[3]. Different oral niches, such as saliva, the tongue, and tooth surfaces, host distinct microbial communities. These communities exist in a dynamic balance, and external factors, including acidity, heat, and antibiotic use can disrupt this balance, increasing the risk of caries and other oral and systemic diseases[4].
However, limited research has been conducted on the microbiology of dental caries in children. This study aims to identify factors associated with caries and employs 16S rDNA sequencing to evaluate the differences in oral microbiota between healthy children and those with caries across different oral niches, such as the dorsal tongue mucosa, buccal mucosa, and tooth surfaces. Additionally, the study aims to screen for microbial species with diagnostic value and explore their gene functions. This research is expected to deepen our understanding of the etiology of ECC, improve diagnostic methods, and develop targeted prevention strategies.
This study was approved by the Ethics Committee of Shandong Second Medical University (Approval No.: 2020YX072). The study subjects were children aged 3 to 5 years. Informed consent was obtained from all legal guardians before sampling, and all the study procedures were conducted in adherence to the relevant provisions of the Declaration of Helsinki. The sample size was determined using the Fleiss correction formula $ {N}_{corrected}=\dfrac{P\left(1-P\right)\times {Z}^{2}}{{E}^{2}}\times \dfrac{{P}_{max}\left(1-{P}_{max}\right)}{P\left(1-P\right)} $, with a Z value corresponding to the standard normal distribution at a 95% confidence level (1.96). Pmax was set at 0.5, and the dental caries rate among 5-year-old children was reported as 70.9% based on the results of the Fourth National Oral Health Survey in China[5]. The allowable sampling error was set to less than 6%, leading to a theoretical sample size of 267. In anticipation of invalid samples, the sample size was increased to 315. The study subjects were randomly selected from three kindergartens in Weifang, China. The inclusion criteria were children who had lived in the area for more than six months and who were able to cooperate with oral examinations. The exclusion criteria were children who had used antibiotics, probiotics, or other medications in the past month, as well as those with systemic diseases who were unable to cooperate with the examination.
The oral examinations were conducted by two dentists. Additionally, a questionnaire was designed based on local economic conditions and lifestyle habits, collecting basic information from the participants, including oral hygiene practices, dietary habits, parental educational background, and oral health knowledge. The dentists provided detailed explanations of the questionnaire to the parents to ensure accurate information collection. To capture representative samples across different stages of caries severity and to ensure the feasibility of high-throughput sequencing and detailed microbial analysis, 35 children were selected for sampling in this study. These children were categorized into a healthy group (dmft = 0, Group H), a mild early childhood caries group (1 ≤ dmft ≤ 4, Group M), and a severe early childhood caries group (dmft ≥ 5, Group S). Samples were collected from the 35 children (including 9 healthy children, 15 with mild ECC, and 11 with severe ECC) by oral health professionals using sterile cotton swabs; samples were collected from the dorsal tongue mucosa, teeth, and buccal mucosa. Samples contaminated with blood or other impurities were excluded. The samples were labeled, placed in sterile EP tubes containing buffer solution, and stored accordingly. A total of 105 samples were collected, including dorsal tongue mucosa plaques (T niche, T01-T35), buccal mucosa plaques (C niche, C01-C35), and dental plaque samples (D niche, D01-D35). Following DNA extraction from the oral microbiota samples, 16S rDNA genes were amplified using specific primers. Libraries were prepared according to sequencing requirements and sequenced on the Illumina MiSeq PE300 platform. Finally, functional predictions were conducted using PICRUSt2 and STAMP software.
The data were organized into a database using Excel, and accuracy was ensured by double-entry verification. The dataset was then imported into R software (v4.2.1) and SPSS 27.0 (SPSS Inc, Chicago, IL, USA) for analysis. Count data were expressed as the number of cases, and univariate analysis was conducted using the χ2 test. Subsequently, factors with P < 0.05 were included in a multivariate logistic regression analysis, with a significance level set at α = 0.05. The differences in oral microbiota sequencing data between groups were assessed using the Kruskal-Wallis test and one-way ANOVA. Furthermore, differences at various bacterial community classification levels were evaluated using the LEfSe method. In this study, LDA score > 2.0, P < 0.05 was considered statistically significant.
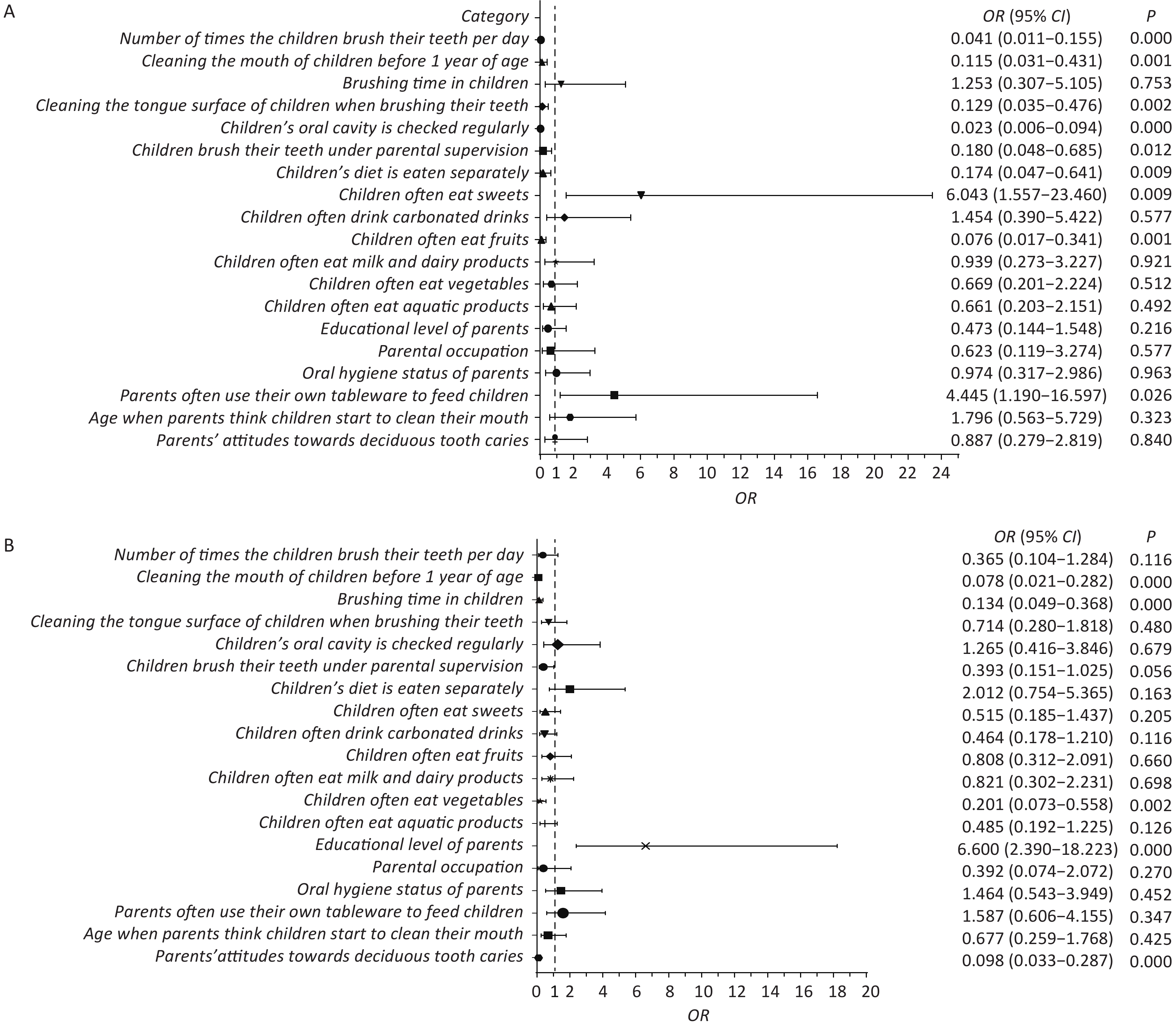
A total of 315 children aged 3–5 years were surveyed, including nearly equal proportions of boys and girls. The analysis revealed a caries prevalence of 67.93%. The children were divided into healthy, mild ECC, and severe ECC groups. Univariate analysis (Supplementary Table S1, available in www.besjournal.com) identified 19 factors significantly associated with dental caries, including oral hygiene and dietary habits. Multivariate analysis (Figure 1) revealed that frequent consumption of sweets and the use of parental utensils were major factors associated with a 6.043 and 4.445 times increase in the risk of caries, respectively. Conversely, regular tooth brushing, oral cleaning before the age of one, and frequent fruit consumption were identified as protective factors. The study also indicated that parental education level influenced the severity of caries, with higher education levels associated with more severe caries. Interestingly, despite the lack of statistically significant difference in parental literacy between the healthy and ECC groups, literacy emerged as a determining factor in caries severity. These findings suggest that higher education levels do not always correlate with better oral hygiene practices. This difference highlights the need for targeted education on specific oral health behaviors, regardless of the parents’ overall educational attainment.
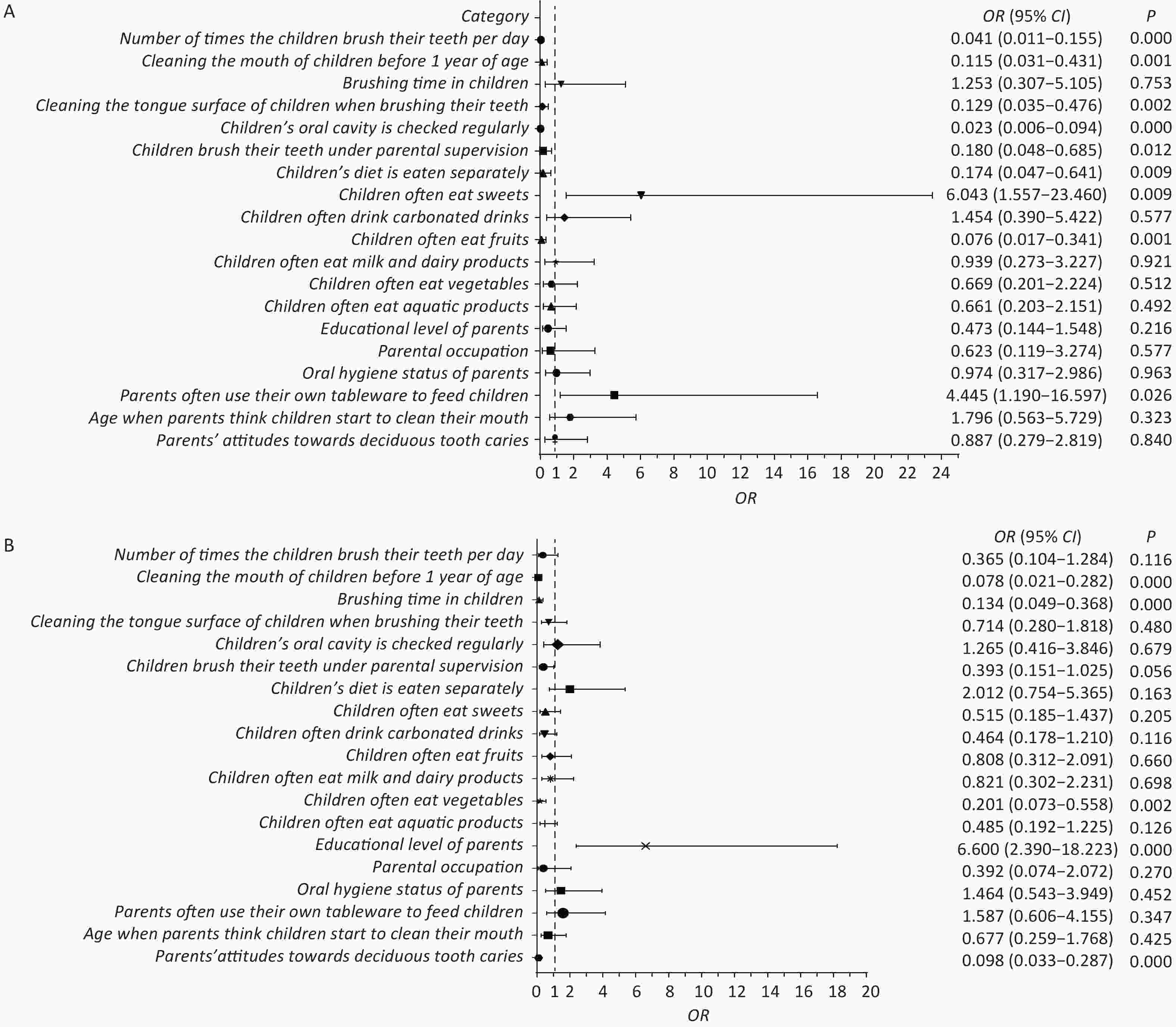
Figure 1. Forest plot. (A) Multifactorial regression analysis of health and ECC groups. (B) Multifactorial regression analysis of mild ECC and severe ECC groups. In the left planar rectangular coordinate system diagram, the horizontal scale with 1 as the center of the vertical null line indicates the protective factors on the left side of the line; the factors on the right side are associated factors, and the factors crossing the line are null factors. On the right side are the odds ratios with 95% confidence intervals and the P-values. ECC, early childhood caries.
Table S1. Univariate analysis of caries associated factors in early childhood
Factors Categories Number of cases Degree of early childhood caries χ2 P Health Mild Severe Gender of child Male 162 57 60 45 2.209 0.331 Female 153 44 56 53 Age of child (years) 3– 52 22 14 16 8.254 0.083 4– 81 29 34 18 5– 182 50 68 64 Childhood feeding mode Breast feeding 192 64 69 59 0.376 0.829 Mixed feeding 123 37 47 39 Age at which the child started tooth brushing 3+ years old 258 81 96 81 0.293 0.864 Age three and under 57 20 20 17 Number of times the children brush their teeth per day 2 and more times 106 73 11 22 103.347 0.000** Less than 2times 209 28 105 76 Cleaning the mouth of children before 1 year of age No 174 21 61 92 107.973 0.000** Yes 141 80 55 6 Cleaning the mouth of children aged 1 to 3 years Gargle 36 10 19 7 4.849 0.303 Just use a toothbrush 207 67 72 68 Toothbrush and toothpaste 72 24 25 23 Children's oral cleaning method after the age of 3 years Ordinary toothbrush 187 57 66 64 4.044 0.400 Children's toothbrush 80 31 30 19 Electric toothbrush 48 13 20 15 Brushing time in children Less than 2 minutes 109 29 18 62 55.799 0.000** 2 minutes or more 206 72 98 36 Cleaning the tongue surface of children when brushing their teeth No 156 30 65 61 24.184 0.000** Yes 159 71 51 37 Children's oral cavity is checked regularly No 196 21 97 78 108.927 0.000** Yes 119 80 19 20 Children brush their teeth under parental supervision No 138 22 53 63 36.765 0.000** Yes 177 79 63 35 Children's diet is eaten separately No 160 27 80 53 39.145 0.000** Yes 155 74 36 45 Children often eat sweets No 156 70 51 35 24.723 0.003** Yes 159 31 65 63 Children often drink carbonated drinks No 178 73 55 50 15.324 0.000** Yes 137 28 61 48 Children often eat fruits No 120 18 50 52 28.144 0.000** Yes 195 83 66 46 Children often eat meat No 147 47 57 43 0.592 0.744 Yes 168 54 59 55 Children often eat eggs No 136 36 56 44 3.684 0.1585 Yes 179 65 60 54 Children often eat milk and dairy products No 140 31 59 50 11.386 0.003** Yes 175 70 57 48 Children often eat tofu or soy products No 116 41 35 40 3.495 0.174 Yes 199 60 81 58 Children often eat coarse grains class No 137 39 49 49 2.741 0.254 Yes 178 62 67 49 Children often eat vegetables No 144 29 45 70 40.115 0.000** Yes 171 72 71 28 Children often eat aquatic products No 134 31 49 54 12.13 0.002** Yes 181 70 67 44 Educational level of parents General 154 39 79 36 27.2 0.000** Higher 161 62 37 62 Parental occupation Medical personnel 48 25 17 6 13.414 0.001** Non-medical personnel 267 76 99 92 Oral hygiene status of parents General 170 45 64 61 6.373 0.041* Good 145 56 52 37 Parents often use their own tableware to feed children No 174 79 58 37 29.533 0.000** Yes 141 22 58 61 Age when parents think children start to clean their mouth After the teeth sprout 161 62 52 47 6.49 0.039* After the age of 3 154 39 64 51 When do parents think the child starts going to the dentist After the teeth sprout 32 8 9 15 7.325 0.292 About 3 years old 162 53 56 53 When there is toothache 64 19 28 17 Unsureness 57 21 23 13 Parents' attitudes towards deciduous tooth caries Don't care 159 36 43 80 55.286 0.000** Regular inspection,
early treatment156 65 73 18 Note. *P < 0.05, **P < 0.01. Additionally, frequent intake of high-sugar foods was strongly associated with an increased risk of caries, as these foods provide nutrients for bacterial growth and acid production, leading to enamel demineralization. On the other hand, moderate fruit intake, particularly fiber-rich fruits, protects against caries by increasing saliva production, which neutralizes acids and removes food debris. Regular oral hygiene practices, including brushing and tongue cleaning, as well as routine dental checkups, play an essential role in reducing the occurrence of caries. Using separate utensils to feed children was also emphasized as an important preventive measure, reducing the risk of transmitting harmful oral bacteria from parents to children.
This study identified 18 phyla, 30 classes, 70 orders, 128 families, and 253 genera from the oral microbiota samples collected from children. The six most abundant phyla were Firmicutes, Proteobacteria, Fusobacteria, Bacteroidota, Actinobacteria, and Patescibacteria, accounting for more than 98% of the total sequences. In addition, significant differences in microbial composition were observed across the niches of the dorsal tongue mucosa, buccal mucosa, and tooth surfaces (Supplementary Figure S1, available in www.besjournal.com). Firmicutes were predominant on the dorsal tongue and buccal mucosa, whereas Actinobacteria were more prevalent on tooth surfaces. Notably, children with severe caries (Group S) showed a significant reduction in Firmicutes within the dental plaque niche, suggesting that the shift in microbial balance may be associated with caries progression. At the genus level, Streptococcus, Actinomyces, and Neisseria were the most common genera, with Veillonella being particularly prevalent in the T niche and Streptococcus in the C niche. Alpha and beta diversity analyses (Supplementary Figure S2, available in www.besjournal.com) revealed no statistical significance in overall microbial diversity across the different niches; however, significant differences in community composition were observed, especially at the genus level. For example, Rothia was identified as a biomarker in the T niche of healthy children, whereas Streptococcus cristatus (S. cristatus) was prominent in the mild ECC group. In the C niche, Prevotella scopos and Neisseria pharynges (N. pharyngis) were identified as key species associated with varying degrees of ECC severity. Streptococcus, Veillonella, and Actinomyces were dominant genera associated with caries, with Streptococcus playing a crucial role in plaque formation and acid production, leading to enamel demineralization. In addition, Veillonella interacts with other cariogenic bacteria, exacerbating acid production, and further promoting caries. LEfSe analysis (Supplementary Figure S2C–E) identified biomarkers in different niches, such as Rothia sp. in healthy children and S. cristatus in those with mild ECC, emphasizing the niche-specific nature of these microbial communities.
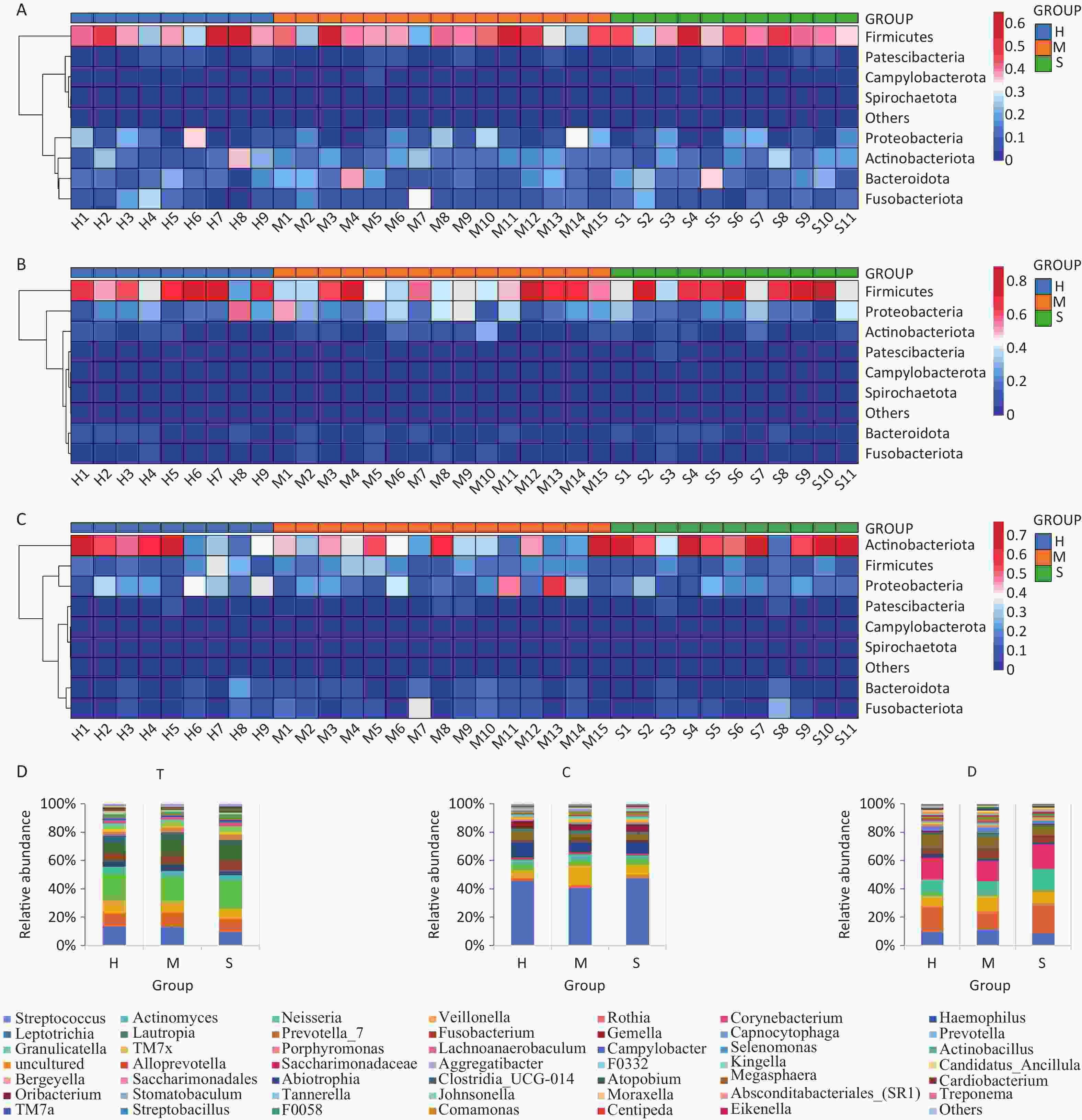
Figure S1. Heatmap of oral microorganisms at the phylum and genus levels. (A–C) T, C, and D niches at the phylum level, respectively. (D) T, C, and D niches at the genus level, respectively.
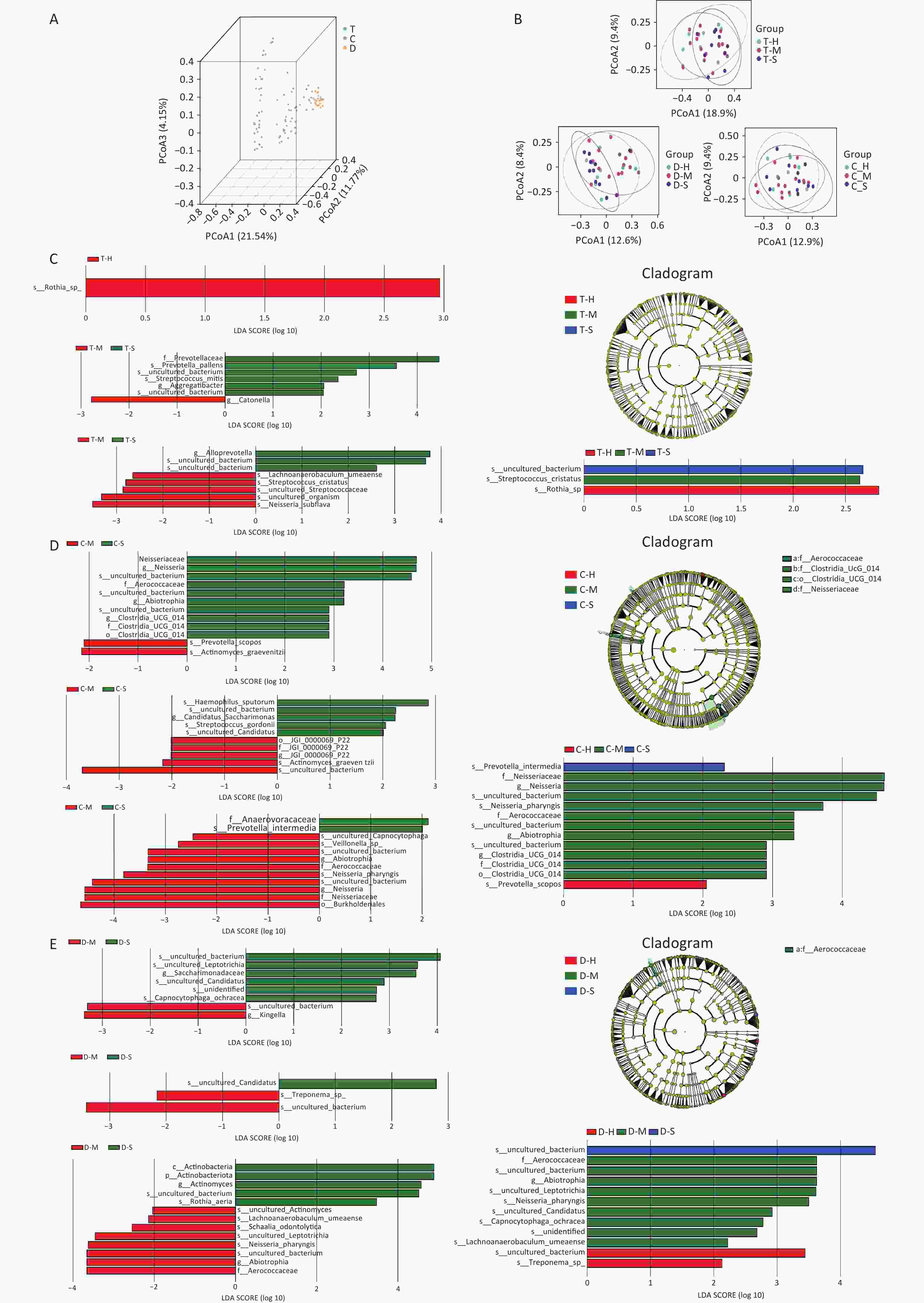
Figure S2. Beta diversity of oral microbiota. (A) Results of PCoA analysis of different niches; (B) PCoA of the healthy, mild ECC, and severe ECC groups; (C–E) Results of the comparison between the healthy group, mild ECC group, and severe ECC group at different niches. The left side shows two-group comparisons and the right side shows three-group comparisons. The left and lower right parts are histograms of LDA distribution; the upper right is an evolutionary branching diagram, showing the phyla to genera as a sequential hierarchical relationship from the inner circle to the outer circle in the sample community. ECC, Early childhood caries.
This study clarifies the relationship between dental caries and the oral microbiota across different niches. Notably, in children with severe ECC, the oral microbiota exhibited reduced diversity and shifts in dominant species. Streptococcus and Veillonella, known contributors to caries, were particularly prevalent in caries-affected niches, highlighting their role in acid production and enamel demineralization. Additionally, biomarkers such as Prevotella intermedia and N. pharyngis were identified in caries-affected niches, suggesting their potential role in the pathogenesis of ECC. These findings highlight the complex interactions between the oral microbiota and dental caries, indicating the close association between microbial species and the development and progression of caries in specific oral niches[6].
Functional predictions using PICRUSt2 (Figure 2) indicated variations in the metabolic potential of microbiota across niches, particularly in metabolism-related pathways, which could influence caries development. Notably, diagnostic analysis (Figure 3A) revealed that certain bacterial families, such as Saccharimonadaceae and Aggregatibacter in the T niche, had high predictive performance for caries, as evidenced by their AUC values. Clostridia_UCG.014 is a known gut microbial strain[7], and previous studies have explored the relationship between nutrition, oral microbiota, and dental caries. Nonetheless, its association with dental caries remains unclear. With ongoing research on the “oral-intestinal microbiota axis”, some important physiological functions and potential pathogenic roles may be revealed.
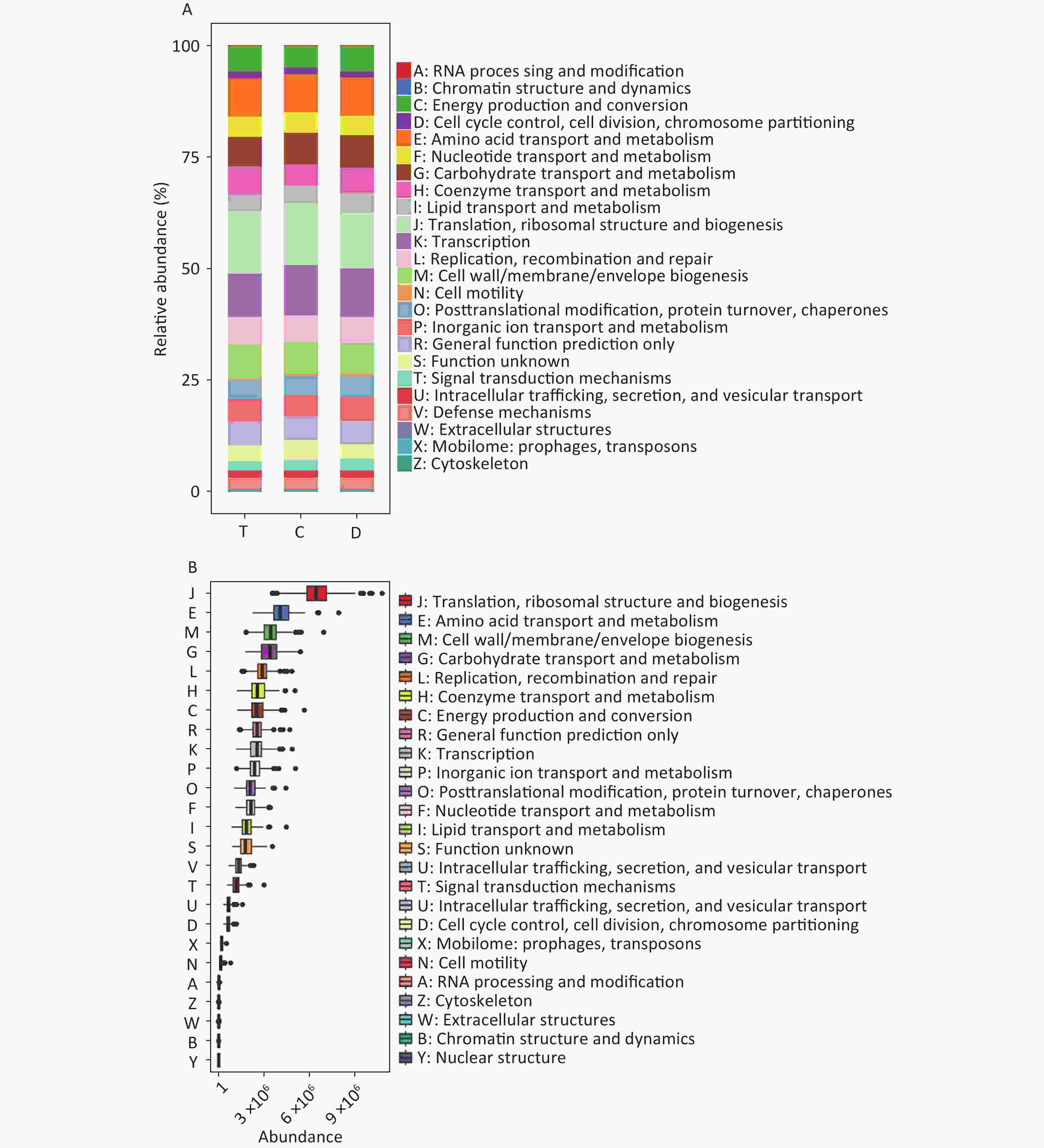
Figure 2. PICRUSt2 functional prediction and phylogenetic tree at the genus level. (A) Composition of COG functions in the three groups. (B) Abundance of COG functions in all samples. T: T niche, samples were collected from the dorsal tongue mucosa. D: D niche, samples from the teeth. C niche, samples were collected from the buccal mucosa. COG, Clusters of Orthologous Groups.
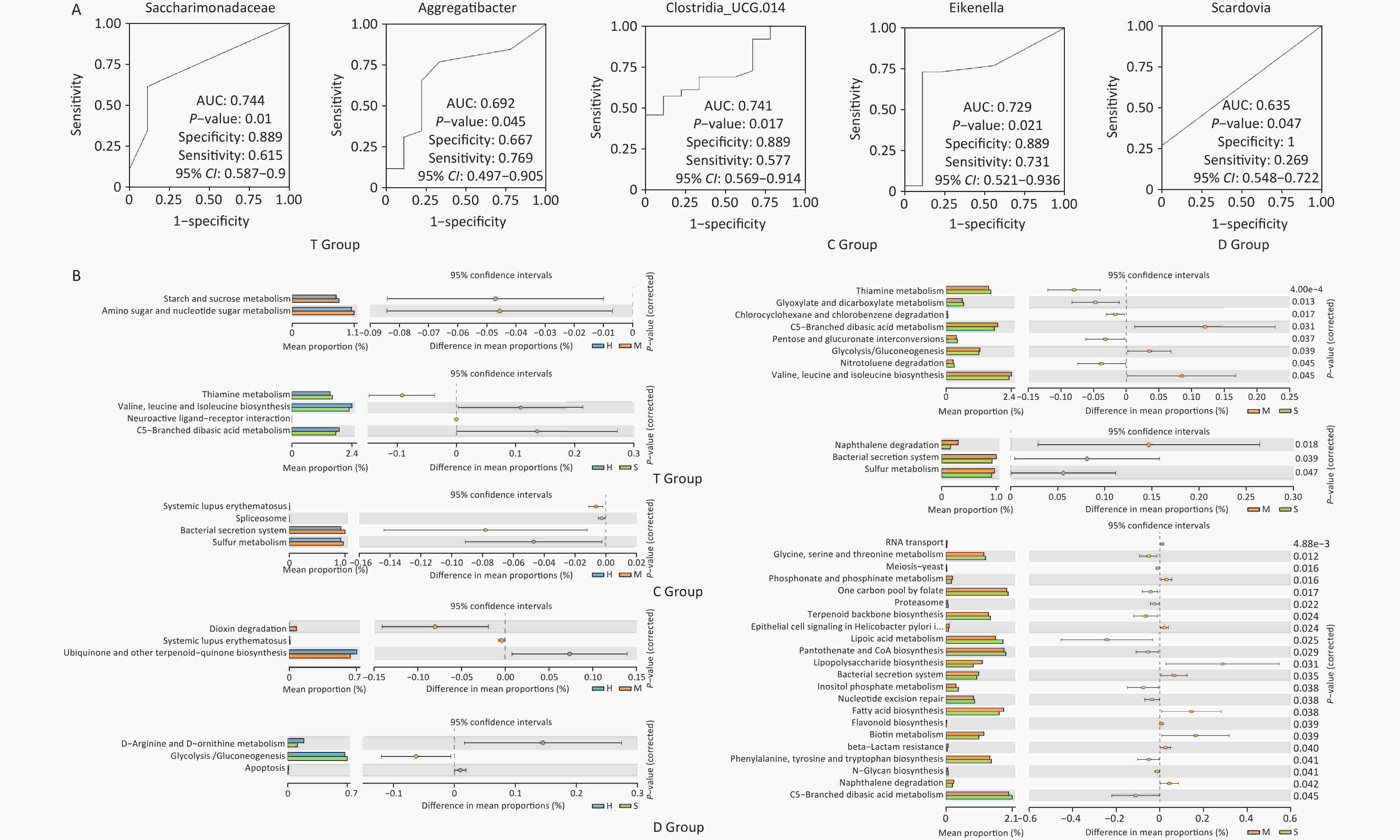
Figure 3. Predictive power of microbiota markers and metabolic pathway prediction. (A) ROC curve analysis. From left to right, Saccharimonadaceae, Aggregatibacter, Clostridia_UCG.014, Eikenella, and Scardovia; (B) KEGG pathway analysis. The bars on the left represent the abundance of a metabolic pathway as a percentage of all metabolic pathways in each of the two groups of samples, with the corrected P-values on the right. KEGG, Kyoto Encyclopedia of Genes and Genomes.
This study underscores the role of specific oral niches in shaping microbial communities and their potential impact on caries. Despite the similar overall diversity across groups, the presence of specific genera related to ECC severity, such as Streptococcus and Veillonella, highlights the importance of niche-specific microbial dynamics. The presence of specific bacteria such as Streptococcus and Veillonella in caries-affected niches indicates their role in disease progression[8]. These findings suggest that individualizing interventions based on the specific microbial profiles of different oral niches could be more effective. However, the unexpected increase in the abundance of Bacteroidota in caries-affected niches suggests complex interactions, which require further investigation. Functionally, the differences in metabolic pathways between healthy and caries-affected children indicate that targeting microbial metabolism could be a potential strategy for preventing caries. Furthermore, identifying specific microbial markers also presents avenues for developing more accurate diagnostic tools. Functional predictions (Figure 3B) further support this, indicating that microbial metabolic activity may be a key factor in caries progression. Future research should focus on exploring these niche-specific interactions to develop more precise caries prevention strategies. Functional predictions revealed significant differences in metabolic pathways between the groups, which were primarily related to glucose, amino acid, and lipid metabolism; these findings highlight the importance of microbial metabolic activity in caries progression. Nevertheless, the present study relied on questionnaire data, which carries potential biases such as recall bias and the accuracy of parental reporting[9,10]. Still, the questionnaire data were combined with microbiological analysis to mitigate these limitations, thereby providing a more comprehensive understanding of the factors contributing to dental caries. Future research should focus on refining these methods and further exploring the metabolic functions of the oral microbiota in caries prevention.
Our findings depict the distinct microbial characteristics and biomarkers across the three oral niches in preschool children, demonstrating differences between healthy children and those with varying degrees of caries. The study emphasizes the importance of good dietary habits, reducing high-sugar food intake, and using personal tableware to maintain a balanced oral microbiota. Additionally, promoting healthy oral hygiene practices, such as regular brushing, flossing, and early detection of oral issues through routine checkups, is crucial. The results underscore the role of family and community environments in shaping children’s oral health and reducing the incidence of dental caries in young children.
doi: 10.3967/bes2024.186
Investigation of Associated Factors and Microbiota of Different Oral Niches in Early Childhood Caries
-
The authors declare that they have no conflict of interest.
注释:1) Competing Interests: -
Figure 1. Forest plot. (A) Multifactorial regression analysis of health and ECC groups. (B) Multifactorial regression analysis of mild ECC and severe ECC groups. In the left planar rectangular coordinate system diagram, the horizontal scale with 1 as the center of the vertical null line indicates the protective factors on the left side of the line; the factors on the right side are associated factors, and the factors crossing the line are null factors. On the right side are the odds ratios with 95% confidence intervals and the P-values. ECC, early childhood caries.
S2. Beta diversity of oral microbiota. (A) Results of PCoA analysis of different niches; (B) PCoA of the healthy, mild ECC, and severe ECC groups; (C–E) Results of the comparison between the healthy group, mild ECC group, and severe ECC group at different niches. The left side shows two-group comparisons and the right side shows three-group comparisons. The left and lower right parts are histograms of LDA distribution; the upper right is an evolutionary branching diagram, showing the phyla to genera as a sequential hierarchical relationship from the inner circle to the outer circle in the sample community. ECC, Early childhood caries.
Figure 2. PICRUSt2 functional prediction and phylogenetic tree at the genus level. (A) Composition of COG functions in the three groups. (B) Abundance of COG functions in all samples. T: T niche, samples were collected from the dorsal tongue mucosa. D: D niche, samples from the teeth. C niche, samples were collected from the buccal mucosa. COG, Clusters of Orthologous Groups.
Figure 3. Predictive power of microbiota markers and metabolic pathway prediction. (A) ROC curve analysis. From left to right, Saccharimonadaceae, Aggregatibacter, Clostridia_UCG.014, Eikenella, and Scardovia; (B) KEGG pathway analysis. The bars on the left represent the abundance of a metabolic pathway as a percentage of all metabolic pathways in each of the two groups of samples, with the corrected P-values on the right. KEGG, Kyoto Encyclopedia of Genes and Genomes.
S1. Univariate analysis of caries associated factors in early childhood
Factors Categories Number of cases Degree of early childhood caries χ2 P Health Mild Severe Gender of child Male 162 57 60 45 2.209 0.331 Female 153 44 56 53 Age of child (years) 3– 52 22 14 16 8.254 0.083 4– 81 29 34 18 5– 182 50 68 64 Childhood feeding mode Breast feeding 192 64 69 59 0.376 0.829 Mixed feeding 123 37 47 39 Age at which the child started tooth brushing 3+ years old 258 81 96 81 0.293 0.864 Age three and under 57 20 20 17 Number of times the children brush their teeth per day 2 and more times 106 73 11 22 103.347 0.000** Less than 2times 209 28 105 76 Cleaning the mouth of children before 1 year of age No 174 21 61 92 107.973 0.000** Yes 141 80 55 6 Cleaning the mouth of children aged 1 to 3 years Gargle 36 10 19 7 4.849 0.303 Just use a toothbrush 207 67 72 68 Toothbrush and toothpaste 72 24 25 23 Children's oral cleaning method after the age of 3 years Ordinary toothbrush 187 57 66 64 4.044 0.400 Children's toothbrush 80 31 30 19 Electric toothbrush 48 13 20 15 Brushing time in children Less than 2 minutes 109 29 18 62 55.799 0.000** 2 minutes or more 206 72 98 36 Cleaning the tongue surface of children when brushing their teeth No 156 30 65 61 24.184 0.000** Yes 159 71 51 37 Children's oral cavity is checked regularly No 196 21 97 78 108.927 0.000** Yes 119 80 19 20 Children brush their teeth under parental supervision No 138 22 53 63 36.765 0.000** Yes 177 79 63 35 Children's diet is eaten separately No 160 27 80 53 39.145 0.000** Yes 155 74 36 45 Children often eat sweets No 156 70 51 35 24.723 0.003** Yes 159 31 65 63 Children often drink carbonated drinks No 178 73 55 50 15.324 0.000** Yes 137 28 61 48 Children often eat fruits No 120 18 50 52 28.144 0.000** Yes 195 83 66 46 Children often eat meat No 147 47 57 43 0.592 0.744 Yes 168 54 59 55 Children often eat eggs No 136 36 56 44 3.684 0.1585 Yes 179 65 60 54 Children often eat milk and dairy products No 140 31 59 50 11.386 0.003** Yes 175 70 57 48 Children often eat tofu or soy products No 116 41 35 40 3.495 0.174 Yes 199 60 81 58 Children often eat coarse grains class No 137 39 49 49 2.741 0.254 Yes 178 62 67 49 Children often eat vegetables No 144 29 45 70 40.115 0.000** Yes 171 72 71 28 Children often eat aquatic products No 134 31 49 54 12.13 0.002** Yes 181 70 67 44 Educational level of parents General 154 39 79 36 27.2 0.000** Higher 161 62 37 62 Parental occupation Medical personnel 48 25 17 6 13.414 0.001** Non-medical personnel 267 76 99 92 Oral hygiene status of parents General 170 45 64 61 6.373 0.041* Good 145 56 52 37 Parents often use their own tableware to feed children No 174 79 58 37 29.533 0.000** Yes 141 22 58 61 Age when parents think children start to clean their mouth After the teeth sprout 161 62 52 47 6.49 0.039* After the age of 3 154 39 64 51 When do parents think the child starts going to the dentist After the teeth sprout 32 8 9 15 7.325 0.292 About 3 years old 162 53 56 53 When there is toothache 64 19 28 17 Unsureness 57 21 23 13 Parents' attitudes towards deciduous tooth caries Don't care 159 36 43 80 55.286 0.000** Regular inspection,
early treatment156 65 73 18 Note. *P < 0.05, **P < 0.01. -
[1] Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet, 2007; 369, 51−9. doi: 10.1016/S0140-6736(07)60031-2 [2] Uribe SE, Innes N, Maldupa I. The global prevalence of early childhood caries: a systematic review with meta‐analysis using the WHO diagnostic criteria. Int J Paediatr Dent, 2021; 31, 817−30. doi: 10.1111/ipd.12783 [3] Ribeiro AA, Paster BJ. Dental caries and their microbiomes in children: what do we do now?. J Oral Microbiol, 2023; 15, 2198433. doi: 10.1080/20002297.2023.2198433 [4] Maier T. Oral microbiome in health and disease: maintaining a healthy, balanced ecosystem and reversing dysbiosis. Microorganisms, 2023; 11, 1453. doi: 10.3390/microorganisms11061453 [5] The Central People's Government of the People's Republic of China. The results of the fourth national oral health epidemiological survey are released[EB/OL]. (2017-09-20) [2024-09-01]. https://www.gov.cn/xinwen/2017-09/20/content_5226224.htm. (In Chinese) [6] Khan MW, De Jesus VC, Mittermuller BA, et al. Role of socioeconomic factors and interkingdom crosstalk in the dental plaque microbiome in early childhood caries. Cell Rep, 2024; 43, 114635. doi: 10.1016/j.celrep.2024.114635 [7] Leibovitzh H, Lee SH, Xue MY, et al. Altered gut microbiome composition and function are associated with gut barrier dysfunction in healthy relatives of patients with Crohn’s disease. Gastroenterology, 2022; 163, 1364-76. E10. [8] Tuganbaev T, Yoshida K, Honda K. The effects of oral microbiota on health. Science, 2022; 376, 934−6. doi: 10.1126/science.abn1890 [9] Wade WG, Prosdocimi EM. Profiling of oral bacterial communities. J Dent Res, 2020; 99, 621−9. doi: 10.1177/0022034520914594 [10] Şenel S. An overview of physical, microbiological and immune barriers of oral mucosa. Int J Mol Sci, 2021; 22, 7821. doi: 10.3390/ijms22157821 -




 下载:
下载:



 Quick Links
Quick Links