-
Pancreatic cancer is a highly malignant tumor with low early detection rates and poor prognoses, reflected in a five-year survival rate below 10. The morbidity and mortality rates for this cancer type are nearly identical, indicating a substantial disease burden[1]. In China, pancreatic cancer ranks as the sixth leading cause of cancer deaths for both sexes. In 2019, approximately 114,964 cases were recorded, resulting in an incidence rate of 5.78 per 100,000, reflecting an increase of 329.40% in cases and 82.11% in incidence since 1990[2]. High fasting plasma glucose (HFPG) is the fastest-growing risk factor for cancer deaths globally and has been linked to various malignancies. Studies have shown that HFPG is the fifth largest contributor to age-standardized disability-adjusted life year (DALY) rates (ASDR) for males and the fourth for females[3].
The crude mortality rate of pancreatic cancer attributed to HFPG nearly tripled from 1990 to 2019, with an estimated annual percentage change (EAPC) in ASDR of 2.04%. Projections indicate significant increases in ASDR and the age-standardized mortality rates (ASMR) for pancreatic cancer due to HFPG from 2019 to 2040[4].
Epidemiological studies have demonstrated a significantly higher risk of pancreatic cancer in individuals with long-standing type 2 diabetes, a correlation further supported by prospective studies[5]. Insulin resistance, hyperglycemia, hyperinsulinemia, and inflammation are potential factors driving pancreatic cancer development. Findings suggest that each 0.56 mmol/L increase in HFPG correlates with a 14% increase in pancreatic cancer incidence[3].
Although previous studies have explored the overall cancer burden associated with HFPG, specific analyses focusing on pancreatic cancer are lacking. The study aims to use data from the Global Burden of Disease Study (GBD) 2021 database and apply Joinpoint regression to assess the burden and temporal trends of pancreatic cancer attributed to HFPG in China from 1990 to 2021. Additionally, the age-period-cohort (APC) model will evaluate the effects of age, period and cohort on disease burden, providing essential data to guide the monitoring and management of fasting plasma glucose (FPG) levels in pancreatic cancer patients in China.
HFPG is defined as the mean FPG level in a population, measured in millimoles per liter (mmol/L). According to the Institute for Health Metrics and Evaluation (IHME), HFPG refers to any level exceeding the theoretical minimum-risk exposure range of 4.8–5.4 mmol/L (86.4–97.2 mg/dL)[3]. Data on pancreatic cancer attributed to HFPG in China from 1990 to 2021 were sourced from the GBD 2021 public database, which provides standardized estimates across 204 countries and territories for 371 diseases and 88 risk factors. Specifically, data on pancreatic cancer attributable to HFPG were stratified by gender and age and included mortality rates, DALYs, and corresponding 95% uncertainty intervals (UIs) for China from 1990 to 2021. The GBD 2021 database defines pancreatic cancer as a primary malignant neoplasm of the pancreas, designated by the ICD-10 code C25 (https://www.healthdata.org/research-analysis/gbd).
This study utilized mortality rates, DALYs, and age-standardized rates (ASRs). The mortality rate represents the proportion of deaths in a population over a specified period and is a commonly used metric to assess mortality risk. DALYs, calculated by summing years of life lost (YLLs) and years lived with disability (YLDs), provide a comprehensive measure of the disease’s impact on population life expectancy. ASRs were calculated using the age composition of the GBD 2021 standard population, derived from the unweighted mean of age-specific proportional distributions among national sites with populations exceeding 5 million.
Joinpoint analysis was employed to evaluate trends in pancreatic cancer attributed to HFPG from 1990 to 2021. This method segments longitudinal data through piecewise regression to identify statistically significant trends over time[6]. The annual percentage change (APC) and its 95% confidence interval (CI) were calculated for each period, while the average annual percentage change (AAPC) described the overall trend. Statistically significant APC and AAPC values were identified based on non-overlapping 95% CIs and a P-value of less than 0.05, rejecting the null hypothesis of no variation. The analysis was performed using Joinpoint software version 5.1.0.
Based on the Poisson distribution, the APC model decomposed changes in pancreatic cancer mortality and DALYs attributed to HFPG across three dimensions: age, period, and cohort[7]. The study population included individuals aged 25 to 99 years, divided into intervals consistent with the structural requirements of the APC model, where age group intervals equaled period group intervals. The years from 1990 to 2021 were divided into six groups using the same 5-year interval. Birth cohorts were calculated by subtracting age from the period, resulting in 20 cohorts. Relative risk (RR) and 95% CIs were derived from the estimated coefficients to quantify the effects of age, period, and cohort on mortality and DALY rates. The implementation of the APC model was carried out using StataSE 16 and R version 4.3.2.
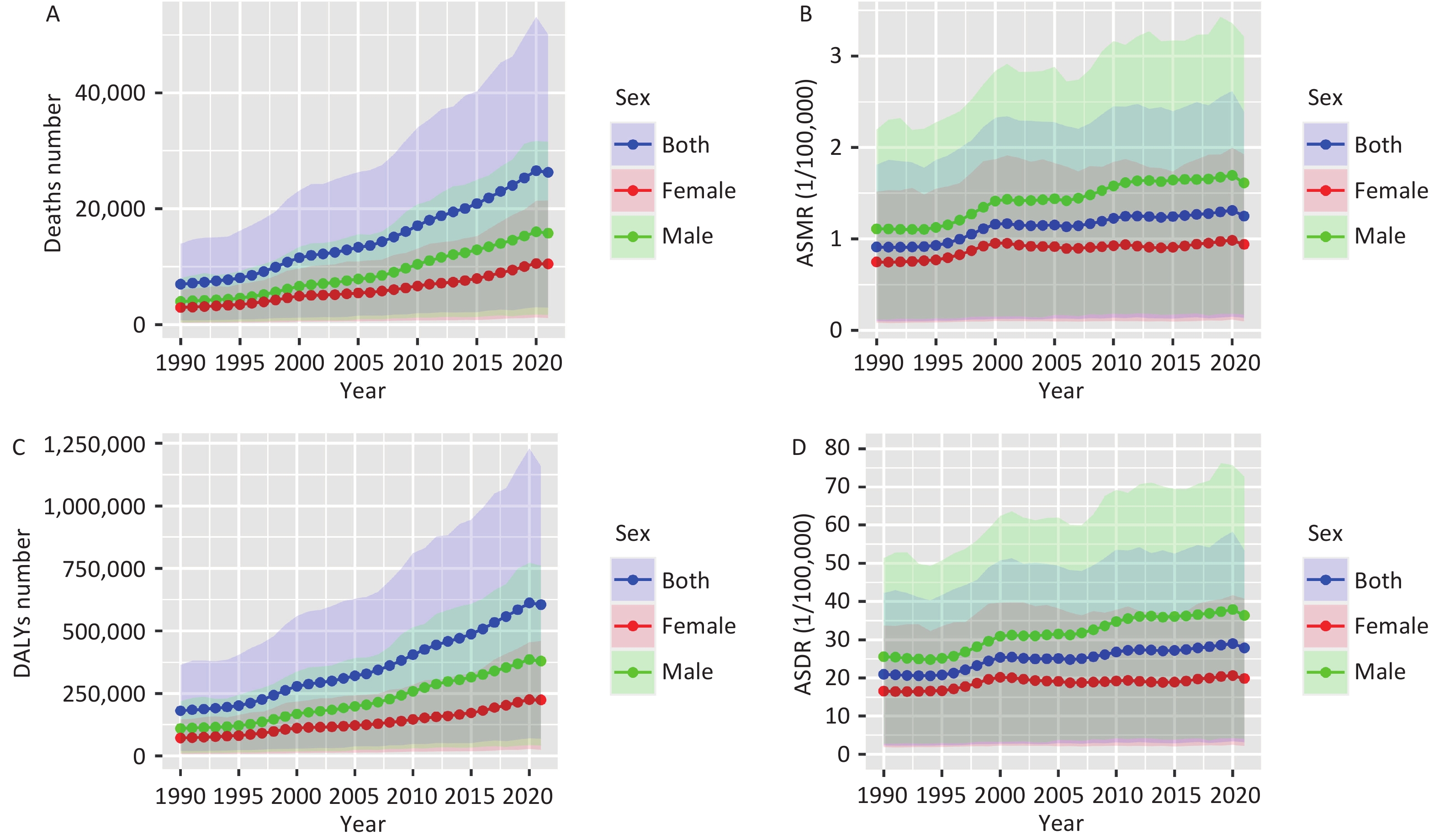
Figure 1 illustrates the trends in DALY and mortality rates for pancreatic cancer attributed to HFPG in China from 1990 to 2021, showing an upward trajectory. After age standardization, the rate of increase decreased slightly, but the trend remained consistent. Deaths due to HFPG-related pancreatic cancer rose from 6,991 in 1990 to 26,256 in 2021. The ASMR increased from 0.91 per 100,000 individuals (95% UI: 0.10–1.80) to 1.25 per 100,000 individuals (95% UI: 0.14–2.39), indicating a 37.36% overall increase. Additionally, DALYs surged from 181,127.90 to 605,286.10, with the DALY rates climbing from 20.98 per 100,000 individuals (95% UI: 2.35–42.23) to 27.88 per 100,000 individuals (95% UI: 3.16–53.41), indicating a growth of 32.89% (Supplementary Table S1).
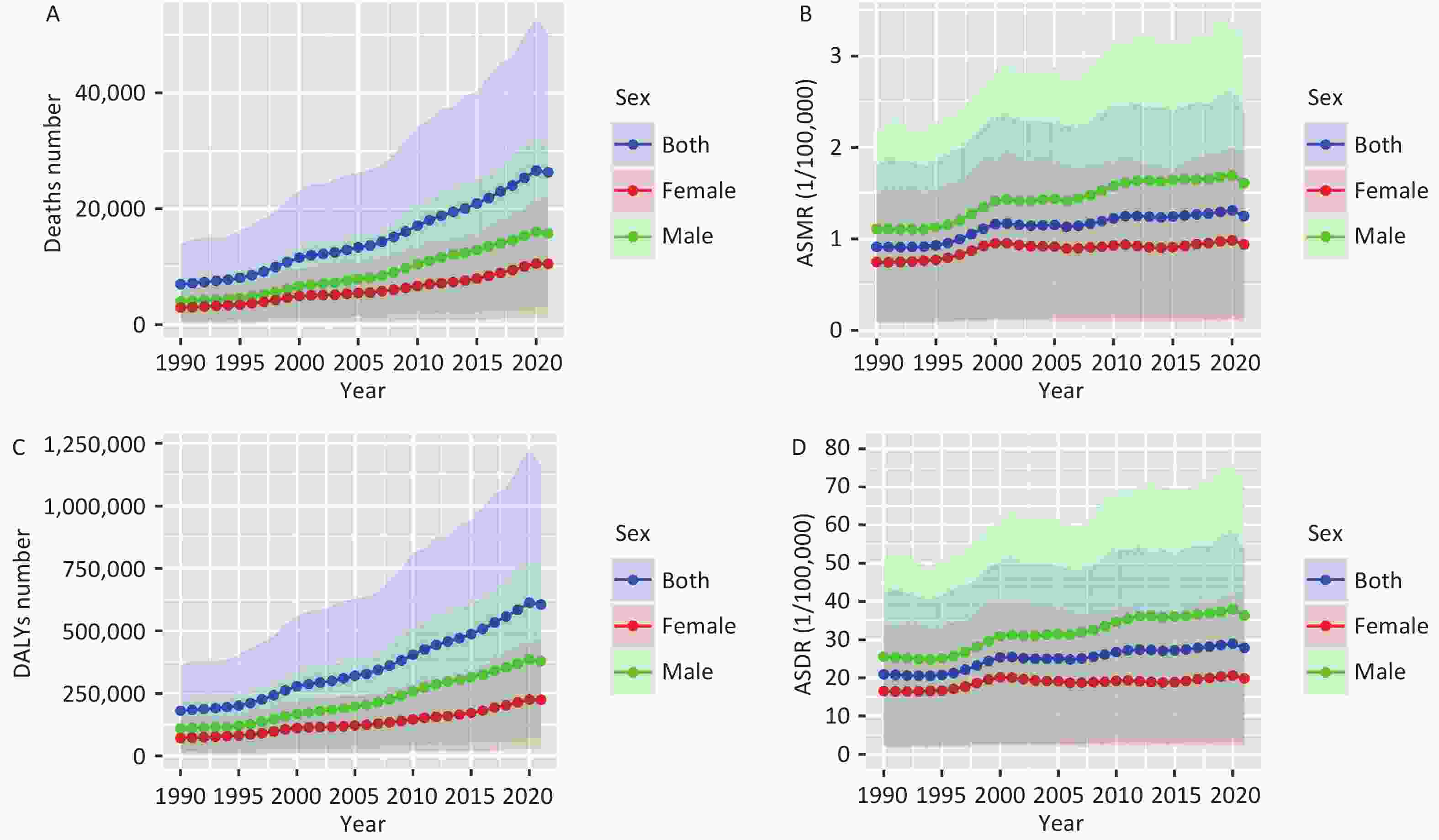
Figure 1. Trends in mortality and DALYs of pancreatic cancer attributed to HFPG in China at all ages, 1990–2021. (A) Deaths number; (B) ASMR, age-standardized mortality rate; (C) DALYs number; (D) ASDR, age-standardized DALY rate; DALYs, disability-adjusted life years; HFPG, high fasting plasma glucose.
The burden of pancreatic cancer due to HFPG was higher in males, as the ASMR and ASDR consistently exceeded those of females from 1990 to 2021. As illustrated in Figure 1, there was a marked upward trajectory in ASMR and ASDR for both males and females until 2000, followed by a less pronounced increase between 2000 and 2021, with similar trends observed for both genders. Between 1990 and 2021, the ASMR for males increased from 1.11 per 100,000 (95% UI: 0.12–2.20) to 1.61 per 100,000 (95% UI: 0.17–3.21), while for females, it rose from 0.75 per 100,000 (95% UI: 0.09–1.52) to 0.94 per 100,000 (95% UI: 0.10–1.92). Similarly, the ASDR for males surged from 25.58 per 100,000 (95% UI: 2.70–51.36) to 36.37 per 100,000 (95% UI: 3.98–72.72), while for females, it escalated from 16.61 per 100,000 (95% UI: 1.89–33.73) to 19.90 per 100,000 (95% UI: 2.14–40.76). (Supplementary Table S1)
Figure 2 depicts the burden of pancreatic cancer attributed to HFPG across different age groups, showing an overall increase in 2021, particularly escalating with age. Figures 2A and 2B reveal lower death rates for individuals under 50 years old, which rise after this age, peaking in the 70–74 age group. Meanwhile, Figures 2C and 2D show that both the number and rate of DALY peaked for males and females in the 65–69 age group. The ASDR exhibited a consistent upward trend until age 74, followed by a decline after 79 years old. Notably, the ASDR and ASMR attributed to pancreatic cancer due to HFPG remained significantly higher in males across all age groups compared to females.
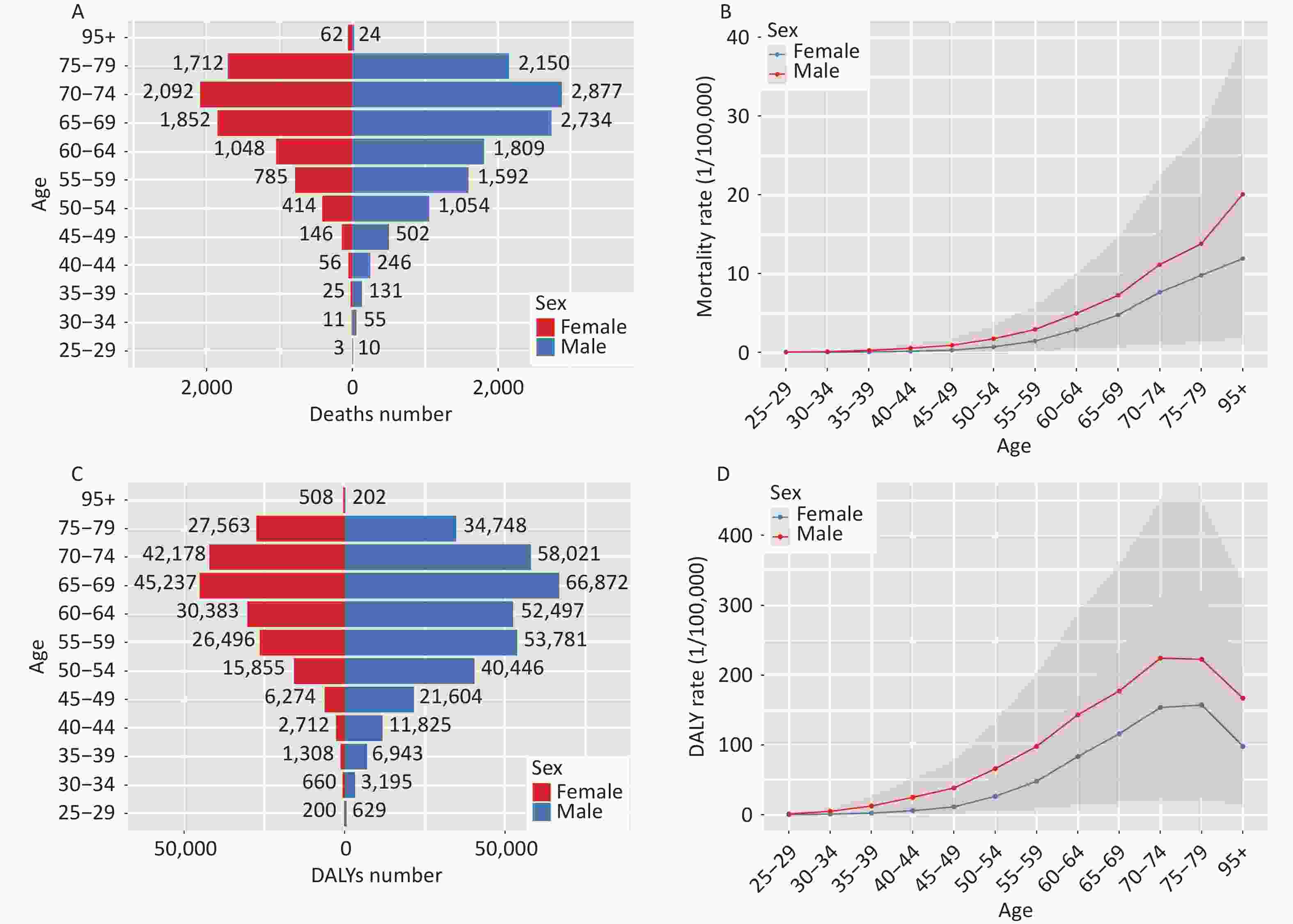
Figure 2. Comparison of the number and rates of mortality and DALY of pancreatic cancer attributed to HFPG in China in different age groups and genders, 2021. (A) Age-specific deaths number; (B) Age-specific mortality rate; (C) Age-specific DALYs number; (D) Age-specific DALY rate; DALYs, disability-adjusted life years; HFPG, high fasting plasma glucose.
Joinpoint regression analysis was conducted to assess trends in pancreatic cancer attributed to HFPG from 1990 to 2021. The models segmented temporal trends of ASMR, ASDR and APCs by gender. As shown in Supplementary Figure S1A and Table S2, the ASMR of pancreatic cancer attributed to HFPG in China demonstrated a consistent overall upward trend from 1990 to 2021, with males exhibiting higher rates than females. For the total population, the AAPC for ASMR was 1.07% (95% CI: 1.01%–1.13%) with a notable increase between 1995 and 2000 (APC: 4.82%). For males, the AAPC was 1.32% (95% CI: 1.25%–1.39%), with significant changes observed during 1995–2000 (APC: 5.03%) and 2007–2011 (APC: 3.14%). For females, the AAPC was 0.79% (95% CI: 0.73%–0.86%), with marked variability from the 1995–2000 period (APC: 4.55%).
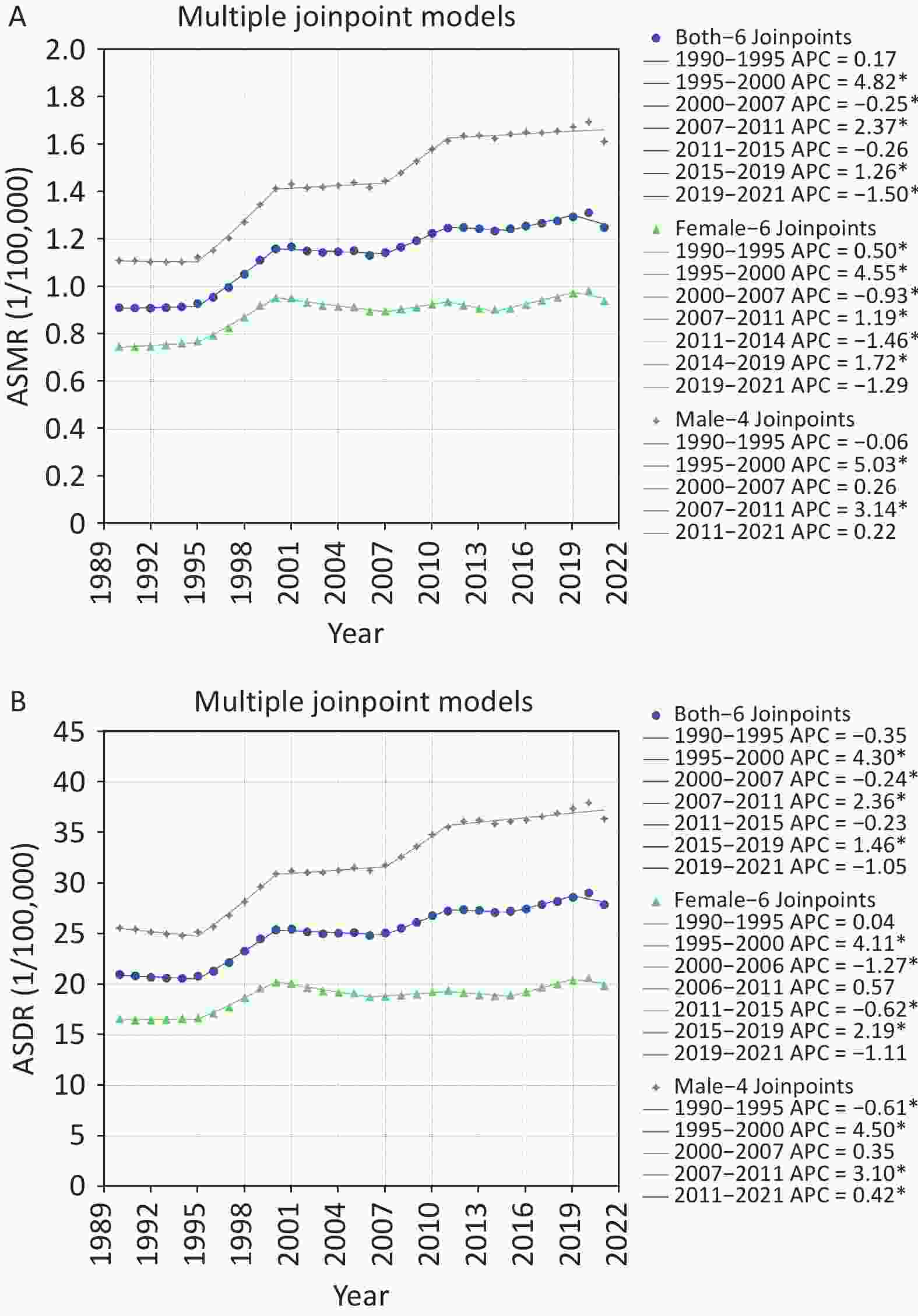
Figure S1. Joinpoint regression analysis of ASMR (A) and ASDR (B) of pancreatic cancer attributed to HFPG in China from 1990 to 2021 by gender. ASMR, age-standardized mortality rate; ASDR, age-standardized DALY rate; DALYs, disability-adjusted life years; HFPG, high fasting plasma glucose; *Indicates that the annual percent change (APC) is significantly different from zero at the alpha = 0.05 level.
Table S2. Total AAPCs and APCs of ASDR and ASMR due to pancreatic cancer attributed to HFPG in China from 1990 to 2021
Sex ASMR (1/100,000) ASDR (1/100,000) Period APC (95% CI) AAPC (95% CI) Period APC (95% CI) AAPC (95% CI) Both 1990–1995 0.17 (−0.17 to 0.50) 1.07* (1.01–1.13) 1990–1995 −0.35 (−0.70 to 0) 0.96* (0.90–1.02) 1995–2000 4.82* (4.51 to 5.12) 1995–2000 4.30* (3.99 to 4.64) 2000–2007 −0.25* (−0.52 to −0.02) 2000–2007 −0.24* (−0.56 to −0.01) 2007–2011 2.37* (1.68 to 3.19) 2007–2011 2.36* (1.71 to 3.19) 2011–2015 −0.26 (−1.02 to 0.26) 2011–2015 −0.23 (−1.00 to 0.30) 2015–2019 1.26* (0.84 to 2.06) 2015–2019 1.46* (1.03 to 2.26) 2019–2021 −1.50* (−2.48 to −0.35) 2019–2021 −1.05 (−2.06 to 0.11) Female 1990–1995 0.50* (0.15 to 0.87) 0.79* (0.73–0.86) 1990–1995 0.04 (−0.59 to 0.46) 0.63* (0.56–0.69) 1995–2000 4.55* (4.27 to 4.90) 1995–2000 4.11* (3.67 to 4.56) 2000–2007 −0.93* (−1.23 to −0.73) 2000–2006 −1.27* (−1.80 to −0.84) 2007–2011 1.19* (0.69 to 1.98) 2006–2011 0.57 (−0.60 to 1.49) 2011–2014 −1.46* (−1.98 to −0.58) 2011–2015 −0.62* (−1.45 to −0.02) 2014–2019 1.72* (1.45 to 2.53) 2015–2019 2.19* (1.76 to 3.09) 2019–2021 −1.29 (−2.27 to 0.02) 2019–2021 −1.11 (−2.24 to 0.10) Male 1990–1995 −0.06 (−1.02 to 0.52) 1.32* (1.25–1.39) 1990–1995 −0.61* (−1.08 to −0.13) 1.22* (1.16–1.29) 1995–2000 5.03* (4.31 to 6.06) 1995–2000 4.50* (3.96 to 5.25) 2000–2007 0.26 (−0.53 to 0.62) 2000–2007 0.35 (−0.20 to 0.64) 2007–2011 3.14* (2.24 to 4.25) 2007–2011 3.10* (2.33 to 4.09) 2011–2021 0.22 (−0.04 to 0.43) 2011–2021 0.42* (0.20 to 0.60) Note. APC, annual percent changes; AAPC, average annual percent changes; ASMR, age−standardized mortality rate; ASDR, age−standardized DALY rate; DALYs, disability−adjusted life years; CI, confidence interval; HFPG, high fasting plasma glucose; *Indicates that the APC and AAPC are significantly different from zero at the alpha = 0.05 level. In Supplementary Figure S1B and Table S2, the ASDR of pancreatic cancer attributed to HFPG in China demonstrated an overall upward trend from 1990 to 2021 across the total population, males and females, with higher rates consistently observed in males. The AAPC for the total population's ASDR was 0.96% (95% CI: 0.90%-1.02%), with the most prominent upward trend occurring during the period 1995–2000, showing an APC of 4.30% (95% CI: 3.99%–4.64%). Among males, the AAPC was 1.22% (95% CI: 1.16%–1.29%), with the ASDR initially declining before subsequently increasing significantly (APC: 4.50%) from 1995 to 2000. For females, the AAPC was 0.63% (95% CI: 0.56%–0.69%), with notable changes occurring during 1995–2000 (APC: 4.11%), reflecting statistically significant disparities.
The APC analysis of pancreatic cancer attributed to HFPG in China from 1990 to 2021 reveals distinct trends in the effects of age, period, and cohort on mortality and DALYs associated with the disease. Findings in Figure 3A indicate that mortality risk initially increases with age, peaking at 85–89 years with an effect coefficient of 1.57, before declining in older age groups. The lowest mortality risk was observed in the 25–29 age group, with an effect coefficient of −3.97. Period effects showed a gradual increase in mortality risk over time, with the lowest effect observed during 1992–1996 (coef = –0.59) and the highest from 2017 to 2021 (coef = 0.51). In contrast, cohort effects demonstrated a progressive decrease in mortality risk with advancing birth cohorts. For instance, individuals born between 1897 and 1901 had a cohort effect coefficient of 1.52, compared to –1.29 for those born between 1992 and 1996 (Supplementary Table S3).
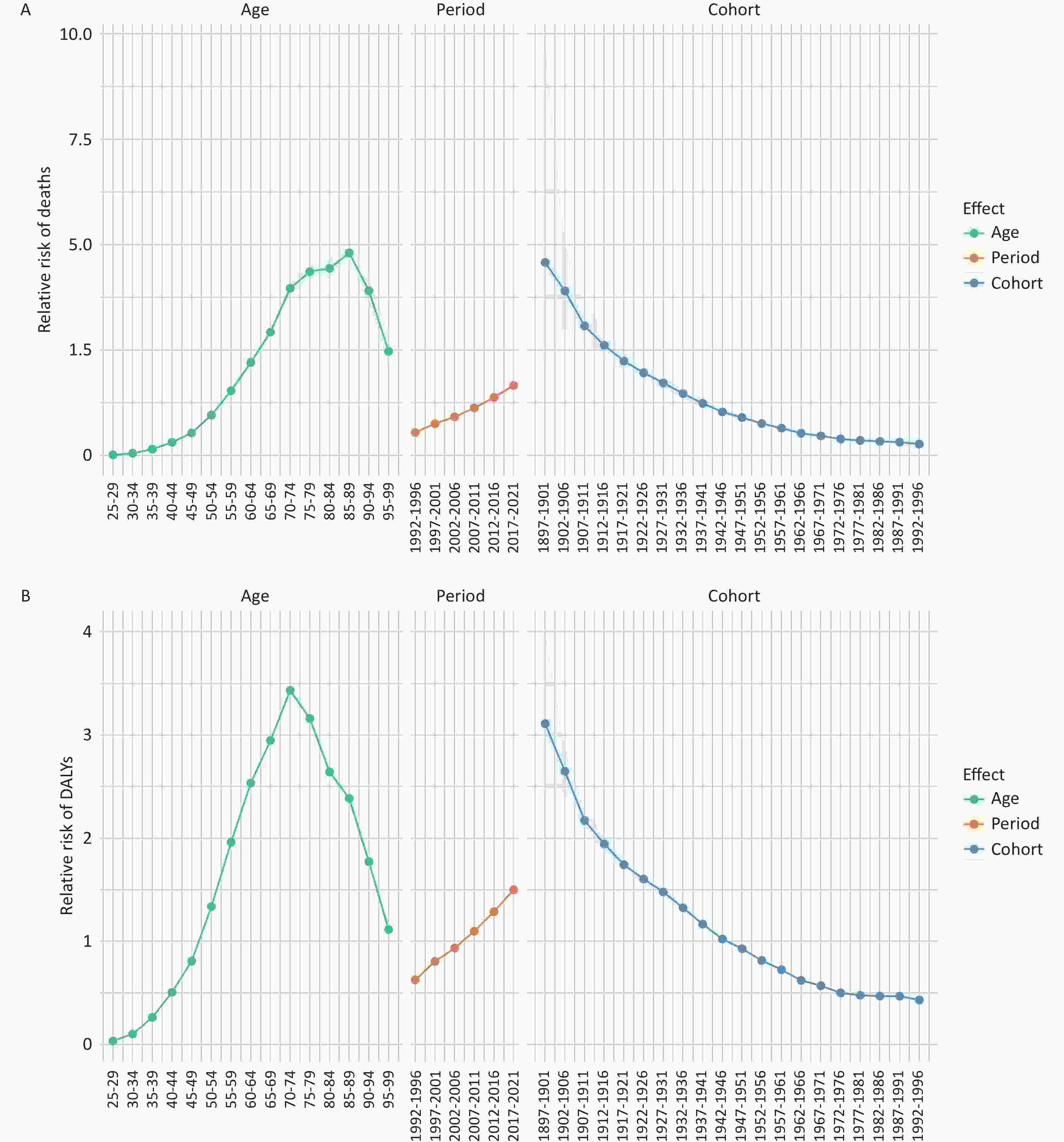
Figure 3. Age-period-cohort effect of pancreatic cancer attributed to HFPG in China from 1990 to 2021. (A) Age, period and cohort effects for pancreatic cancer mortality rate. (B) Age, period and cohort effects for pancreatic cancer DALY rate. DALYs, disability-adjusted life years; HFPG, high fasting plasma glucose.
The age effect of DALYs depicted in Figure 3B initially increased before declining, peaking at ages 70–74 with an effect coefficient of 1.23. The lowest effect was observed in the 25–29 age group, with an effect coefficient of –3.37. Period effects on DALYs showed a similar increase over time, with a coefficient of –0.47 during 1992–1996 and 0.41 during 2017–2021. Cohort effects indicate a consistent decrease in DALYs risk with advancing birth cohorts, with coefficients declining from 1.13 for those born in 1897–1901 to –0.84 for those born in 1992–1996 (Supplementary Table S3).
This study systematically analyzed the burden of pancreatic cancer attributable to HFPG by gender and age group in China from 1990 to 2021. The findings revealed a consistent increase in the burden, particularly among elderly and male populations.
The gender-based analysis highlights significant disparities in the burden of pancreatic cancer attributed to HFPG. Males consistently exhibit a higher burden, with growth rates surpassing those of females. In 2021, pancreatic cancer-related deaths among Chinese males attributed to HFPG were 1.5 times higher than in females. Various risk factors, including lifestyle differences, may contribute to these disparities. Males generally consume more red and processed meats and have higher smoking and alcohol consumption rates, all of which are linked to increased pancreatic cancer risk. In contrast, females typically demonstrate stronger health awareness and lower smoking and alcohol rates, potentially accounting for their lower disease incidence[1]. Additionally, males in China are more likely to have diabetes and elevated FPG levels compared to females[8]. This may stem from females' greater health sensitivity and better glycemic control due to healthier dietary choices and higher risk awareness. Smoking has been reported to impair FPG levels and may result in long-term decreases in insulin, raising FPG levels in males but lowering them in females. Hormonal differences, particularly the protective effects of estrogen in females, may also influence gender-specific pancreatic cancer incidence by modulating cell proliferation and metabolism. Evidence indicates that estrogen exerts protective effects, thereby decreasing the risk of developing pancreatic cancer[9].
APC analysis indicates that the elderly population is more susceptible to hypertension, diabetes, and cardiovascular diseases. With this demographic expected to double to 1.5 billion by 2050, pancreatic cancer mortality in China may further rise[10]. Consequently, prioritizing screening efforts, dietary control, and health education for the elderly is critical. Recent studies, however, suggest increasing incidence and mortality rates among younger populations, potentially driven by unhealthy lifestyles, socio-economic challenges, limited access to medical care, and high-stress environments[1].
The period effect suggests that enhanced medical screening has led to earlier detection of pancreatic cancer cases in recent years, contributing to higher HFPG-related mortality and DALY rates. In contrast, the cohort effect indicates that earlier birth cohorts experience higher mortality and DALY risks, while later cohorts exhibit progressively lower risks. Recent research has identified key risk factors for pancreatic cancer and emphasized the efficacy of interventions in reducing its burden[1]. Increased investment in education and healthcare is essential for improving care quality and mitigating pancreatic cancer morbidity and mortality. Cohort factors significantly influence the mortality and DALY risks of pancreatic cancer attributed to HFPG in China. Earlier cohorts face greater risks, while later cohorts benefit from improved public health education and awareness, reducing risks. Expanding cancer registries to cover a broader population is critical for improving national pancreatic cancer incidence and mortality statistics.
However, this study has several limitations. The absence of experimental validation restricts the ability to establish causal relationships between HFPG and pancreatic cancer outcomes. Additionally, potential biases in data collection and regional reporting practices may have influenced the observed trends in mortality and DALYs. Furthermore, the lack of clinical verification analyses limits the generalizability of the findings, as individual patient variables affecting disease progression were not accounted for.
In conclusion, this research highlights a significant increase in pancreatic cancer mortality and DALYs associated with HFPG in China from 1990 to 2021. The findings emphasize notable gender and age disparities, underscoring the need for targeted public health interventions to address the specific requirements of high-risk populations. Strengthening disease surveillance frameworks, implementing proactive prevention strategies, and promoting early diagnosis and treatment are critical steps toward reducing the pancreatic cancer burden in China.
doi: 10.3967/bes2025.027
Trends of Disease Burden of Pancreatic Cancer Attributed to High Fasting Plasma Glucose in China,1990–2021: Insights from the Global Burden of Disease Study 2021
-
Conceptualized and planned the study: Xiaochen Chen and Feifei Zhong. Collected data and analyzed data: Xiaochen Chen and Feifei Zhong. Manuscript drafted: Xiaochen Chen. Reviewed and revised the manuscript: Juan Li. All authors provided final approval and agreed to be accountable for all aspects of the work, ensuring its integrity and accuracy.
The authors declare that this research was conducted without any commercial or financial relationships that could be interpreted as potential conflicts of interest.
注释:1) Authors’ Contributions: 2) Competing Interests: -
Figure 1. Trends in mortality and DALYs of pancreatic cancer attributed to HFPG in China at all ages, 1990–2021. (A) Deaths number; (B) ASMR, age-standardized mortality rate; (C) DALYs number; (D) ASDR, age-standardized DALY rate; DALYs, disability-adjusted life years; HFPG, high fasting plasma glucose.
Figure 2. Comparison of the number and rates of mortality and DALY of pancreatic cancer attributed to HFPG in China in different age groups and genders, 2021. (A) Age-specific deaths number; (B) Age-specific mortality rate; (C) Age-specific DALYs number; (D) Age-specific DALY rate; DALYs, disability-adjusted life years; HFPG, high fasting plasma glucose.
S1. Joinpoint regression analysis of ASMR (A) and ASDR (B) of pancreatic cancer attributed to HFPG in China from 1990 to 2021 by gender. ASMR, age-standardized mortality rate; ASDR, age-standardized DALY rate; DALYs, disability-adjusted life years; HFPG, high fasting plasma glucose; *Indicates that the annual percent change (APC) is significantly different from zero at the alpha = 0.05 level.
Figure 3. Age-period-cohort effect of pancreatic cancer attributed to HFPG in China from 1990 to 2021. (A) Age, period and cohort effects for pancreatic cancer mortality rate. (B) Age, period and cohort effects for pancreatic cancer DALY rate. DALYs, disability-adjusted life years; HFPG, high fasting plasma glucose.
S2. Total AAPCs and APCs of ASDR and ASMR due to pancreatic cancer attributed to HFPG in China from 1990 to 2021
Sex ASMR (1/100,000) ASDR (1/100,000) Period APC (95% CI) AAPC (95% CI) Period APC (95% CI) AAPC (95% CI) Both 1990–1995 0.17 (−0.17 to 0.50) 1.07* (1.01–1.13) 1990–1995 −0.35 (−0.70 to 0) 0.96* (0.90–1.02) 1995–2000 4.82* (4.51 to 5.12) 1995–2000 4.30* (3.99 to 4.64) 2000–2007 −0.25* (−0.52 to −0.02) 2000–2007 −0.24* (−0.56 to −0.01) 2007–2011 2.37* (1.68 to 3.19) 2007–2011 2.36* (1.71 to 3.19) 2011–2015 −0.26 (−1.02 to 0.26) 2011–2015 −0.23 (−1.00 to 0.30) 2015–2019 1.26* (0.84 to 2.06) 2015–2019 1.46* (1.03 to 2.26) 2019–2021 −1.50* (−2.48 to −0.35) 2019–2021 −1.05 (−2.06 to 0.11) Female 1990–1995 0.50* (0.15 to 0.87) 0.79* (0.73–0.86) 1990–1995 0.04 (−0.59 to 0.46) 0.63* (0.56–0.69) 1995–2000 4.55* (4.27 to 4.90) 1995–2000 4.11* (3.67 to 4.56) 2000–2007 −0.93* (−1.23 to −0.73) 2000–2006 −1.27* (−1.80 to −0.84) 2007–2011 1.19* (0.69 to 1.98) 2006–2011 0.57 (−0.60 to 1.49) 2011–2014 −1.46* (−1.98 to −0.58) 2011–2015 −0.62* (−1.45 to −0.02) 2014–2019 1.72* (1.45 to 2.53) 2015–2019 2.19* (1.76 to 3.09) 2019–2021 −1.29 (−2.27 to 0.02) 2019–2021 −1.11 (−2.24 to 0.10) Male 1990–1995 −0.06 (−1.02 to 0.52) 1.32* (1.25–1.39) 1990–1995 −0.61* (−1.08 to −0.13) 1.22* (1.16–1.29) 1995–2000 5.03* (4.31 to 6.06) 1995–2000 4.50* (3.96 to 5.25) 2000–2007 0.26 (−0.53 to 0.62) 2000–2007 0.35 (−0.20 to 0.64) 2007–2011 3.14* (2.24 to 4.25) 2007–2011 3.10* (2.33 to 4.09) 2011–2021 0.22 (−0.04 to 0.43) 2011–2021 0.42* (0.20 to 0.60) Note. APC, annual percent changes; AAPC, average annual percent changes; ASMR, age−standardized mortality rate; ASDR, age−standardized DALY rate; DALYs, disability−adjusted life years; CI, confidence interval; HFPG, high fasting plasma glucose; *Indicates that the APC and AAPC are significantly different from zero at the alpha = 0.05 level. -
[1] Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology, 2013; 144, 1252−61. doi: 10.1053/j.gastro.2013.01.068 [2] He Y, Zhou XL, Fan XQ, et al. Disease burden of pancreatic cancer - China, 1990-2019. China CDC Wkly, 2022; 4, 527−31. doi: 10.46234/ccdcw2022.056 [3] Anamag FT, Noori M, Nejadghaderi SA, et al. Burden of cancers attributable to high fasting plasma glucose in the Middle East and North Africa region, 1990-2019. Cancer Med, 2023; 12, 10031−44. doi: 10.1002/cam4.5743 [4] Wei YG, Qin ZD, Liao XW, et al. Pancreatic cancer mortality trends attributable to high fasting blood sugar over the period 1990-2019 and projections up to 2040. Front Endocrinol (Lausanne), 2024; 15, 1302436. doi: 10.3389/fendo.2024.1302436 [5] Li DH. Diabetes and pancreatic cancer. Mol Carcinog, 2012; 51, 64−74. doi: 10.1002/mc.20771 [6] Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med, 2000; 19, 335−51. doi: 10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z [7] Chernyavskiy P, Little MP, Rosenberg PS. Correlated Poisson models for age-period-cohort analysis. Stat Med, 2018; 37, 405−24. doi: 10.1002/sim.7519 [8] Dong CP, Wu GF, Li H, et al. Type 1 and type 2 diabetes mortality burden: predictions for 2030 based on Bayesian age-period-cohort analysis of China and global mortality burden from 1990 to 2019. J Diabetes Investig, 2024; 15, 623−33. doi: 10.1111/jdi.14146 [9] Andersson G, Borgquist S, Jirström K. Hormonal factors and pancreatic cancer risk in women: the Malmö diet and cancer study. Int J Cancer, 2018; 143, 52−62. doi: 10.1002/ijc.31302 [10] He C. Situation of population aging in China and the strategy. China Popul Today, 1996; 13, 17. -
 24480+Supplementary Materials.pdf
24480+Supplementary Materials.pdf

-




 下载:
下载:





 Quick Links
Quick Links