-
In 2019, China had over 13.14 million dementia cases, with incidence rates of (56.47–207.08)/100,000[1]. Early cognitive impairment —a key dementia symptom—reduces quality of life, increases care dependence, and lowers survival in older adults[2]. A decline in physical function can also be observed in older adults with increasing age. Grip strength has been shown to be a marker of overall physiological function in older adults.
Grip strength and cognitive function are interrelated in older adults. Existing study shows that lower grip strength predicts cognitive decline, while higher grip strength may protect against impairment[3]. Conversely, baseline cognition also predicts grip strength changes, with better cognition linked to slower decline[4]. We hypothesize a long-term bidirectional link between grip strength and cognition.
Our study examined the bidirectional temporal relationships between cognitive function and grip strength using longitudinal data from the World Health Organization (WHO) Study on global AGEing and adult health (SAGE) China waves (2009–2010, 2014–2015, 2018–2019). During 3 waves, we assessed participants’ grip strength and cognitive function, with the latter four domains (verbal recall, verbal fluency, forward digit span and backward digit span). Our study performed cross-lagged panel analysis on grip strength and cognitive function across the 3 waves. The final analysis included 3,090 participants aged ≥ 50 years from eight Chinese provinces after excluding those with stroke/depression history, test failures, or missing data. SAGE used multistage cluster sampling to select nationally representative samples. Our analytical approach elucidates dynamic cognitive-motor interactions crucial for understanding age-related decline and developing targeted interventions in aging populations.
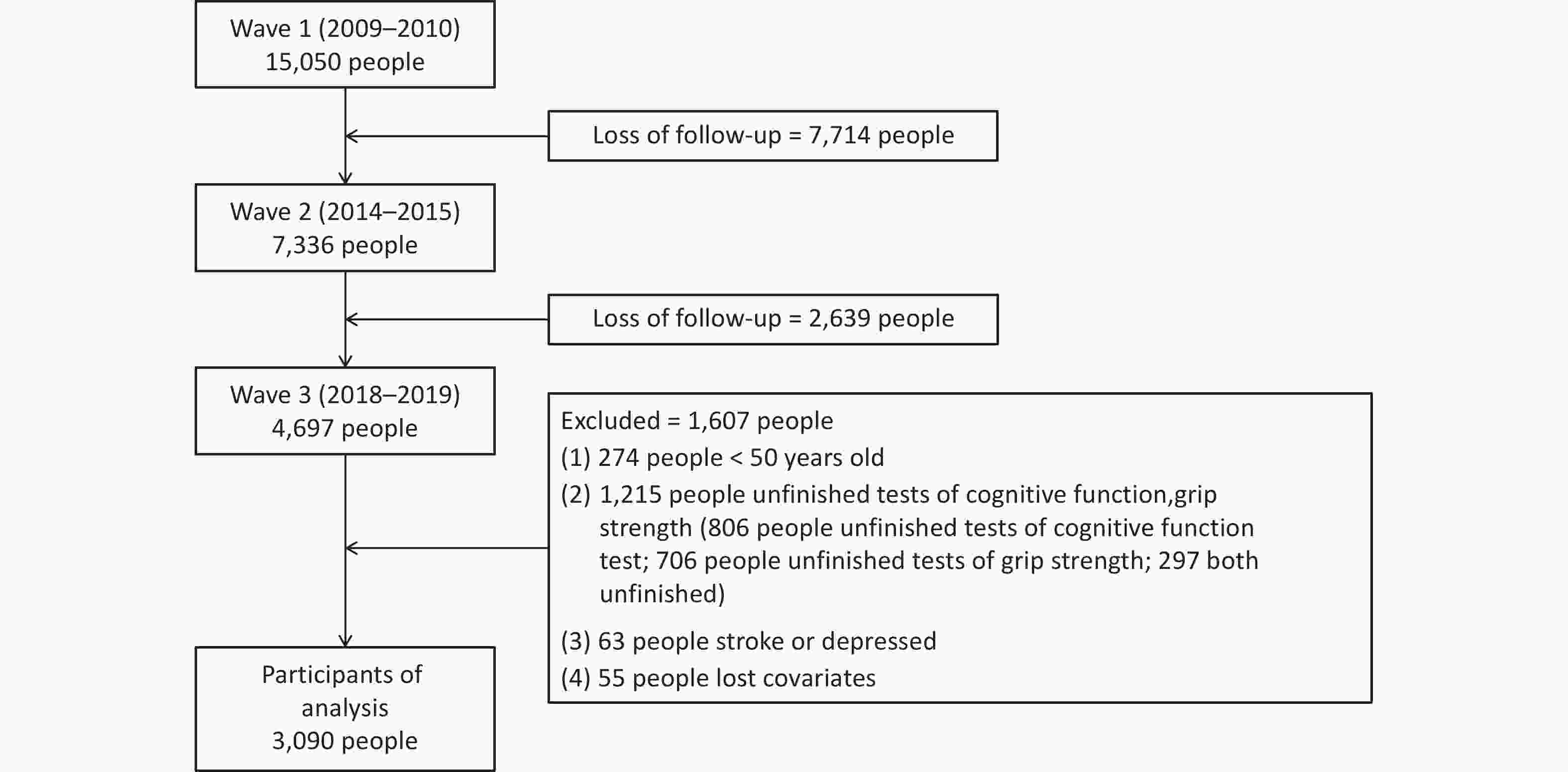
The flow of participants selection is shown in Figure 1 and 3,090 individuals were included in the final analysis. Differences in characteristics between the included and excluded participants are shown in Supplementary Table S1. Supplementary Table S2 summarizes the characteristic of including participants in 3 waves. Mean age of the 3090 participants was 60.83 (SD = 7.54) at Wave 1, 66.28 (SD = 7.62) at Wave 2 and 69.71 (SD = 7.77) at Wave 3. Among the 3090 eligible participants in the final analysis, 1665 (53.9%) were male and 1425 (46.1%) were female. And 1,031 (33.4%) and 2,059 (66.6%) came from urban and rural, respectively.
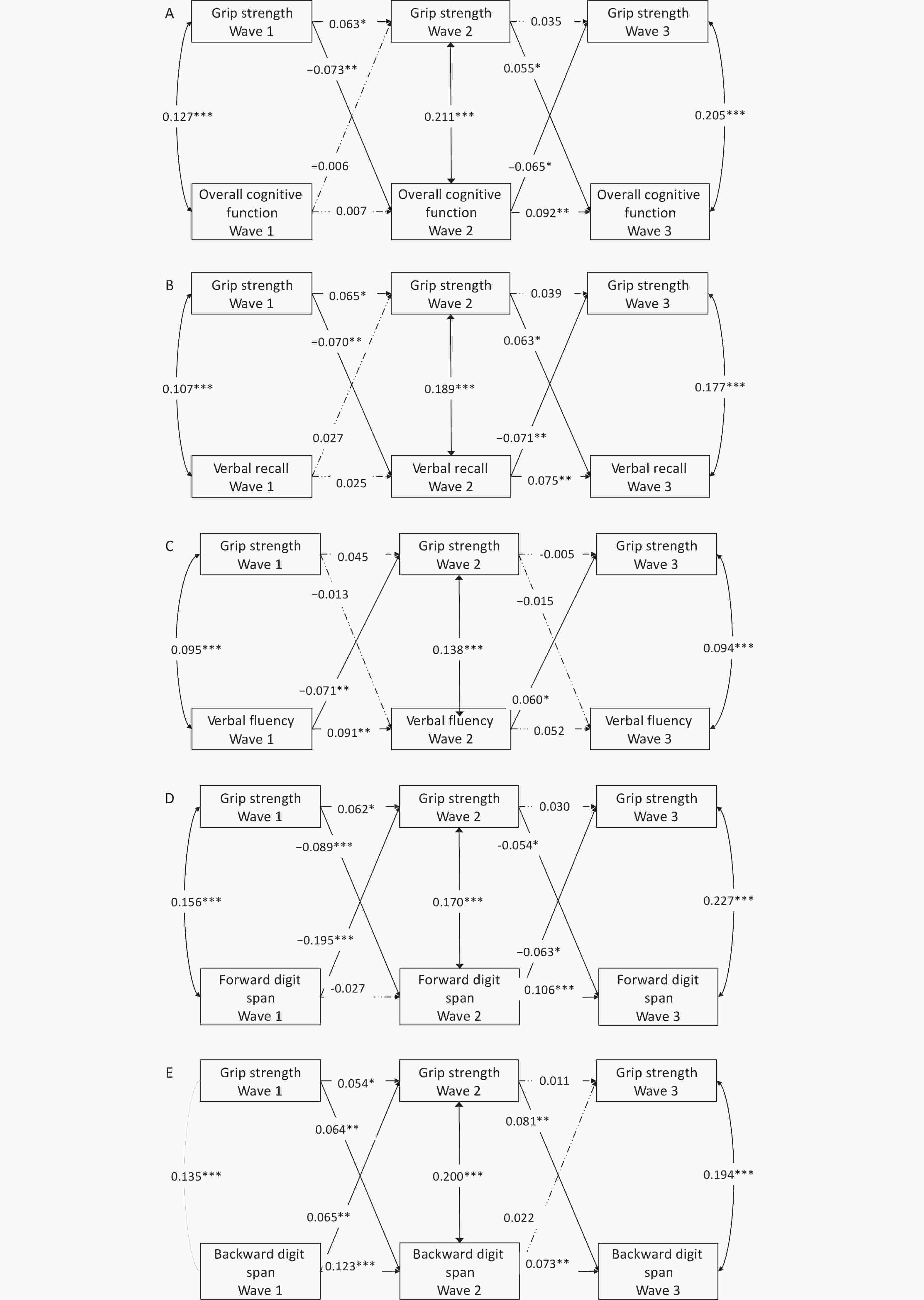
Figure 3. Cross-lagged model of grip strength and cognitive function. (A) Grip strength and overall cognitive function; (B) Grip strength and verbal recall; (C) Grip strength and verbal fluency; (D) Grip strength and forward digit span; (E) Grip strength and backward digit span. ***, P < 0.001; **, P < 0.01; *, P < 0.05.
In 3 waves of survey, the mean scores for each component of cognitive function and grip strength measures for males and females are shown in Supplementary Table S3. Figure 2 shows the trends of cognitive function and grip strength in the 3 waves for all participants, males and females. The four components of cognitive function were presented in a standardized form.
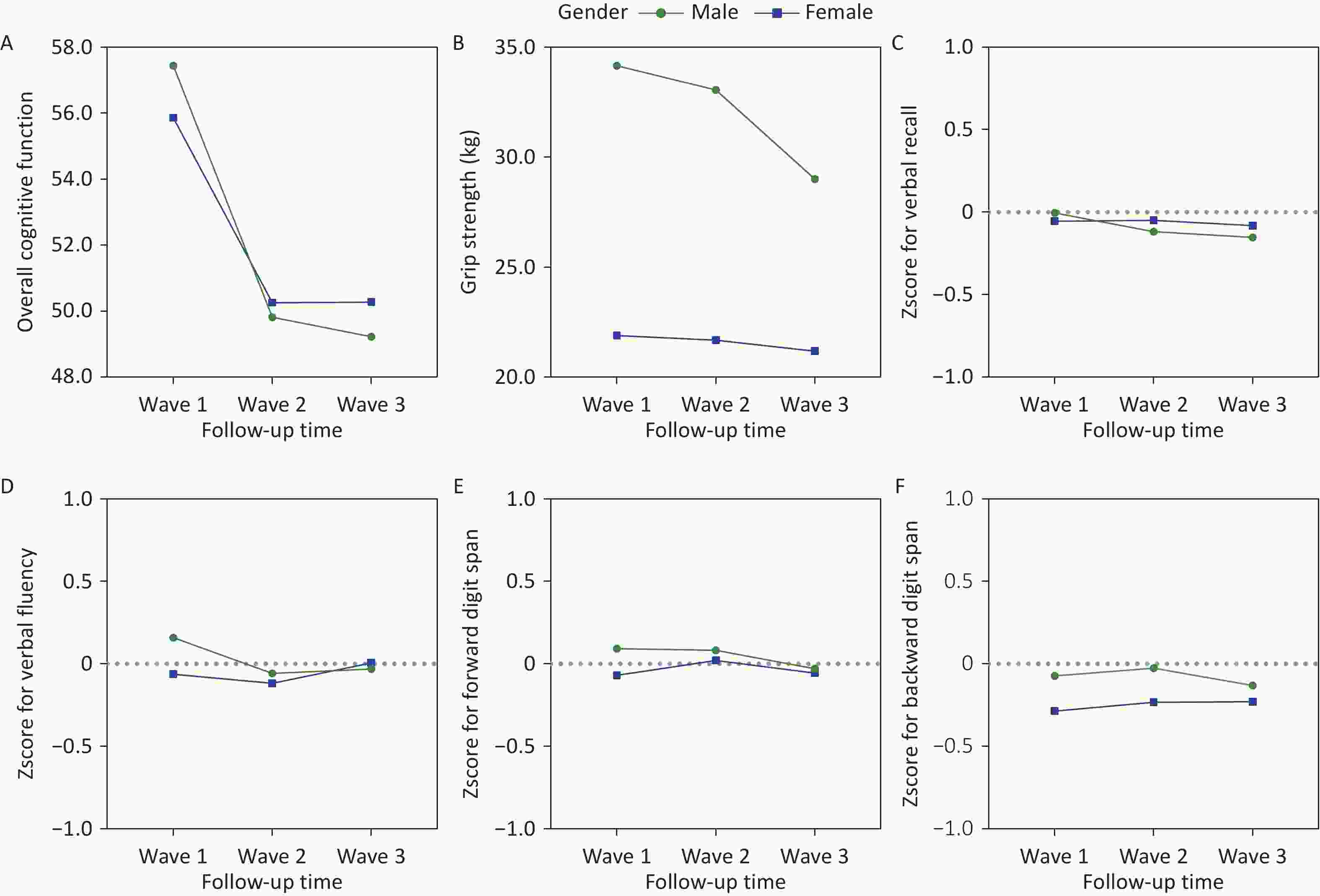
Figure 2. Change of grip strength and cognitive function over time. (A) Overall cognitive function; (B) Grip strength; (C) Zscore for verbal recall; (D) Zscore for verbal fluency; (E) Zscore for forward digit span; (F) Zscore for backward digit span.
Pearson correlation analysis was used to investigate the relationship between cognitive function and grip strength. Supplementary Table S4 demonstrates statistically significant correlations for: (1) grip strength between any 2 waves; (2) cognitive function between any 2 waves; and (3) grip strength and cognitive function within each wave. These results satisfy the prerequisite assumptions of correlation and synchronicity for cross-lagged analysis. Similar correlation patterns were observed between grip strength and individual cognitive domains (Supplementary Tables S5, S6, S7, and S8).
Cross-lagged model was used to explore the time-across association between grip strength and cognition (including its four components), and gender, urban and rural areas, and education level were used as time-invariant covariates, age group, marital status, vegetable and fruit intake, physical activity level and cormobidities as time-dependent covariates. After adjustment, five cross-lagged models were finally obtained.
Fit indices of all crossed-lagged models in examining temporal associations between grip strength and cognitive function were showed in Supplementary Table S9. The model of grip strength and overall cognitive function, grip strength and verbal recall, grip strength and backward digit span had a good fit (comparative fit index = 0.904 to 0.906, root-mean-square error of approximation = 0.046 to 0.049, standardized root mean square residual = 0.025 to 0.026). Of these, the cross-lagged model of grip strength and backward digit span was of better fit.
First, grip strength at Wave 2 and overall cognitive function at Wave 3 were predicted by the same variables measured at an earlier time point, as indicated by significant autoregressive coefficients shown in Figure 3A.
Second, grip strength and overall cognitive function had mutual relationship in the later part of our long-term follow-up (Wave 2 to Wave 3). However, the mutual relationship was not consistent for grip strength and different components of cognitive function. Figure 3A showed that grip strength at Wave 2 could positively predict overall cognitive function at Wave 3 (standardized path coefficients b=0.055, P < 0.05), while overall cognitive function at Wave 2 could negatively predict grip strength at Wave 3 (b=-0.073, P < 0.05). Among the components of cognitive function, only the association between verbal recall and grip strength was similar, as shown in Figure 3B. The back digit span model with the best model fitting indicies only showed a positive relationship between grip strength at Wave 2 and backward digit span at Wave 3 (b=0.081, P < 0.01). In the early follow-up period, the mutual relationship between grip strength and backward digit span was positive, and the variable at Wave 1 was predictive of another variable at the next time period (bgrip strength at Wave 1→backward digit span at Wave 2=0.064, bbackward digit span at Wave 1→grip strength at Wave 2=0.065, P < 0.01).
The aim of this study was to examine the temporal association between handgrip strength and cognitive function in middle aged and elderly adults. The current analyses included a baseline survey and two follow-up visits over a 10-year period. In our study, there was a mutual relationship between grip strength and cognitive function in the middle aged and elderly adults. But this relationship was not consistent across different cognitive components. The results showed that overall cognitive function at Wave 3 was positively predicted by grip strength at Wave 2, but the grip strength at Wave 3 was negatively predicted by overall cognitive function. But looking at the components of cognitive function, we found that early in follow-up, backward digit span and grip strength could predict each other's value at the next time point, and this time correlation is positive.
At present, there are few cross-lagged studies between grip strength and cognitive function. A UK Biobank study that used cross-lagged analysis to explore the relationship between grip strength and cognitive function showed that, grip strength and reaction time influenced each other over a longer period of time, while baseline grip strength was related to pairs-matching at 9 years follow-up, but an association in the opposite direction was not shown[5]. In our study, a temporal effect of grip strength on cognitive components such as verbal recall and back digit span was also shown, but the effect of cognition on grip strength was not clear. The study argues that the cognitive effect on functional abilities is stronger than the opposite effect.
Our study found that grip strength partially contributed to cognitive function. Other studies have also shown that grip strength is only associated with some cognitive components, such as attention, but not with other domains such as cognitive severity[6]. Our study included verbal recall (four times), verbal fluency, and digit span (sequential and backward) tests to assess cognitive function, which was obtained from the MMSE scale. The scale is simple to operate, but the cognitive scope is not comprehensive and the items of cognitive domain are relatively simple. The Digit span test consists of forward and backward contents. Although forward digit span and backward digit span are superficially very similar tasks, the two may rely on separable and completely different mechanisms for the regulation of cognitive ability, with forward digit span requiring verbal working memory and attention, while backward digit span, which tests control and executive function, which is harder[7]. Meanwhile, some studies have shown that the backward digit span test is associated with brain damage, but the forward digit span is not[8]. Our study also showed different results for forward digit span and backward digit span. In conclusion, low score of backward digit span is more easily related to decreased grip strength, due to mechanisms such as brain damage.
Previous finding has shown that low grip strength resulted in longer reaction times (27), which may account for the lower scores on other cognitive tests caused by grip strength. At present, there are several possible mechanisms. The white matter hyperintensity of the brain is closely related to physical function[9]. In addition, muscle has been found to contain a gene that encodes brain-derived neurotrophic factor, which promotes nerve growth and thus reduces the incidence of neurodegenerative diseases[10]. This could explain how the cognitive function point to grip strength. Our study also showed some non-significant results. One possible explanation is that cognitive function and grip strength are jointly affected by brain aging or loss, so the association is not necessarily significant despite simultaneous declines or increases in levels. In addition, the effect from the other side is not constant with the constant changes in cognition and grip strength during the long-term follow-up, and therefore was not a stable mutual relationship, which could explain some of our results shown in Figure 3.
However, our study has some limitations. The 10-year time span was large, and two follow-up visits were conducted during this period, with some loss to follow-up due to relocation and other reasons, which may have caused a bias in the results. In addition, although cross-lagged studies can suggesting the direction of causality, they cannot exclude whether other variables play a role in this relationship. Future studies should incorporate systematic assessments of sarcopenia (e.g., grip strength, muscle mass) and family history data. In addition, the lag parameters obtained using cross-lagged panel analysis do not represent actual interpersonal relationships over time, which may lead to biased conclusions regarding causality. Co-variation in grip strength and cognition may occur with age[10], which may point us toward future research. We may next explore the time period at which this association is strengthened, thereby identifying populations that require special attention.
In sum, in spite of the limitations, our study found a mutual relationship between grip strength and cognition, especially the mutual association between grip strength and back digit span, in Chinese people aged 50 years and older by cross-lagged model. These findings indicate the importance of grip strength and cognitive function, two important indicators in the aging process, and provide a theoretical basis for appropriate intervention of cognitive function and grip strength in the middle aged and elderly adults during aging. The findings suggest the need for early strength training in adults with cognitive decline, while adults with reduced grip strength should also beware of cognitive decline.
doi: 10.3967/bes2025.126
Mutual Relationship between Grip Strength and Cognitive Function in Chinese Middle-Aged and Elderly People over 10 Years: A Cross-Lagged Panel Analysis
-
Study conception and design: Yan Shi and Fan Wu. Data collection: Ye Ruan, Yanfei Guo, Shuangyuan Sun. Acquisition, analysis, or interpretation of data: Jiaqi Wang, Ye Ruan and Anli Jiang. Statistical analysis: Jiaqi Wang, Ye Ruan and Yujun Dong. Manuscript drafting: Jiaqi Wang and Ye Ruan. Review and comment to manuscript: Yan Shi and Fan Wu. All authors read and approved the final manuscript.
The authors declare that they have no competing interests.
The SAGE study was approved by the following bodies: the Ethics Review Committee, World Health Organization, Geneva, Switzerland; Ethics Committee, Shanghai Municipal Centre for Disease Control and Prevention, Shanghai, China.
&These authors contributed equally to this work.
注释:1) Authors’ Contributions: 2) Competing Interets: 3) Ethics: -
Figure 3. Cross-lagged model of grip strength and cognitive function. (A) Grip strength and overall cognitive function; (B) Grip strength and verbal recall; (C) Grip strength and verbal fluency; (D) Grip strength and forward digit span; (E) Grip strength and backward digit span. ***, P < 0.001; **, P < 0.01; *, P < 0.05.
-
[1] Ren RJ, Qi JL, Lin SH, et al. The China Alzheimer report 2022. Gen Psychiatr, 2022; 35, e100751. doi: 10.1136/gpsych-2022-100751 [2] Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet, 2020; 396, 413−46. doi: 10.1016/S0140-6736(20)30367-6 [3] Sternäng O, Reynolds CA, Finkel D, et al. Grip strength and cognitive abilities: associations in old age. J Gerontol B Psychol Sci Soc Sci, 2016; 71, 841−8. doi: 10.1093/geronb/gbv017 [4] Taekema DG, Ling CHY, Kurrle SE, et al. Temporal relationship between handgrip strength and cognitive performance in oldest old people. Age Ageing, 2012; 41, 506−12. doi: 10.1093/ageing/afs013 [5] Jiang RT, Westwater ML, Noble S, et al. Associations between grip strength, brain structure, and mental health in > 40, 000 participants from the UK Biobank. BMC Med, 2022; 20, 286. doi: 10.1186/s12916-022-02490-2 [6] McGough EL, Cochrane BB, Pike KC, et al. Dimensions of physical frailty and cognitive function in older adults with amnestic mild cognitive impairment. Ann Phys Rehabil Med, 2013; 56, 329−41. doi: 10.1016/j.rehab.2013.02.005 [7] Griffin PT, Heffernan A. Digit span, forward and backward: separate and unequal components of the WAIS digit span. Percept Mot Skills, 1983; 56, 335−8. doi: 10.2466/pms.1983.56.1.335 [8] Costa LD. The relation of visuospatial dysfunction to digit span performance in patients with cerebral lesions. Cortex, 1975; 11, 31−6. doi: 10.1016/S0010-9452(75)80018-9 [9] Zheng JJJ, Lord SR, Close JCT, et al. Brain white matter hyperintensities, executive dysfunction, instability, and falls in older people: a prospective cohort study. J Gerontol A Biol Sci Med Sci, 2012; 67, 1085−91. doi: 10.1093/gerona/gls063 [10] Tikkanen E, Gustafsson S, Amar D, et al. Biological insights into muscular strength: genetic findings in the UK Biobank. Sci Rep, 2018; 8, 6451. doi: 10.1038/s41598-018-24735-y -




 下载:
下载:



 Quick Links
Quick Links