-
To investigate the susceptibility of Chukars to duck avian influenza virus H9N2 and explore their role in interspecies transmission of influenza viruses. Chukars were inoculated with duck avian influenza viruses H9N2. The present study demonstrated that inflammatory lesions and virus antigen were present in the trachea, bronchus, and parabronchus, and the viruses could be isolated from throat swabs and lung tissue homogenate supernatants. At 14 d post virus inoculation, anti-H9 influenza virus antibody in the serum was detected. The results indicated that Chukars are susceptible to duck avian influenza virus and serve as an intermediate host, thereby facilitating viral gene evolution and supporting the need for continued surveillance of epidemiology and evolution of the influenza virus in Chukars.
The avian influenza viruses cannot easily cross the interspecific barrier from aquatic birds to humans and other primates, primarily due to the restriction of the receptor binding specificity[1]. The influenza viruses infect the host by viral surface protein hemagglutinin (HA) that specifically binds to the sialic acid receptor of the host cells. Avian influenza A viruses preferentially bind to SAα-2, 3Gal, while the human influenza viruses bind to SAα-2, 6Gal[1]. The SAα-2, 3Gal is predominantly expressed in the gastrointestinal epithelium of ducks, while SAα-2, 6Gal in the tracheal epithelium of humans. These different receptor types and their distribution have been considered as the major barrier for interspecies transmission between avian species and humans[1]. However, the host range of influenza viruses is not restricted, and the influenza viruses can transmit in different species of animals through adaptive genetic mutations and reassortments. Furthermore, it was hypothesized that swine may act as 'mixing vessels' for the reassortment of avian and human influenza viruses, or the adaptation of avian viruses to SAα-2, 6Gal linkages, thereby facilitating the emergence of human pandemic influenza strains[2].
However, since 1997, the avian influenza viruses, such as H5N1, H7N9, and H9N2 frequently infected humans directly from terrestrial animals, suggesting another mechanism of generation of the potential pandemic influenza viruses. Some studies have confirmed that terrestrial birds play a major role in avian influenza viruses that cross the interspecific barriers from bird species to directly infect the humans[3]. Quail was speculated to serve as an intermediate host for the transmission of avian influenza viruses from aquatic to terrestrial birds[3]. Other terrestrial birds, such as Chukars, may also play a major role in the transmission of avian influenza virus across species, as well as, viral gene evolution. In recent years, due to the upsurge in the market quotation of commodity Chukars and an increase in domestic Chukar industry, they were usually produced by a mixed culture of goose and chicken on the farms and sold in live poultry market. This provides an optimal environment for avian influenza viruses to form new variant virus by transmitting among different hosts, creating adaptable changes, and reassortants. A molecular epidemiological survey found that A/Chicken/ Beijing/1/94 (H9N2) (Ck/Bei-like) viruses have been introduced into several types of poultry in southern China, including quail and Chukar, to establish a stable lineage[4]. In a previous study[5], we examined the distribution of influenza virus receptors in the respiratory and digestive tracts of Chukars and found that they carried not only the avian-type SAα-2, 3Gal receptors but also the human-type SAα-2, 6Gal receptors. Moreover, the avian-type SAα-2, 3Gal receptors were distributed in the respiratory and digestive tracts, while the human-type SAα-2, 6Gal receptors were distributed primarily in the respiratory tract. Then, we used fluorescein Alexa488-labeled avian influenza virus DK/ST/1588/00 (H9N1) and human influenza viruses A/ST/92/09 (H1N1) to bind to the tissue section of the respiratory and digestive tracts in Chukars. The study revealed that the avian influenza virus H9N1 bound to the epithelial cells of the respiratory and digestive tracts, while the human influenza virus H1N1 bound to the trachea, bronchus, and secondary bronchus, but not to the parabronchus and colon. Taken together, these results indicated that Chukars, which carry both SAα2, 3Gal and SAα2, 6Gal receptors compatible to binding with avian and human influenza viruses, can be probably infected with avian influenza and human influenza viruses, and viral genetic reassortment could occur easily. The expression of the human SAα-2, 6Gal receptor in the trachea and the secondary bronchus of Chukars suggested that these birds can facilitate the evolution of avian influenza viruses. Therefore, similar to quail, Chukar may act as a potential 'mixing vessel' of influenza A viruses and intermediate host.
Based on the above results, the present study used waterfowl-derived duck virus DK/ST/3208/2010 (H9N2) (abbreviated as ST3208) that was non-pathogenic/low pathogenic and could bind to a SAα-2, 3Gal receptor to infect the Chukars through natural infection route for investigating their susceptibility to the duck influenza virus subtype H9N2. Groups of 8 birds, including clean-class 6-week-old Chukar (Alectoris chukar) (Guangdong Zhaoqing Livestock Culture Co., Ltd, Zhaoqing, China) and 6-week-old specific-pathogen-free chickens (Beijing Meiliyaweitong Experimental Animal Technology Co., Ltd, Beijing, China), were inoculated intraocularly, orally, intranasally, and intratracheally with 0.5 mL (Chukar) or 1.0 mL (chicken) allantoic fluid containing 107 50% egg infections dose (EID50) of the avian influenza viruses subtype H9N2, ST3208, and QA/GX/3139/2012 (H9N2) (abbreviated as GX3139). The isolation of influenza viruses from the selected animals before the infection experiment was negative, and the influenza antibodies titer in the serum was also negative. An equivalent volume of PBS was used to replace the virus fluid in the negative control group. Tracheal and cloacal swabs specimens of the animals were collected at days 1, 3, 5, 7, and 9 post-infection. At days 3, 5, and 7 post-infection, two animals were randomly selected for sacrifice, and trachea, lung, and colon tissue specimens were collected. Each specimen was divided into two portions: one was fixed with 10% formalin, and another was ground and with mixed antibiotic fluid. The serum of Chukar at 14 d post-infection was obtained and inactivated at 56 ℃ for 30 min. The experiments were carried out under BSL-2 conditions with investigators adorning appropriate protective equipment, compliant with the Institutional Animal Care and Use Committee of Guangxi Medical University approved protocols and under the Animal Welfare regulations.
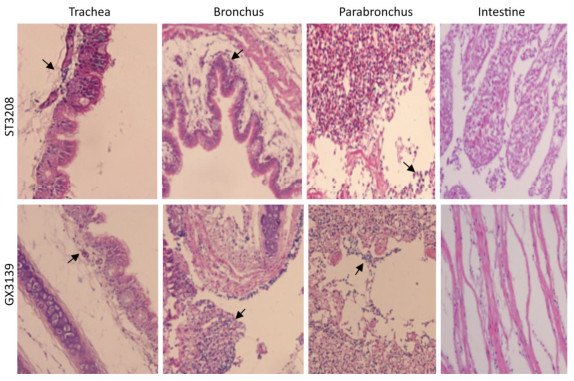
The clinical signs, gross pathological changes, and histopathology in Chukars were observed. The Chukars were essentially normal; no clinical manifestations of mental depression and diarrhea were noted post‐infection with waterfowl‐derived ST3208 viral strain. Necropsy findings revealed scattered hemorrhagic spots in the lung of No.3 and No.7 animals; however, no abnormality was found in other animals. The pathological examination for No.1, No.3, and No.7 Chukars inoculation with ST3208 viral strain revealed tracheal and bronchial inflammation, a small amount of inflammatory cell infiltration in the tracheal wall, and focal inflammatory cell infiltration in the bronchial wall. In addition, inflammatory cells were primarily infiltrated by lymphocytes and fibrin exudate with minor bleeding in the parabronchus (Figure 1). The antigen of influenza virus was detected by immunohistochemistry. Antigen retrieval was performed, followed by the addition of primary anti‐NP (nuclear protein) antibody (National Institute of Diagnostics and Vaccine Development in Infectious Diseases, Xiamen University, China). Subsequently, secondary anti‐mouse IgG‐biotin and streptavidin/peroxidase (Sigma) were added. Positive tissue sections (quail lung tissues infected with H9N2 influenza virus in vivo) were used as the positive control. The primary antibody nucleoprotein (NP) was replaced by 10% normal rat serum that was utilized as negative control. After Chukars were infected with waterfowl‐derived duck virus ST3208, the virus antigens NP in the trachea, bronchus, and intrapulmonary parabronchus were positive in 3/11 animals (Figure 2), thereby substantiating that the influenza virus was replicated in the respiratory epithelial cells of Chukars and produced a specific pathogenicity.
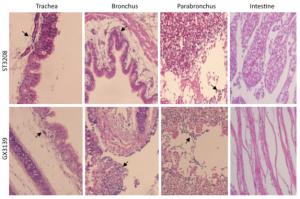
Figure 1. Trachea, bronchus, and lung tissues show different degrees of inflammatory lesions in Chukars infected with DK/ST/3208/2010 (H9N2) and DK/GX/3139/2010 (H9N2) viral strains (indicated by the arrows); however, there was no inflammatory response in the colon (hematoxylin and eosin staining, magnification ×200).
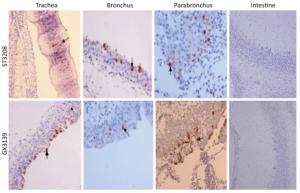
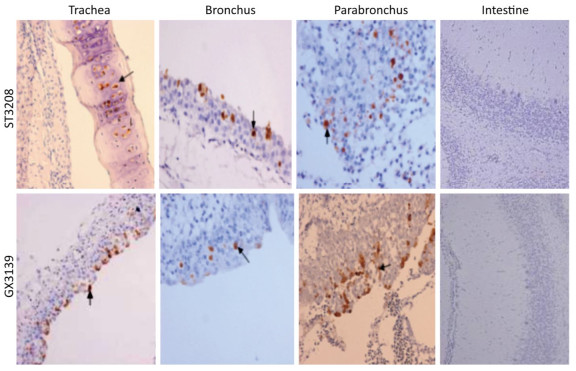
Figure 2. Post-infection with DK/ST/3208/2010 (H9N2) and DK/GX/3139/2010 (H9N2) viral strains; immunohistochemistry showed that the tracheal, bronchial, and lung epithelial cells of the Chukars were positive for NP protein expression (indicated by the arrows), while the colonic influenza virus antigen NP was negatively expressed (hematoxylin and eosin staining, magnification ×200).
Viruses in samples were titrated for infectivity in 10‐day embryonated chicken eggs and the presence of virus in the allantoic fluids was determined by virus hemagglutination assay. The viruses can be isolated from throat swabs and lung tissue homogenate supernatant at 5 d and 7 d post- inoculation in 3/11 animals. The viruses can be isolated at 5 d in the throat swab and lung tissue homogenate supernatant in 2/3 animals, and isolated at 7 d in the remaining 1/3 animal. However, no virus was isolated from the cloacal swabs and intestine tissue homogenate supernatant (Table 1), indicating that the inoculated duck H9N2 influenza virus mainly replicated in the respiratory tract and not the intestine, which was consistent with the histopathological and immunohistochemistry results. Obviously, Chukars will not shed the viruses by feces, which explains the absence of clinical symptoms of diarrhea. The hemagglutination inhibition (HI) antibody level was one of the critical indicators for reflecting humoral immunity. The antibodies produced as a response to viral infection were determined by HI assay. This study found that the highest titer of HI antibody was 1:320 in the serum of Chukars at 14 d post virus inoculation, thereby suggesting that chukars can produce infectious antibodies after infection by duck H9N2 influenza viruses.
Animal No. ST3208 (dpi) GX3139 (dpi) 3 5 7 3 5 7 1 0/0/0/0 0/0/0/0 1:24/0/1:24/0 1:24/0/1:24/0 1:25/0/1:24/0 1:23/0/1:24/0 2 0/0/0/0 0/0/0/0 0/0/0/0 0/0/0/0 0/0/0/0 0/0/0/0 3 0/0/0/0 1:25/0/1:24/0 0/0/0/0 1:25/0/1:25/0 1:23/0/1:24/0 0/0/0/0 4 0/0/0/0 0/0/0/0 0/0/0/0 0/0/0/0 0/0/0/0 0/0/0/0 5 0/0/0/0 0/0/0/0 0/0/0/0 0/0/0/0 0/0/0/0 0/0/0/0 6 0/0/0/0 0/0/0/0 0/0/0/0 1:26/0/1:25/0 0/0/0/0 1:27/1:24/1:24/0 7 0/0/0/0 1:23/0/1:23/0 0/0/0/0 0/0/0/0 1:24/0/1:24/0 1:25/0/1:25/0 8 0/0/0/0 0/0/0/0 0/0/0/0 0/0/0/0 0/0/0/0 0/0/0/0 9 0/0/0/0 0/0/0/0 0/0/0/0 0/0/0/0 1:25/0/1:25/0 1:26/0/1:26/0 10 0/0/0/0 0/0/0/0 0/0/0/0 1:26/0/1:26/0 1:28/1:24/1:24/0 1:27/0/1:24/0 11 0/0/0/0 0/0/0/0 0/0/0/0 0/0/0/0 0/0/0/0 0/0/0/0 12 0/0/0/0 1:25/0/1:25/0 1:25/0/1:25/0 Table 1. Results of Virus Isolation from Chukar Post-infection with DK/ST/3208/2010 (H9N2) and DK/GX/3139/2010 (H9N2) Viral Strain (throat swabs/cloacal swabs/lung tissue homogenate supernatant/intestinal tissue homogenate supernatant)
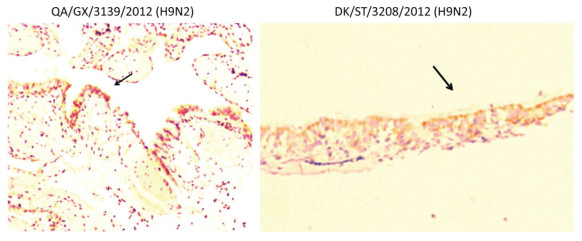
Meanwhile, we found that inflammatory lesions, virus NP antigen were present in the trachea, bronchus, and intrapulmonary parabronchus (Figure 2). Also, virus could be isolated from the throat swab and lung tissue of 7/12 Chukars after inoculation with GX3139 viral strain (Table 1), and HI antibody was positive in the serum of 7/12 animals at 14 d after inoculation with GX3139 virus. These indicated that terrestrial bird-derived GX3139 virus can effectively replicate and shed viruses in the respiratory tract of Chukars. However, the duck ST3208 and quail GX3139 virus failed to replicate and shed viruses in the chicken. Perez demonstrated that quail were more susceptible than chickens to duck H9N2 viruses[3], and served as intermediate hosts, in which, the avian influenza viruses can be amplified and transmitted to other animal species. Similar to the quail, Chukars were more susceptible to the aquatic birds H9N2 virus than chicken. Therefore, in vivo animal infection confirmed that Chukars are susceptible to the aquatic birds' influenza virus subtype H9N2. Although there was no virus replication after the chickens were inoculated with ST3208 and GX3139 viruses, the adsorption of the viruses to the tracheal and bronchial epithelial cells was observed (Supplementary Figure S1 available in www.besjournal.com).
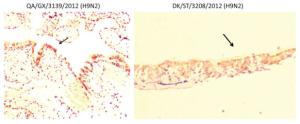
Figure Supplementary Figure S1. Post-infection with QA/GX/3139/2012 (H9N2) and DK/ST/3208/2012(H9N2), the virus adsorption to the trachea and bronchial epithelial cells in chicken (indicated by the arrows) (magnification ×200). Staining by immunohistochemistry.
The transmission of influenza viruses across host species primarily depended on the receptor binding specificity of the hemagglutinin (HA) protein. The receptor binding properties and host range of H9N2 virus were also analyzed by gene sequencing in this study (Supplementary Table S1 available in www.besjournal.com). The receptor binding sites (RBS), such as 224N, 226Q, and 228G, located on the head of HA at the position of duck virus ST3208-HA were characteristic of the avian influenza virus receptor SAα-2, 3Gal[6]. The cleavage sites of HA1 and HA2 connecting polypeptides were the key sites where the viruses infection the host cells. Duck virus ST3208-HA1-HA2 with PARSNR/GL in the cleavage sites could be classified as low pathogenic avian influenza virus. The ST3208 virus did not show any amino acid deletions in the neck of neuramidinase (NA), which was not compliant with the characteristic of the highly pathogenic avian influenza virus. Influenza viruses with changes in the amino acid sequence of polymerase basic 2 (PB2) determined the host range and pathogenicity of the virus. Herein, the ST3208 virus carried a glutamic acid at site 627 of PB2, which was optimal for infecting the birds[7]. PB1-F2 is a nonstructural protein, found recently, encoded by alternative reading frame (ARF) of PB1. The N66S mutation of PB1-F2 was speculated to contribute towards the high mortality of H1N1 virus in 1918 and H5N1 virus in 1997. The PB1-F2 position of duck virus ST3208, N (asparagine), was not mutated. Except for the neck of NA that displayed a deletion of 2 amino acids, the sequencing of the terrestrial bird-derived GX3139 virus was similar to that of the waterfowl-derived ST3208 strain. The two viruses bind to the avian-type SAα-2, 3Gal receptors and cannot replicate effectively in mammals.
Virus Residue at RBS HA-Connecting NA Deletion (aa) PB2 PB1-F2 224 226 228 627 66 DK/ST/3208/2012 N Q G PARSNR/GL no E N QA/GX/3139/2012 N Q G PARASR/GL 2 aa (39-40) E N Table Supplementary Table S1. Amino Acid Sequences of HA, NA, PB2, and PB1-F2 Genes of DK/ST/3208/2010 (H9N2) and DK/GX/3139/2010 (H9N2) Strains
Taken together, Chukars, which carry both SAα2, 3Gal and SAα2, 6Gal receptors compatible along with susceptibility to aquatic bird duck influenza virus subtype H9N2, facilitated the evolution of influenza viruses. After transmitting from aquatic to terrestrial birds, the virus adapted to the new host, evolved into a stable lineage, and even circulated. The molecular epidemiological studies found that some avian influenza viruses such as H9N2 and H6N1 established a stable lineage in quail, chicken, and Chukar[4, 8]. The currently circulating avian influenza virus H9N2 and H7N9 subtypes, some viral strains from terrestrial birds appeared to bind specifically to the human-type SAα-2, 6Gal receptors, which also replicated and transmitted in several terrestrial birds[9-10]. Thus, it could be deduced that viruses putatively bring about adaptive changes in the terrestrial birds, especially repeated replication in the respiratory tract with high-density SAα-2, 6Gal receptor. Consequently, the changes in the receptor binding site of avian influenza virus HA were promoted, which in turn, infected the humans. Therefore, Chukars may act as potential intermediate hosts for the transmission of influenza viruses across species, thereby expanding the host range and transmitting the virus to the mammals (including humans), as well as, generating new influenza viruses with pandemic potential. With the rapid development of poultry farming, the hosts of influenza viruses are expanding with increased complexity; the waterfowl influenza viruses infect the terrestrial birds such as quail, Chukar, and chicken, and these viruses may cause adaptive changes and/or reassortment to generate new variants that can infect the humans after long-term replication in these animals. Therefore, since influenza viruses are a severe threat to humans, we must systematically monitor the dynamics of the epidemic and grasp the evolutionary pattern of the viruses in Chukars over a prolonged period.
No conflict of interest to declare.
Replication and Pathology of Duck Influenza Virus Subtype H9N2 in Chukar
doi: 10.3967/bes2018.039
the National Natural Science Foundation of China 31260033
the National Natural Science Foundation of China 31660041
- Received Date: 2017-09-07
- Accepted Date: 2018-02-07
| Citation: | ZHU Yin Chuan, ZHANG Bin, SUN Zeng Hui, WANG Xi Jing, FAN Xiao Hui, GAO Ling Xi, LIANG Ying, CHEN Xiao Yan, ZHANG Zeng Feng. Replication and Pathology of Duck Influenza Virus Subtype H9N2 in Chukar[J]. Biomedical and Environmental Sciences, 2018, 31(4): 306-310. doi: 10.3967/bes2018.039 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: