-
Infrasound is a mechanical vibration wave with a frequency range from 0.0001 to 20 Hz. Human ears are not able to hear infrasound since its frequency is below human hearing threshold. Infrasound widely exists in nature, our living condition, productive and traffic environment[1]. Infrasound has the physical characteristics of less attenuation in long distance propagation and stronger penetration. As an important part of public noise, infrasound is receiving more and more attention in the field of clinical medicine, preventive medicine and military medicine. Some people living in the environment of wind energy infrastructure experience negative health effects such as annoyance, sleep disturbance, stress-related health impacts and reduced quality of life[2]. Current studies have confirmed that infrasound with a certain sound pressure level can cause function disorders, and even induce tissue damage. The available studies mainly focus on its effect on central nervous system, stress reaction, auditory system, reproductive system, cardiovascular, and respiratory system, while studies on the effects of infrasound on gastrointestinal tract are relatively less. Gastrointestinal tract is relatively sensitive to infrasound. Previous study indicated that gastric mucosal blood flow significantly decreased in Sprague-Dawley rats exposed to infrasound[3]. However, the effect of infrasound on gastrointestinal function is unclear.
The effect of nitric oxide (NO) on gastrointestinal motility is dual. On the one hand, NO within physiological values plays an important role in protecting gastrointestinal mucosa. NO plays an important regulatory role in gastrointestinal mucosa vasodilation, since it can regulate blood flow and maintain the mucosa integrity[4]. In addition, as a nonadrenergic and noncholinergic inhibitory neurotransmitter, NO maintains normal gastrointestinal motility. On the other hand, excessive NO can induce mucosa inflammation and abnormal hyperplasia and also cause gastrointestinal motility disorders[5]. Nitric oxide synthase (NOS) is a key rate-limiting enzyme for NO synthesis, directly influencing the NO production and its physiological and pathological function. Hence, the study of the expression of NOS after exposure to infrasound, may explore underlying mechanisms of infrasound on gastroenterology.
The Infrasonic system included sound source and check system, provided by the Fourth Military Medical University (Xi'an, China). Seventy male Sprague-Dawley rats (6-7 weeks old, weighing 180-200 g) obtained from the Center of Experimental Animal in the Fourth Military Medical University were maintained with food and water available ad libitumin an air-conditioned room at a temperature of 23 ± 2 ℃ and a relative humidity of 60% ± 5%, under a 12/12 h light/dark cycle. The rats were randomly divided into the experimental group and control group (35 rats/group). The experimental group was placed into the infrasonic chamber and exposed to the infrasound of 8 Hz - 130 dB for 2 hours per day for 14 consecutive days. The control group was under the same conditions without infrasonic exposure. Seven rats were randomly collected to examine gastric emptying rate, gastric morphology and NOS expression at day 1, 7, 14, 21, and 28 after the end of the exposure. All experiments were conducted following the institutional ethical guidelines. All the necessary efforts were made to minimize the number of animals per group and their suffering.
Gastric motility was evaluated by gastric fluid-emptying rate, which was measured according to previous methods[6]. The gastric fluid-emptying rate percentage was calculated according to the following formula: 20 min gastric fluid-emptying rate percentage = (intestinal γ-ray quantity/stomach γ-ray quantity + intestinal γ-ray quantity) × 100. Then the rats were killed, and the stomach was rapidly removed and washed in Krebs solution. Gastric antrum tissue (0.5 cm above the pylorus) was fixed in 10% paraformaldehyde phosphate buffer solution for HE stained and detecting of expression of iNOS mRNA and of iNOS and eNOS proteins. Two experienced pathologists observed and evaluated the gastric morphology using a double-blind method in HE staining section. Gastric mucosal damage score was calculated by the sum of the score of depth of inflammatory cell infiltration, intrinsic glands number variation and structure damage (Supplementary Table 1 available in www.besjournal.com).
Score Parameter Depth of inflammatory cell infiltration 0 No inflammatory cell infiltration 1 Depth of inflammatory cell infiltration involves 1/3 of superficial mucosa 2 Depth of inflammatory cell infiltration involves 1/3 to 2/3 of the mucosa 3 Depth of inflammatory cell infiltration involves over 2/3 of the mucosa Intrinsic glands number variation 0 Glands number is normal 1 Glands number decrease is within 1/3 of the normal number 2 Glands number decrease ranges from 1/3 to 2/3 of the normal number 3 Glands number decrease is over 2/3 of the normal, which is severe Structure damage 0 Intact mucosa 1 Mild epithelial layer damage, while the lamina propria and muscularis mucosa are normal 2 The epithelial layer is clearly damaged. The lamina propria is clearly infiltrated by inflammatory cells and hyperemic, while the muscularis mucosa is normal 3 The muscularis mucosa is hyperemic and incomplete, showing edema, uneven thickness, and muscle fiber glassy degeneration Table Supplementary Table 1. The Gastric Mucosa Damage Grade Criteria in Rat
The expression of iNOS mRNA in gastric antrum tissues was measured by in situ RNA hybridization technique. In the microarray, androblastoma sample was used as a positive control, and PBS buffer solution instead of cRNA probe was used as a negative control. Under the microscope, the cells exhibited light brown, brown or dark brown staining in the nucleus or cytoplasm, thus the cells were identified as iNOS mRNA positive cells. Three visual fields were selected under ×200 magnification and positive cell number average was considered as total positive cell number.
The expression of iNOS and eNOS proteins in gastric antrum tissues was measured by tissue microarray technology. In the microarray, androblastoma sample was used as a positive control, and PBS buffer solution instead of primary and secondary antibodies as a negative control. NOS positive standards were evaluated according to the staining intensity of cytoplasm: negative (-) means no color; weakly positive (+) means light brown color; positive (++) means brown color; strong positive (+++) means dark brown color. Since NOS distribution was uneven in the stomach, every sample was divided into three observation area, such as the epithelium, submucosa and muscularis area. Thus, for a total of 7 rats in each group, 21 observation areas (n) were observed. The expression of iNOS and eNOS proteins was calculated by the sum of positive areas in each group.
Statistical differences were evaluated by one-way analysis of variance (ANOVA) followed by LSD-t test using statistical SPSS 16.0 software package (SPSS, Chicago, IL). The expression of NOS protein was shown as percentage or grading count. The grading count was evaluated by Ridit test. The percentage comparison between groups was evaluated by Chi-square test. P < 0.05 was considered statistically significant.
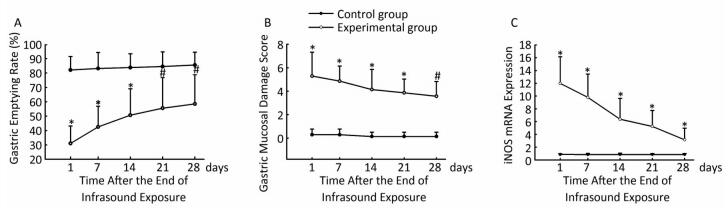
A follow-up influence was observed after the rats' exposure to the infrasound of 8 Hz - 130 dB for 14 consecutive days. The gastric emptying rate was clearly lower at day 1, 7, and 14 days (P < 0.01) and at day 21 and 28 (P < 0.05) in the experimental group compared with the control group (Figure 1A). Our results showed that the infrasound decreased gastric motility. Previous research demonstrated that infrasound induce the tissue damages mainly by direct biomechanical resonance[7]. Infrasound of 8 Hz is within the natural frequency range of abdominal organs, so it is easier to induce biomechanical resonance to produce stronger biological effects. Gastric dysfunction may cause anorexia and nausea. This study may help scientists to understand the anorexia and nausea of human and animals after exposure to infrasound. Moreover, after the rats' exposure to the infrasound of 8 Hz - 130 dB for 14 consecutive days, the gastric emptying rate was gradually recovered in the subsequent 28 days, although the gastric emptying rate was still lower than the control group. It indicated that the rats were adapt to the infrasound, which was in agreement with another research[8].

Figure 1. The effect of infrasound on rats' gastric emptying rate (A), gastric mucosal damage score (B), and expression of gastric antrum iNOS mRNA protein (C) at different days after the end of the exposure. #P < 0.05 vs. control group. *P < 0.01 vs. control group.
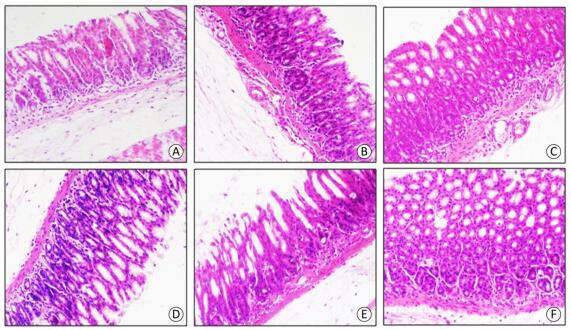
Previous studies have confirmed that infrasound can induce tissue damages, including central nervous system, lung, cardiac ultrastructure and liver cell damage[8-10]. However, the influence of infrasound on gastric mucosa structure was rarely reported. Our studies indicated that the experimental group rats' gastric mucosal damage score was clearly higher at day 1, 7, 14, and 21 (P < 0.01) and day 28 (P < 0.05) compared with the control group (Figure 1B). At the early stage it was mainly inflammatory cell infiltration, glands hypertrophy, capillary expansion and muscular layer edema. However, as time was passing, the inflammatory cell infiltration slightly relieved (Figure 2). Therefore, infrasound not only affected gastric function, but also caused gastric mucosa damage. In addition, the gastric mucosal damage score showed a certain trend of recovery after the end of the infrasound exposure. The gastric tissue was also adapted to the infrasound. The mechanism of gastric mucosal lesion might be related to biomechanical stimuli directly damaging gastric antrum tissue structure.
Figure 2. HE staining of gastric antrum mucosa. magnification x200. (A) Control group. (B) Experiment group at day 1. (C) Experiment group at day 7. (D) Experiment group at day 14. (E) Experiment group at day 21. (F) the experiment group at day 28.
It is known that iNOS is involved in the acute and chronic gastric mucosal damage induced by several stimuli, including cold water stimulation, hydrochloric acid, empyrosis and Helicobacter pylori infection[21, 22]. iNOS produced by inflammatory cells might result in a great NO amount synthesis. Excessive NO can induce mucosa inflammatory damage and abnormal hyperplasia and also cause gastrointestinal motility disorder. Our results found that similarly to the gastric emptying rate and gastric antrum mucosa damage, the expression of gastric antrum iNOS mRNA was clearly higher in the experimental group compared with the control group at day 1, 7, 14, 21, and 28 (P < 0.01) (Figure 1C). The expression of gastric antrum iNOS protein in the experimental group was higher compared with control group at day 1, 7, and 14 (Ridit test, P < 0.01) and 21, 28 (Ridit test, P < 0.05) (Table 1). The expression of gastric antrum iNOS mRNA and protein in the experimental group tended to recover after the end of the exposure, but the expression was still higher within the follow-up observation of 28 days compared with the control group. These results suggested that infrasound might influence iNOS expression of gastric antrum. Then iNOS might further involve in gastric motility dysfunction and gastric mucosal damage. Besides, the expression of gastric antrum mucosa eNOS protein was not affected by infrasound, which further confirmed that eNOS activation was related to the internal environment and it is not related to the inflammatory environment.
Time (day) Control Group Experiment Group n - + ++ +++ n - + ++ +++ 1* 21 20 1 0 0 20 0 19 1 0 7* 20 19 1 0 0 19 3 15 1 0 14* 20 19 1 0 0 21 10 11 0 0 21# 19 18 1 0 0 20 12 8 0 0 28# 21 20 1 0 0 21 13 8 0 0 Note.*P < 0.01 vs. control group (Ridit test), #P < 0.05 vs. control group (Ridit test). Table 1. The Expression of Gastric Antrum iNOS Protein After the End of Infrasound Exposure
In summary, despite some deleterious effects of infrasound on some human organs and tissues are known, not much attention is paid to its effect on the gastrointestinal tract. Therefore, our experiments revealed additional and unknown potential adverse human health effects due to infrasound exposure.
We wish to express our gratitude to ZHI Ming, YIN Yan, and ZHAO Ping for their assistance with experiments.
Effects of Infrasound on Gastric Motility, Gastric Morphology and Expression of Nitric Oxide Synthase in Rat
doi: 10.3967/bes2018.052
financially supported by the Shaanxi province science and technology project 2005k12-G1-3
- Received Date: 2017-10-15
- Accepted Date: 2018-03-09
Abstract: Infrasound widely exists in nature, our living condition, productive and traffic environment. Gastrointestinal tract is relatively sensitive to infrasound. However, the effect of infrasound on gastrointestinal function is unclear. Therefore, the purpose of our study was to observe the effects of infrasound on gastric motility and gastric morphology and to assess the expression of nitric oxide synthase (NOS) in gastric antrum after exposure to infrasound of 8 Hz - 130 dB for 2 hours per day for 14 consecutive days. Gastric motility was assessed by gastric fluid-emptying rate. Gastric morphology was evaluated by HE. The expression of NOS was measured by tissue microarray technology. The results would contribute to understand the role of infrasound in gastroenterology, and help to explain the mechanism of infrasound on gastroenterology.
| Citation: | ZHAO Ju Hui, WANG Jin Hai, LUO Jin Yan, GUO Xiao Yan, WANG Yan, CHENG Yan. Effects of Infrasound on Gastric Motility, Gastric Morphology and Expression of Nitric Oxide Synthase in Rat[J]. Biomedical and Environmental Sciences, 2018, 31(5): 399-402. doi: 10.3967/bes2018.052 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: