HTML
-
Campylobacter jejuni (C. jejuni) is one of the major causes of bacterial gastroenteritis in both developed and developing countries[1-3]. People become infected by consuming contaminated food, particularly poultry meat, raw milk, and water[4-7]. In addition to gastrointestinal illness, C. jejuni may also lead to autoimmune conditions known as Guillain-Barré syndrome (GBS) and Miller Fisher syndrome[8]. Zhang et al.[9] reported an outbreak caused by C. jejuni in Jilin, China during 2007, in which 32 patients with GBS were recorded, indicating that Campylobacter is a threat to human health in China.
C. jejuni has a very wide distribution in animals. Poultry is considered as the major reservoir of Campylobacter. The prevalence of C. jejuni in chicken meat was reported as 13.7% (31/227) in Tianjin[10] and 17.2% (52/302) in central China[11]. In addition, Campylobacter can also inhabit cattle, pigs, ducks, dogs, and other wild and domestic animals[12-15], making it challenging to trace the sources and routes of transmission.
Antibiotic resistance of C. jejuni is an increasing concern[16]. Infections by antibiotic resistant strains are usually associated with a longer illness duration, a higher risk of invasive disease, and higher healthcare costs[17-19]. In Shanghai (Eastern China), more than 96% of the Campylobacter spp. isolates are resistant to quinolones and tetracyclines[20]. In central China, even higher resistance of C. jejuni isolates from retail chicken meat has been reported: 100% resistance to ciprofloxacin and 90% resistance to tetracycline[11].
Shenzhen is the fastest-growing major city in China, with a highly concentrated population and high consumption of meat. Hence, studying the genetic and antibiotic resistance characteristics of C. jejuni in Shenzhen is important. However, there is little reported data about C. jejuni in Shenzhen.
The aim of this study was to characterize the genetic profile and antibiotic resistance of C. jejuni isolated from cattle, poultry, and patients with diarrhea in Shenzhen.
-
The study was approved by the institutional review board of the Nanshan Center for Disease Control and Prevention. Written informed consent was obtained from each adult patient or from the children's parents or guardians.
-
During March 1 to November 20, 2016, we enrolled patients who were outpatients presenting with acute diarrhea, which was defined as ≥ 3 passages of watery, loose, mucosal, or bloody-stools during a 24 h period. Stool samples from child patients (≤ 5 years old), adolescent patients (5-17 years old), and adult patients (≥ 18 years old) were collected from four medical facilities, designated as Hospital A, B, Health care C, and Maternal and children's hospital D. A total of 437 patients with diarrhea were enrolled, including 173 child patients, 58 adolescent patients, and 206 adult patients. In total, 437 stool samples were collected.
The poultry meat samples were collected from three retail markets (n = 63), and the cattle meat samples were collected from a slaughterhouse (n = 30) and three retail markets (n = 63). The poultry cloacal swabs (n = 74) were collected from a poultry wholesaler, while the cattle stool samples (n = 103) were collected from a cattle farm (n = 64) and a slaughterhouse (n = 39).
For the stool samples, from both patients and cattle, approximately 0.5 g of stool was collected. Both the stool samples and the cloacal swabs were transferred in Cary-Blair medium at 4 ℃ within 8 h for bacteria isolation. Approximately 200 g of meat samples were collected in a sterile plastic bag, and transported to laboratory at 4 ℃ within 4 h for isolation.
-
Two Campylobacter isolation kits (ZC-CAMPY-001 and ZC-CAMPY-002, Qingdao Sinova Bio-technology Co. Ltd, Qingdao, China) were used to isolate Campylobacter from stool and meat samples, respectively[21]. Briefly, the stool or cloacal swab samples were inoculated into enrichment broth. Each meat sample was placed in a sterile plastic bag containing enrichment media. The enrichment broth was then incubated at 42 ℃ in a microaerophilic atmosphere (5% O2, 10% CO2, and 85% N2) for 48 h. Approximately 300 μL of the enrichment medium was then spotted onto the surface of a membrane filter from the kit, which was pasted onto Karmali and Columbia agar plates. Suspected colonies were picked after incubation at 42 ℃ for 48 h in a microaerophilic atmosphere. Finally, Gram staining and biochemical tests were conducted. Species identification was performed using PCR, according to a previously described method[22].
-
MLST was performed according to the Pubmlst protocol (https://pubmlst.org/campylobacter/info/primers.shtml)[14]. Briefly, genomic DNA was extracted using a commercial kit (QIAamp DNA mini kit, QIAGEN, German), and seven housekeeping genes (aspA, glnA, gltA, glyA, pgm, tkt, and uncA) were amplified using PCR. The amplification reactions were performed in a 20 μL mixture containing 10 μL of 2× PCR master (Quanshijin Biotechnology Ltd. Corp., Beijing, China), 0.5 μL of forward and reverse primer, 1 μL of DNA template, and 7.5 μL of pure water. PCR was performed as 1 cycle of 5 min at 94 ℃; 35 cycles of 1 min at 94 ℃, 2 min at 50 ℃, and 1 min at 72 ℃; and a final extension of 5 min at 72 ℃. After amplification, 2 μL of the PCR product was subjected to electrophoresis through a 1.5% agarose gel. Then the PCR products were the purified and sequenced. The sequence was subjected to a BLAST search against the PubMLST database, after which the allele numbers were assigned and sequence types (ST) and clonal complexes (CC) were determined. Novel alleles and STs were submitted to the PubMLST C. jejuni/C. coli databases.
-
Commercial kits (Zhongchuang Biotechnology Ltd. Corp., Qingdao, China) were used to determine the minimum inhibition concentration (MIC) of all C. jejuni isolates. The agar dilution method was used[21], and the breakpoints used for each antibiotic were those recommended by National Antimicrobial Resistance Monitoring System (NARMS-2014): Erythromycin (≥ 32 μg/mL), azithromycin (≥ 8 μg/mL), nalidixic acid (≥ 64 μg/mL), ciprofloxacin (≥ 4 μg/mL), gentamycin (≥ 8 μg/mL), streptomycin (≥ 16 μg/mL), chloromycetin (≥ 32 μg/mL), florfenicol (≥ 8 μg/mL), tetracycline (≥ 16 μg/mL), telithromycin (≥ 16 μg/mL), and clindamycin (≥ 8 μg/mL).
Briefly, fresh C. jejuni colonies were suspended in an NaCl solution (0.9%) to obtain a suspension with 0.5 McFarland turbidity. One hundred microliters of the suspension were diluted in 900 μL NaCl solution (0.9%) resulting in a concentration range of 106-107 colony forming units (cfu) per mL. Each well was dispensed with 2 μL of the suspension. The plates were incubated at 37 ℃ for 48 h in microaerophilic atmosphere. The MIC was defined as the lowest concentration of each antibiotic that could prevent visible growth of C. jejuni, and the MIC50 was defined as the MIC required to inhibit the growth of 50% of the C. jejuni isolates. In addition, multi-drug resistance (MDR) was defined as resistance to three or more antibiotics in this study. C. jejuni ATCC33560 was used as the control.
-
Statistical analysis was carried out using SPSS 16.0 (SPSS Inc., Chicago, IL, USA) and Microsoft Excel 2016. The prevalence and MDR rate of C. jejuni isolated from different sources were compared using the Chi-square test (at a confidence level of 95%). The Simpson's index (D), described previously[23], was used to determine the genetic diversity of C. jejuni genotypes. MIC50 values were calculated using Microsoft Excel 2016.
Sample Collection
Culture, Isolation, and Identification
Multilocus Sequence Typing
Antibacterial Susceptibility Testing
Data Analysis
-
A total of 126 C. jejuni strains were isolated from samples taken in this study. Multilocus sequence typing and antibacterial susceptibility testing were successfully performed in all isolates.
-
The prevalence of C. jejuni from the various sources is shown in Table 1. Statistically, the isolation rate from poultry meat was significantly higher than that from cattle meat (χ2 = 36.222, P < 0.001). However, the isolation rate from poultry cloacal swabs was lower than that from cattle stool (χ2 = 15.950, P < 0.001).
Source No. of Samples No. of Isolates Prevalence of C. jejuni (%) Poultry meat 63 23 36.5 Cattle meat 93 1 1.1 Cattle feces 103 59 57.3 Poultry cloacal swab 74 20 27.0 Patients' feces 437 23 5.3 Table 1. Prevalence of C. jejuni in Poultry, Cattle, and Diarrheal Patients in Shenzhen
The patients with C. jejuni ranged in age from 7 months to 43 years old. Three strains were isolated from 7, 8, and 9 month-old infants. The overall prevalence of C. jejuni in the patients with diarrhea was 5.3% (23/437). Separately, the prevalence of C. jejuni was 4.0% (7/173) in child patients, 6.9% (4/58) in adolescent patients, and 5.8% (12/206) in adult patients (≥ 18 years old). No statistically significant difference was found in the prevalence of C. jejuni between the different age groups (χ2 = 0.955, P = 0.620).
-
We identified 62 STs among the 126 isolates (Table 2). Among them, 27 STs were not reported previously, and 10 novel alleles were found in this study: GlyA734, 735, 736, 737, and 738; pgm894, 896, 897, and 898; and tkt699. Isolates from patients with diarrhea had the highest proportion of new STs (12/23, 52%), followed by those isolated from poultry sources (22/43, 51.0%), and those from cattle sources (7/60, 11.7%).
Poultry Source Cattle Source Patients Source ST Clone Complex Number of Isolates ST Clone Complex Number of Isolates ST Clone Complex Number of Isolates 464 464 3 19 21 7 51 443 1 2145 574 1 22 22 13 354 354 1 2895 574 2 45 45 1 403 403 2 4324 NAa 1 51 443 2 1811 21 1 6493 NA 2 61 61 6 1919 52 1 6607 NA 3 113 460 1 2131 NA 1 6909 574 2 257 257 6 2132 NA 1 6913 464 1 354 354 3 2328 NA 1 6962 NA 1 403 403 5 4265 52 1 7456 NA 1 583 45 3 8003 403 1 7866 NA 1 2089 NA 2 8880 574 2 8089 NA 2 5799 443 1 8901 52 1 8847 NA 1 6500 21 1 8904 45 1 8877b 45 2 7469 464 1 8905 354 1 8879 NA 2 7695 21 1 8906 52 1 8880 574 1 8896* NA 2 8907* NA 1 8881* NA 1 8898 21 4 8908 NA 1 8883* NA 2 8934 464 1 8909 NA 1 8884 NA 4 8910 21 1 8886 NA 1 8911* 362 1 8887* NA 2 8935* 403 1 8889* NA 2 8892 NA 1 8893* NA 1 8894* 45 1 8895 NA 1 8936 NA 1 Total 43 60 23 Note. aNA, not assigned; bNew STs are given in bold; *ST contains the new allele unreported previously. Table 2. ST Type of 126 C. jejuni Isolates from Shenzhen
Eighty-six isolates belonged to 13 clonal complexes, while 40 isolates could not be assigned to any known clonal complex. The most frequently observed clonal complexes were ST-21 (11.9%), ST-22 (10.3%), and ST-403 (7.1%).
Separately, 27 STs were obtained from 43 poultry isolates, and 18 STs were obtained from 60 cattle isolates. The calculated Simpson's index of the STs from the poultry isolates was 0.977, versus 0.912 from the cattle isolates.
Moreover, ST-21, ST-45, ST-354, ST-403, and ST-443 overlapped between isolates from patients and cattle, while ST-45 and ST-574 overlapped between isolates from patients and poultry. In addition, ST-45 was the only clonal complex found in all three sources.
-
The MICs of the quality control strain (C. jejuni ATCC 33560) were within the reference quality control range. Overall, all the isolates were resistant to at least one antibiotic. The highest resistance rate was detected for ciprofloxacin (89.7%), followed by tetracycline (74.6%), and nalidixic acid (69.0%). The resistance rates of C. jejuni from different sources are shown in Figure 1.
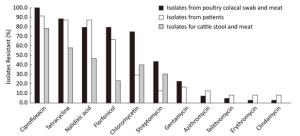
Figure 1. The antibiotic resistance rate of C. jejuni isolated from different sources in Shenzhen. The x-axis represents the antibiotics. The y-axis represents the percentage of resistant isolates. The black area represents the resistance rate of poultry isolates, the white area represents the resistance rate of patient isolates, and the grey area represents the resistance rate of cattle isolates.
The MIC50s of nalidixic acid and tetracycline to all C. jejuni isolates were ≥ 64 μg/mL, while the MIC50s of erythromycin, azithromycin, gentamycin, telithromycin, and clindamycin to all C. jejuni isolates were ≤ 2 μg/mL. For the different sources, C. jejuni from poultry had higher or equal MIC50 s compared with those from patients or cattle against all 11 antibiotics.
For multidrug resistance (MDR), 88.4% of C. jejuni isolated from poultry were resistant to four to nine antibiotics. Among them, one strain was resistant to nine antibiotics, including erythromycin, azithromycin, nalidixic acid, ciprofloxacin, gentamycin, streptomycin, chloromycetin, telithromycin, and clindamycin. This strain was isolated from a chicken cloacal swab from a poultry wholesaler. Similarly, 73.9% of isolates from patients with diarrhea were resistant to four to eight antibiotics. The most prevalent resistance pattern was combined nalidixic acid, ciprofloxacin, florfenicol, and tetracycline resistance (30.4%). In particular, 65.2% of isolates from patients were resistant to florfenicol. In contrast, all C. jejuni from cattle were resistant to less than five antibiotics. None of the isolates from cattle were resistant to erythromycin, azithromycin, gentamycin, telithromycin, or clindamycin.
Statistically, the MDR rate among the isolates from poultry (97.6%, 42/43) was significantly higher than that from cattle (60.0%, 36/60) (χ2 = 19.343, P < 0.001). No statistical significance was observed between the MDR rate of isolates from poultry and patients (91.3%, 21/23) (χ2 = 1.401, P = 0.236).
Prevalence of C. jejuni from Poultry, Cattle, and Patients with Diarrhea
Multilocus Sequence Typing
Antibacterial Susceptibility Testing
-
C. jejuni is one of the major causes of gastroenteritis worldwide. However, there is limited information about the genetic and antibiotic resistance characteristics of C. jejuni in Shenzhen, which makes tracking the origin of transmission challenging. In this study, we conducted multilocus sequence typing and antibiotic susceptibility testing for C. jejuni from poultry, cattle, and patients with diarrhea in Shenzhen.
Several studies of Campylobacter prevalence in children have been conducted previously. For instance, Wang et al.[24] reported no Campylobacter infections in patients with diarrhea in Beijing, either in children (under 5 years, 0/1422) or adults (0/1047). Zhu et al.[25] reported that 2.9% of children with diarrhea were infected by Campylobacter in Wuhan (Southwest China). Our study revealed a C. jejuni prevalence of 4.0% (7/173) in children with diarrhea (≤ 5 years old) and 5.8% (12/206) in adults with diarrhea (≥ 18 years old). The discrepancy in prevalence may be caused by regional differences or the isolation methods that we adopted, which was a standard enhanced filtration based method incorporating a commercial kit.
Poultry are considered as the major reservoir of Campylobacter spp. The prevalence of C. jejuni in poultry is believed to be higher than that in cattle. However, in the present study, the prevalence of C. jejuni in poultry cloacal swabs was only 27.0%, versus 57.3% in cattle feces (Table 1). This unexpected result may have been caused by the sampling method. For poultry, cloacal swabs were collected, whereas feces were collected from cattle. Compared with feces, swabs carry lower sample volume, which may have lead to a lower isolation rate of C. jejuni in poultry.
Moreover, in this study, the prevalence of C. jejuni in cattle meat (1.1%) was significantly lower than that in poultry meat (36.5%) (Table 1). In addition to the different prevalence of C. jejuni in poultry and cattle, fecal contamination during evisceration may have contributed to this discrepancy. Poultry feathers are plucked and then birds are eviscerated. During evisceration, the intestines can rupture, leading to contamination of the skin of the carcass. For cattle, after evisceration, the hide is removed; therefore, contamination of the carcass is less likely. Since the skin on poultry carcasses is retained and the hide of cattle is not, the risk of fecal contamination is higher in poultry than in cattle.
Ma et al.[10] reported that the most prevalent clonal complexes were ST-354 and ST-21 in C. jejuni from retail chickens in Tianjin. Zhang et al.[26] also reported that the ST-21 complex was the most common clonal complex of C. jejuni among human isolates and chicken isolates in north China. Conversely, in our study, no ST-21 complexes were found in the poultry isolates. In addition, 51.0% of STs from poultry isolates were newly reported. Our results suggested that the C. jejuni isolated from poultry in Shenzhen may be independent to other areas, although sampling bias cannot be excluded.
Islam et al.[27] reported that the ST-403 complex was the most prevalent lineage among 49 C. jejuni strains recovered from patients with enteritis or GBS. In the present study, we found nine C. jejuni isolates that belonged to the ST-403 complex; four of them were isolated from patients and were resistant to five to eight antibiotics, while five of them were isolated from cattle and were resistant to three antibiotics. The strong antibiotic resistance of ST-403 complex isolates from patients requires further research.
In a previous study, the ST-45 complex was identified from raw or undercooked meat, as well as from contact with dogs or cats[28]. In addition, the ST-45 complex has been found in penguins on the Antarctic[29], implying that this complex has a wide host range and is environmentally well adapted. In our study, we identified eight isolates that could be assigned to the ST-45 complex; four of them were from cattle, three were from poultry, and one was from a patient. The ST-45 complex was the only clonal complex that overlapped with all three sources in this study, suggesting that the ST-45 complex is widespread in Shenzhen.
A high prevalence of resistant C. jejuni has been reported in China previously[11, 20, 26], and ciprofloxacin, nalidixic acid, and tetracycline were the most commonly reported resistance pattern. Similarly, our study found the highest resistant rate occurred for ciprofloxacin (89.7%), followed by tetracycline (74.6%), and nalidixic acid (69.0%). However, 65.2% of C. jejuni from patients were resistant to florfenicol in this study, which has been barely reported before. Florfencicol is only permitted for veterinary use; therefore, further investigation into the reason for this high resistance to florfenicol is needed.
More research is needed to track the origin of transmission. Nevertheless, we believe this to be the first report on the genetic and antibiotic resistance of C. jejuni in Shenzhen. Based on the genetic characteristics of C. jejuni isolates identified in this study, our data may facilitate the development of pathogen tracking in Shenzhen.
-
We thank our colleagues in the four medical facilities for collecting the patients' stool samples.
The United States Environmental Protection Agency through its Office of Research and Development funded and managed the research described here. It has been subjected to Agency review and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
-
ZHANG Mao Jun, Duan YONG Xiang, LU Jing Rang, and JU Chang Yan participated in the design and coordination of the study, analyzed the data, and wrote the manuscript. MA Yan Ping, YU Mu Hua, CHEN Hui, LIU Chu Yun, GU Yi Xin, and FU Yan Yan participated in the sample collection, performed the experiments, and analyzed the data.
-
The authors have no conflicts of interest to disclose.
Shenzhen Technology and Innovation Plan, China 20140416095154399
Sanming Project of Medicine in Shenzhen SZSM201803081
Nanshan District Technology and Innovation Plan, Shenzhen, China 2016064







 Quick Links
Quick Links
 DownLoad:
DownLoad: