-
Active vitamin D (1, 25D) is a fat-soluble vitamin that is mainly produced in the skin by the conversion of 7-dehydrocholesterol under ultraviolet light stimulation. Its role in calcium homeostasis, bone growth, and prevention of rickets and osteomalacia has been known for over two hundred years. Its biological functions also include immune regulation, anti-cancer properties, and regulation of brain development; its deficiency is associated with many pathological conditions[1]. The activities of 1, 25D are mainly mediated through its binding to a nuclear receptor (VDR) that drives gene expression. It promotes innate antimicrobial responses by increasing the production of cathelicidin and defensin beta 4, induction of reactive oxygen intermediates, and antibacterial autophagy. Its effects on specific immunity include suppression of Th1 and Th17 differentiation, upregulation of Foxp3 transcription, and differentiation of regulatory T cells (Tregs). These effects are mainly effected by blocking dendritic cell (DC) differentiation and inhibiting cytokine production[2]. Supplementation with 1, 25D is considered a simple, safe, and inexpensive procedure that is being used to achieve normal levels of vitamin D. In certain pathological conditions involving bones, metabolism, and the immune system, supraphysiological doses of 1, 25D have triggered favorable results. The administration of a given dose has, however, been associated with variable serum levels of this substance in different patients. Many factors may contribute to this inter-individual variation in response to vitamin D supplementation, including basal 1, 25D levels, body mass index, seasons, calcium and fat intake, and genetic background, including genetic polymorphisms in the 1, 25D pathways[3]. A classically described side effect related to vitamin D supplementation is hypercalcemia triggered by intestinal absorption of dietary calcium. Vitamin D can be therapeutically applied to other conditions besides its classical use to promote calcium and phosphate absorption. Higher doses of this hormone are usually required for these alternative applications[4]. Vitamin D analogs with reduced calcemic activity were, therefore, synthesized. Paricalcitol is a vitamin D analog commercialized under the trade name Zemplar®. It is usually prescribed for treatment of secondary hyperparathyroidism associated with chronic renal failure. Paricalcitol's immunomodulatory activities are still being validated, but it was demonstrated to be able to impair the differentiation of immature DCs[5]. Considering supplementation with 1, 25D or paricalcitol has the potential to be used as an adjunct therapy for many inflammatory diseases, the main objective of this investigation was to compare the immunomodulatory effects of supraphysiological amounts of 1, 25D in C57BL/6 and DBA/1 mice. The effects of 1, 25D were also compared with the ones triggered by paricalcitol as this analogue does not trigger hypercalcemia and may be, theoretically, more appropriate for immunomodulatory purposes.
C57BL/6 (n = 45) and DBA/1 (n = 39) female mice, 9-11 weeks old, bought from Ribeirão Preto Medical School (Brazil), were intraperitoneally injected with eight doses (0.1 μg each) of either 1, 25D or paricalcitol every other day for 15 days. Mice injected with the vehicle (15% ethyl alcohol) were used as controls. Body weight was assessed daily. One day after the last dose (16th day), the animals were euthanized, and the following parameters were analyzed in the spleen: cytokine production, percentages of DCs and Tregs, and median fluorescence intensity (MFI) of major histocompatibility complex class Ⅱ (MHCII); calcium serum levels were also determined. This experimental design is illustrated by a timeline scheme in Figure 1A. A second set of experiments were performed to evaluate the effect of a higher concentration (0.2 μg) of 1, 25D and paricalcitol in DBA/1 mice (n = 22). Animals received sterilized water and food (Presence®) ad libitum. The food already contained physiological levels of this vitamin. Experimental procedures were approved by the Ethics Committee on Use of Animals - Institute of Biosciences of Botucatu (Protocol number 815). 1, 25D (1α, 25-dihydroxyvitamin D3) and paricalcitol (Zemplar®) were purchased from Sigma-Aldrich and Abbott Laboratories, respectively. The following monoclonal antibodies were used in flow cytometry: PerCP-labeled anti-mouse CD3, FITC-labeled anti-mouse CD4, APC-labeled anti-mouse CD25, PerCP-labeled anti-mouse F4/80, FITC-labeled anti-mouse CD11c, APC-labeled anti-mouse MHCII, and PE-labeled anti-mouse CD86. A forkhead box P3 (Foxp3) PE Staining Set was also used for intracellular staining. All reagents for flow cytometry were purchased from eBioscience (San Diego, CA, USA). Calcium levels were determined using Calcio Arsenazo Ⅲ commercial kit (Bioclin, Belo Horizonte, MG, Brazil). Spleens were disrupted by gentle meshing through cell strainers (70-μm). Cells were lysed with buffer containing NH4Cl, adjusted to 5.106 cells/mL, and then cultured in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% fetal calf serum, 2 mmol/L L-glutamine, and 10 μg/mL concanavalin A. Cytokine levels were evaluated 48 hours later by ELISA using IL-2, IL-5, IFN-γ, and IL-10 OptEIA Sets (BD Biosciences, San Diego, CA, USA), and IL-6, IL-17, and TNF-α DuoSets (R & D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions. For Tregs analysis, cells were incubated with 0.2 μg PerCP-Cy5.5-labeled anti-mouse CD3, 0.25 μg FITC-labeled anti-mouse CD4 and 0.25 μg APC-labeled anti-mouse CD25 for 30 min at 4 ℃. The cells were washed and intracellularly stained with 0.2 μg PE-labeled anti-mouse Foxp3 transcription factor according to the manufacturer's instructions (eBiosciences, San Diego, CA, USA). For DC analysis, spleen cells were incubated with 0.1 μg PerCP-labeled anti-mouse F4/80, 0.25 μg FITC-labeled anti-mouse CD11c, 0.03 μg APC-labeled anti-mouse MHCII, and 0.125 μg PE-labeled anti-mouse CD86 for 30 min at 4 ℃. The cells were then washed, resuspended in flow cytometry buffer, and fixed in 1% paraformaldehyde. Cells were analyzed by flow cytometry using FACSCanto Ⅱ (Becton Dickinson, San Jose, CA, USA) for data acquisition and FlowJo software (TreeStar, Ashland, OR, USA) for Tregs and DCs analyses. Results were expressed as mean ± standard deviation or median (25%-75%). Group comparisons were made by one-way ANOVA followed by Tukey's test for parametric variables, and by Kruskal-Wallis test followed by Dunn's test for non-parametric variables. Body weight variation analysis was performed by two-way ANOVA followed by Tukey's test. Statistical analysis was carried out using SigmaPlot 12.0 software (Systat Software Inc., San Jose, CA, USA) and P < 0.05 was considered significant. Prism 6.0 software (GraphPad Software Inc., La Jolla, CA, USA) was used for graphic design.
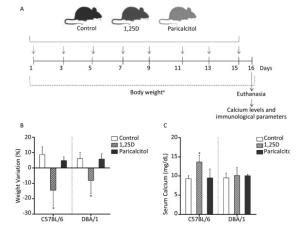
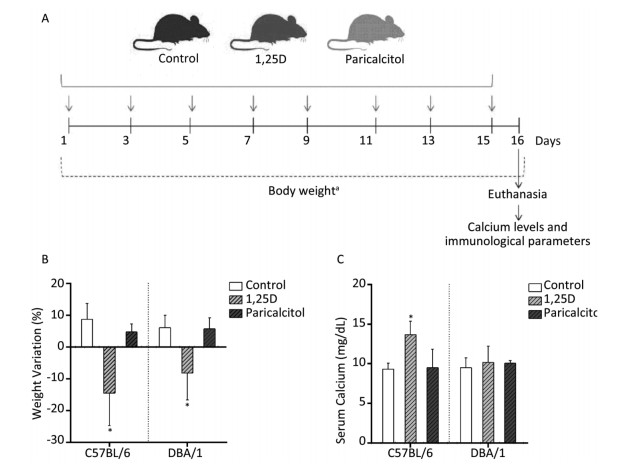
Figure 1. Effect of 1, 25D and paricalcitol on body weight and serum calcium levels in C57BL/6 and DBA/1 mice. Mice were intraperitoneally injected with 0.1 μg of 1, 25D or paricalcitol every other day for 15 days, as depicted in the timeline schematic (A). Variation in body weight (B) and serum calcium levels (C) in response to administration with 1, 25D, paricalcitol or 15% ethyl alcohol (control). Data are presented as mean ± SD of four to six animals per group. *P < 0.05 vs. control. Representative results from three and two experiments for body weight and calcium levels, respectively.↓Administration of 1, 25D or paricalcitol, aDaily evaluations.
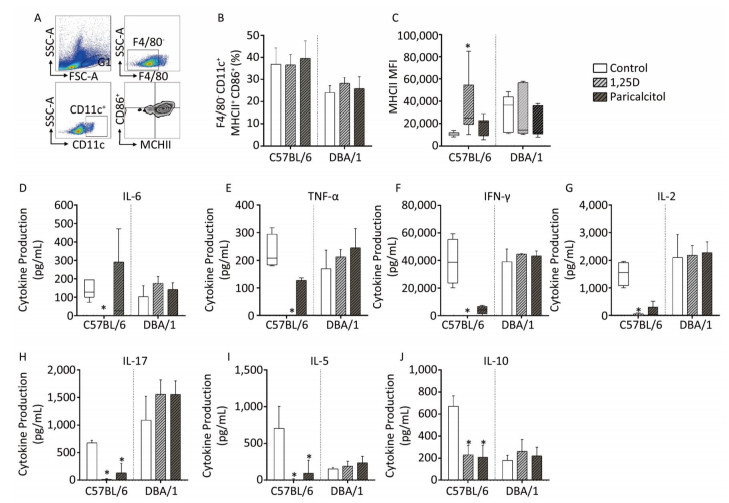
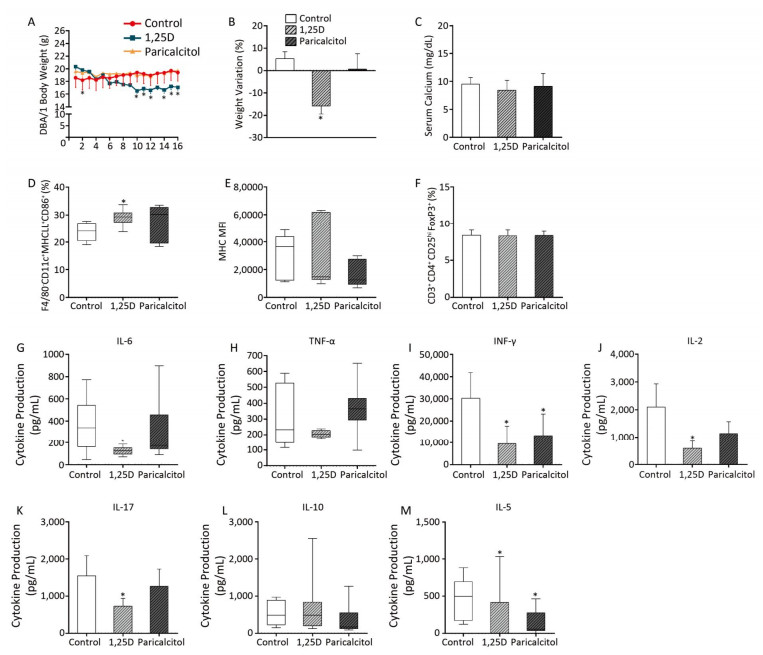
Both mice strains lost weight, but this effect was observed earlier (not shown) and was more pronounced in the C57BL/6 strain. Percentage of total body weight variation at the end of the experiment is illustrated in Figure 1B. The results indicate that 1, 25D resulted in significant body weight loss in both strains. Conversely, paricalcitol administration did not trigger any alteration in the body weight of these two strains. To check if supplementation with 0.1 μg of 1, 25D or paricalcitol was modifying calcium concentration, this element was quantified in serum samples. As displayed in Figure 1C, 1, 25D significantly increased calcium levels in C57BL/6 mice, but not in DBA/1 mice; paricalcitol administration did not affect calcium levels in any strain. The percentage of DCs, immunophenotyped as F4/80-CD11c+MHCII+CD86+ cells was not affected by 1, 25D or paricalcitol in C57BL/6 and DBA/1 mice, as depicted in Figure 2B. However, 1, 25D significantly increased the MIF of MHCII expression in C57BL/6, but not in DBA/1 as shown in Figure 2C. The percentage of Tregs, immunophenotyped as CD3+CD4+CD25hiFoxp3+cells, was not altered by any of these two substances in C57BL/6 and DBA/1 mice (not shown). However, 1, 25D supplementation was able to significantly downregulate the production of IL-6, TNF-α, IFN-γ, IL-2, IL-17, IL-5, and IL-10 production in C57BL/6 mice (Figure 2D-J). Paricalcitol triggered a similar, but not as pronounced, downregulatory effect. Neither 1, 25D nor paricalcitol, at this dose, changed the production of these cytokines in DBA/1 mice. To test the possibility that DBA/1 mice required higher doses of 1, 25D and paricalcitol to be immunomodulated, these mice were injected with 0.2 μg of each substance. As observed in Figure 3A and 3B, administration with a higher dose of 1, 25D, but not paricalcitol, resulted in body weight loss; these higher doses did not increase serum calcium levels, as shown in Figure 3C. Except for the percentage of DCs, which was increased by administration with a dose of 0.2 μg of 1, 25D (Figure 3D), the MHCII MFI and the percentage of Tregs were not altered by this higher concentration of 1, 25D or paricalcitol, as shown in Figure 3E and F. The higher 1, 25D dose was, however, able to significantly decrease IL-6, IFN-γ, IL-2, IL-17, and IL-5 expression (Figure 3G, I, J, and M).
Figure 2. Phenotypic analysis of DCs and Tregs and cytokine production in spleen of C57BL/6 and DBA/1 mice injected with 1, 25D or paricalcitol. Gate strategy for F4/80-CD11c+MHCII+CD86+DCs (A). Percentage of F4/80-CD11c+MHCII+CD86+ cells (B). MHCII MFI in C57BL/6 and DBA mice (C). Levels of IL-6 (D), TNF-α (E), IFN-γ (F), IL-2 (G), IL-17 (H), IL-5 (I), and IL-10 (J) detected through ELISA. Data are presented as mean ± SD or median (25%-75%) of seven to thirteen animals per group. *P < 0.05 vs. control. Representative results from two and three experiments were combined for C57BL/6 and DBA/1, respectively.
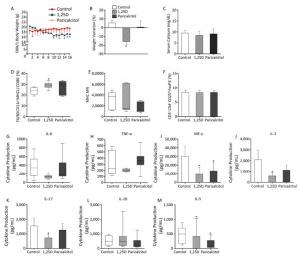
Figure 3. Effect of a higher 1, 25D and paricalcitol dose on clinical and immunological parameters of DBA/1 mice. Body weight kinetics (A), body weight variation (B), calcium levels (C), percentage of F4/80-CD11c+MHCII+CD86+ DCs (D), MHCII MFI (E), and percentage of CD3+CD4+CD25hiFoxp3+ Tregs (F). Levels of IL-6 (G) TNF-α (H), IFN-γ (I), IL-2 (J), IL-17 (K), IL-10 (L), and IL-5 (M) detected through ELISA. Data are presented as mean ± SD or median (25%-75%) of three to five animals per group. *P < 0.05 vs. control. Representative results from two experiments.
This paper shows, as was hypothesized, that distinct mice strains differ in their response to vitamin D supplementation. The initial protocol (0.1 μg) indicated that 1, 25D, but not paricalcitol, significantly decreased body weight in both strains. The therapeutic potential of vitamin D alone or associated with Ca2+ has been pointed out in obesity. Binding of 1, 25D to VDR interrupts adipogenesis and high levels of 1, 25D and Ca2+ intake activate mediators of apoptosis in adipose tissue. Recent data also indicate that vitamin D can act directly on the hypothalamus to lower food consumption[6].
The presence of more immature DCs expressing lower levels of MHCII and a higher frequency of Tregs were expected but not observed in vitamin D/paricalcitol-treated mice. An increased level of MHCII expression was associated with 1, 25D supplementation in C57BL/6 mice. These results may be due to the adopted experimental model, the absence of a concomitant antigenic stimulus, 1, 25D/paricalcitol dose, or administration route. These possibilities are, at least partially, supported by other authors. Sochorová et al., [7] for example, observed that differentiated DCs are less sensitive to 1, 25D than early progenitor cells. Gambhir et al.[8] demonstrated that certain 1, 25D inhibitory activities are triggered only if binding to VDR occurs before antigenic stimulation. Epicutaneous and oral routes, but not other routes, triggered a substantial increase in the percentage of Tregs[9]. The fact that cytokines were highly downregulated by 1, 25D and, to some extent, by paricalcitol in C57BL/6 without a simultaneous alteration in DC phenotype or percentage of Tregs suggests that these are two independent phenomena. We therefore suggest that vitamin D acts by directly downregulating cytokine gene transcription. An indirect pathway, such as that to maintain DCs in an immature state, would allegedly require the presence of an antigen. Unlike those from C57BL/6, DBA/1 spleen cells were not downmodulated by vitamin D and paricalcitol. This variable response to vitamin D verified in mice strains can also occur during human supplementation, particularly in the case of autoimmune pathologies related to immune regulation. The higher 1, 25D dose was able to downregulate some of the evaluated cytokines in the DBA strain; however, this mouse strain remained unresponsive to paricalcitol, even at a higher dose. Considering that 1, 25D interaction with its target cells is mediated by VDR and that VDBP provides a reservoir for this hormone, it is possible that this phenomenon is related to differences in VDR and VDBP expression. The work of Safadi et al.[10] supports this idea by demonstrating that 1, 25D was rapidly metabolized by the liver in C57BL/6J mice deficient in VDBP. Interestingly, a recent paper showed that 1, 25D serum levels are higher in C57BL/6 than in DBA/1 mice[11].
Even though the translation of these findings to clinical practice requires further investigation, we believe that they suggest the need of a more rational vitamin D prescription. In this sense, we highlight the need to check both the blood vitamin D levels and the biological response of each patient during supplementation. We also believe that the analogs need to be carefully compared to vitamin D with regard to their use in controlling inflammation, kidney pathologies, and osteoporosis.
Three main conclusions can be drawn from this study: C57BL/6 and DBA/1 mice differ in their response to supraphysiological supplementation with 1, 25D and paricalcitol; cytokine production by C57BL/6, but not by DBA/1, is the immunological parameter more intensely downmodulated by 1, 25D and its analog; DBA/1 mice require higher doses of 1, 25D and paricalcitol to be slightly immunomodulated.
In the context of these findings, we believe that further investigation is of paramount importance to reveal if a similar variation exists in human beings. Characterization of the immunomodulatory properties of vitamin D analogs is also necessary. These studies would allow a more rational and appropriate use of 1, 25D and its analogs.
C57BL/6 and DBA/1 Mice Differ in Their Response to Supplementation with 1, 25D and Paricalcitol
doi: 10.3967/bes2018.083
São Paulo Research Foundation (FAPESP) 2013/26257-8
- Received Date: 2018-03-19
- Accepted Date: 2018-08-01
| Citation: | Aline Parisoto Missio, Thais Fernanda de Campos Fraga-Silva, Larissa Lumi Watanabe Ishikawa, Luiza Ayumi Nishiyama Mimura, Thais Graziela Donegá França, Larissa Ragozo Cardoso de Oliveira, Alexandrina Sartori, Sofia Fernanda Gonçalves Zorzella-Pezavento. C57BL/6 and DBA/1 Mice Differ in Their Response to Supplementation with 1, 25D and Paricalcitol[J]. Biomedical and Environmental Sciences, 2018, 31(8): 613-618. doi: 10.3967/bes2018.083 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: