-
Vibrio parahaemolyticus, the leading cause of seafood-borne gastroenteritis, has the ability to form biofilms on biotic and abiotic surfaces. Biofilm formation is a complicated process involving many specific structures and regulatory processes. The most significant of the structures and processes include polar and lateral flagella, mannose-sensitive hemagglutinin type Ⅳ pili, chitin-regulated pili, capsular polysaccharide (CPS), exopolysaccharide (EPS), cyclic-di-GMP, and quorum sensing[1]. Some transcriptional regulators, such as OxyR[2], VPA0606[3], and CpsR[4], are involved in the regulation of V. parahaemolyticus biofilms. However, the full details of the regulation of V. parahaemolyticus biofilm formation are unknown.
ToxR was originally described as a virulence regulator of V. cholerae. ToxR activates ctx and tcp genes, which encode the toxin co-regulated pilus and cholera toxin, respectively[5]. Later studies showed that ToxR is also involved in regulating biofilm formation in V. cholerae[6], V. alginolyticus[7], and V. anguillarum[8]. We previously reported that ToxR controls V. parahaemolyticus virulence at least partly by regulating the thermostable direct hemolysin, type Ⅲ secretion system 1, and system 2[9, 10]. However, whether ToxR has regulatory actions on biofilm formation in V. parahaemolyticus is still obscure.
In the present study, the pandemic V. parahaemolyticus strain RIMD 2210633 (wild type, WT) and its nonpolar toxR mutant (designated ΔtoxR)[10] and the complementary mutant (ΔtoxR/pBAD33-toxR, designated C-ΔtoxR)[10] were employed to investigate the roles of ToxR in V. parahaemolyticus biofilm formation using different biofilm-related assays.
The WT and ΔtoxR strains were grown overnight in HI broth (2.5% Bacto Heart Infusion) or M broth (3.75% Difco Marine broth 2216) at 37 ℃ with shaking at 200 rpm. The resulting cultures were diluted 50-fold into 15 mL of corresponding fresh HI or M broth, and allowed to grow to reach an optical density at 600 nm (OD600) value of approximately 1.0. Thereafter, the cultures were diluted 1000-fold into 15 mL of corresponding fresh culture medium for further cultivation. The OD600 values were measured hourly for each culture (Supplementary Figure S1, available in www.besjournal.com). Bacterial growth was slightly slower in the M broth relative to the HI broth, but the temperature seemed to have no effect on the growth rates in the same medium. In addition, the two strains showed similar growth rates under each growth condition. Thus, the deletion of toxR had no evident effect on the in vitro growth of the pandemic V. parahaemolyticus strains.
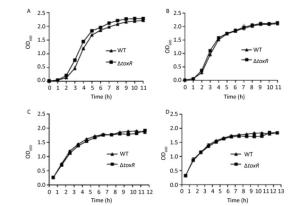
Figure Supplementary Figure S1. Growth curves of WT and ∆toxR V. parahaemolyticus. A representative sample from three independent experiments is shown. (A) Grown in the HI broth at 30 ℃; (B) Grown in the HI broth at 37 ℃; (C) Grown in the M broth at 30 ℃; (D) Grown in the M broth at 37 ℃.
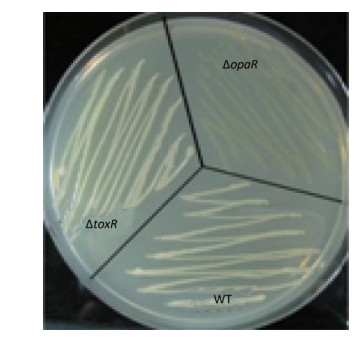
V. cholerae EI Tor strain A1552 is unable to form rugose colonies, while approximately 35% ± 10% of toxR mutants display this colonial morphology[6]. Thus, ToxR is likely required for the variation of the smooth and rugose colony morphologies in Vibrio spp. Here, overnight growth cultures of V. parahaemolyticus strains in HI broth were diluted 50-fold into 3 mL of M broth and incubated without shaking at 30 ℃ for another 4 days. Thereafter, the cultures were mixed thoroughly, and 2 μL of each culture was spotted on HI agar containing 0.1% arabinose and 5 μg/mL chloramphenicol to investigate the effects of ToxR on V. parahaemolyticus colony morphology. As shown in Figure 1, ΔtoxR/pBAD33 (ΔtoxR harboring the empty pBAD33 plasmid) produced smooth colonies, WT/pBAD33 that produced rugose colonies, and C-ΔtoxR bacteria produced slightly smoother colonies than the WT strain. The switching of colony appearance from smooth to rugose colonies was due to the yield of EPS, which is attributed to the expression of the VPA1403-1412 (cpsA-J) operon in V. parahaemolyticus[1]. The data indicated that the expression of cpsA-J was under the positive control of ToxR in V. parahaemolyticus.
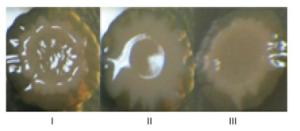
Figure 1. Colony morphology of V. parahaemolyticus strains. Bacteria were spotted on HI agar and incubated at 30 ℃ for 4 days. Ⅰ, Ⅱ, and Ⅲ represent WT/pBDA33, ΔtoxR/pBDA33, and C-ΔtoxR, respectively.
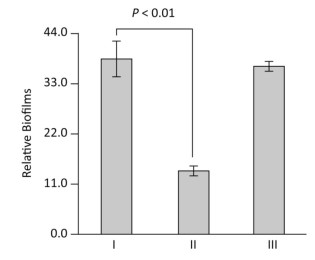
The production of mature biofilms is closely correlated with the expression of EPS genes in V. parahaemolyticus[1]. We thus investigated the ToxR-dependent biofilm formation in V. parahaemolyticus using the crystal violet staining assay. The overnight growth cultures were diluted 50-fold into 2 mL of M broth containing 0.1% arabinose and 5 μg/mL chloramphenicol in glass tubes and cultured at 30 ℃ with shaking at 100 rpm for 48 h. The planktonic cells were removed and the surface-attached cells were stained with 0.1% crystal violet. The bound crystal violet in each tube was dissolved with dimethylsulfoxide and the OD at 570 nm (OD570) was measured. As shown in Figure 2, the ΔtoxR/pBAD33 strain displayed a remarkable decrease in crystal violet staining of biofilm relative to the WT/pBAD33 and C-ΔtoxR strains, while C-ΔtoxR showed a restored phenotype in the crystal violet staining experiment. These results suggested a positive correlation between ToxR and the in vitro biofilm production in V. parahaemolyticus.
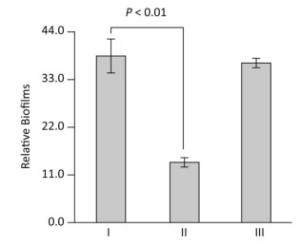
Figure 2. ToxR activates biofilm formation in vitro. V. parahaemolyticus was grown in the M broth in glass tubes. The bacterial mass attached to the liquid-solid interface was stained by crystal violet and the OD570 was determined. Simultaneously, the planktonic cells were used for measurement of the OD600 values. The relative binding of crystal violet by biofilms was determined by using the formula: 100 × (OD570/OD600). The experiment was performed using at least three independent bacterial cultures, and the values are expressed as the mean ± standard deviation (SD). Paired Student's t-test was used to calculate statistically significant differences. P < 0.01 indicated statistical significance. Ⅰ, Ⅱ, and Ⅲ represent WT/pBDA33, ΔtoxR/pBDA33, and C-ΔtoxR, respectively.
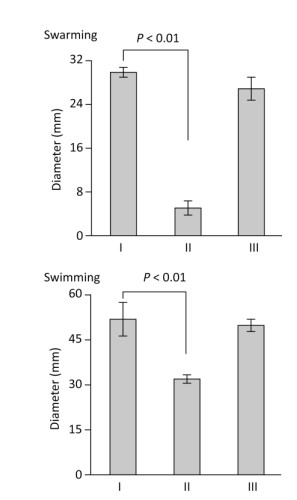
Flagella-mediated motility enables bacteria to rapidly colonize host cells and to form mature biofilms[11, 12]. Polar flagellum gene mutants are defective in attachment and biofilm formation[3]. Thus, the swarming and swimming motility capacities of V. parahaemolyticus strains were detected on the surface of solid swarm medium comprised of 2.5% Bacto HI, 1.5% NaCl (Merck), 2.0% Difco noble agar (BD Bioscience), and 0.1% arabinose, and using semi-solid swimming plate medium comprised of 1% Oxoid tryptone, 2% NaCl (Merck), 0.5% Difco noble agar (BD Bioscience), and 0.1% arabinose (Figure 3). The capacities of the two types of motility were dramatically decreased in ΔtoxR relative to WT and C-ΔtoxR, while C-ΔtoxR displayed similar motility phenotypes as WT. These findings indicated that ToxR acts as an activator of both swarming and swimming motility in V. parahaemolyticus.
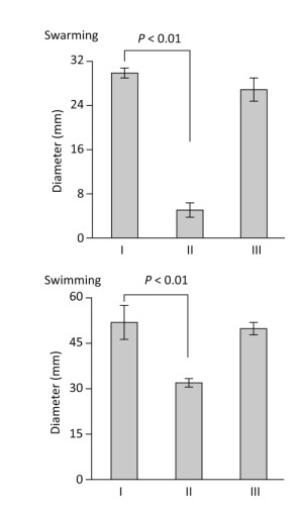
Figure 3. ToxR is required for swarming and swimming motility. The motility capacities of V. parahaemolyticus strains were evaluated by measuring the diameter of bacterial colonies. The experiment was performed at least three times, and the results are expressed as the mean ± standard deviation (SD) and analyzed by Paired Student's t-test. Ⅰ, Ⅱ, and Ⅲ represent WT/pBDA33, ΔtoxR/pBDA33, and C-ΔtoxR, respectively.
V. parahaemolyticus switches between an opaque colony type and a translucent colony type. The switch has been associated with the production of CPS[1]. CPS content has been inversely related to biofilm formation in other bacteria[1]. Strains that failed to produce CPS did not produce opaque colonies and were defective in pellicle formation in microtiter wells and in a biofilm attachment assay[4]. Thus, the regulatory action of ToxR on the opaque-translucent switching was presently investigated. Aliquots (2 μL) of overnight cultures were inoculated onto HI agar and incubated at 37 ℃ for 24-48 h. The surface morphologies of the bacterial colonies were photographed. As shown in Supplementary Figure S2 (available in www.besjournal.com), both ΔtoxR and WT displayed the opaque colonies, while the ΔopaR positive control displayed translucent colonies[13]. These results indicated that ToxR has no regulatory activities on the opaque-translucent switching and CPS production in V. parahaemolyticus.
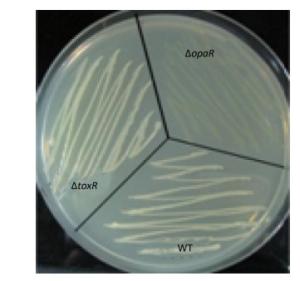
Figure Supplementary Figure S2. Opaque and translucent phenotypes of V. parahaemolyticus strains. Strains were grown overnight at 30 ℃ on HI plate. In the photographs, the opaque colony appears much whiter than the translucent colony.
In summary, our data show that V. parahaemolyticus ToxR is required for biofilm formation and flagellar-mediated motility, which is beneficial for the survival and pathogenesis of V. parahaemolyticus. This study provides a deeper understanding of the function of ToxR in the pandemic V. parahaemolyticus. The molecular mechanisms corresponding to the phenotypic result needs to be further elucidated.
ToxR Is Required for Biofilm Formation and Motility of Vibrio Parahaemolyticus
doi: 10.3967/bes2018.112
the Natural Science Foundation of Jiangsu Province BK20160505
the National Natural Science Foundation of China 81601809
- Received Date: 2018-07-24
- Accepted Date: 2018-11-05
| Citation: | CHEN Long, QIU Yue, TANG Hao, HU Ling Fei, YANG Wen Hui, ZHU Xiao Jue, HUANG Xin Xiang, WANG Tang, ZHANG Yi Quan. ToxR Is Required for Biofilm Formation and Motility of Vibrio Parahaemolyticus[J]. Biomedical and Environmental Sciences, 2018, 31(11): 848-850. doi: 10.3967/bes2018.112 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: