-
Hypoxia is a common pathological process in various clinical diseases and is characterized by abnormal changes in metabolism, function, and morphological structure of tissues resulting from insufficient oxygen supply or oxygen barriers in tissues. In particular, hypoxia in vital organs such as the brain and heart is an important cause of death[1]. The prevention of tissue hypoxia and the treatment of hypoxia-induced tissue damage are urgent issues.
Ginseng (Panax ginseng C. A. Mey) is commonly used as a nutritional dietary supplement and additive. In recent years, there has been increasing interest and research into the pharmacological and active ingredients of ginseng. It is necessary to identify the active ingredients in ginseng before developing new drugs that target hypoxia.
In the present study, we attempted to identify the main effective components of ginseng and elucidate the biological mechanisms by which they act using a combination of network pharmacology and molecular docking, with the aim of ameliorating tissue hypoxia. Moreover, the structural docking of related proteins and compounds provides a theoretical basis for the development of new bioactive components of traditional Chinese medicine.
We identified the active ingredients of ginseng by querying phytochemical databases and screening the relevant literature. We queried the following phytochemical databases: the traditional Chinese medicine systems pharmacology database and analysis platform (TCMSP; http://ibts.hkbu.edu.hk/LSP/tcmsp.php) and the TCM Database @ Taiwan (http://tcm.cmu.edutw/). We also used computer simulations to integrate absorption, distribution, metabolism, and excretion (ADME) models to screen pharmaceutically active ingredients. The ADME model used in the present study was mainly based on oral bioavailability prediction (PreOB). Oral bioavailability (OB) is one of the most important pharmacokinetic properties of oral drugs because it embodies the efficacy of oral drug delivery into the body's circulation[2]. We used a computer screening model (OBioavail 1.1) to calculate the OB values of the active ingredients of ginseng[3]. Finally, compounds with OB values > 30% were considered active ingredients that required further investigation[4]. However, it should be noted that the ginsenosides Re, Rg1, Rg2, Rb1, Rb2, and Rc are all widely regarded as active ingredients of ginseng despite having OB values < 30%. Therefore, the present study also included the ginsenosides mentioned above for further analysis of biological activity. We downloaded the molecular structure files for all the candidate active ingredients from the ChemSpider database (http://www.chemspider.com), and saved them in mol format.
The hypoxia-related potential therapeutic targets included in the present study were derived from two databases: DrugBank (http://www.drugbank.ca/) and the Online Mendelian Inheritance in Man (OMIM) database (http://www.omim.org/). We used the following search keywords: 'hypoxia', 'brain hypoxia', and 'myocardial hypoxia'. We also searched the literature to identify potential therapeutic targets, and converted all the incorporated proteins to the UniProt ID representation. We obtained X-ray crystal structures for all the candidate therapeutic targets from the RCSB protein database (http://www.pdb.org/). The protein structures were pretreated: hydrogen atoms were fixed and cocrystallized ligands and water molecules in protein-ligand complexes were removed. Finally, we used Cytoscape 3.2.0 to build a 'component-target-disease' interaction network.
To clarify the in vivo pathways involved in the treatment of hypoxia with ginseng, we used database for annotation, visualization, and integrated discovery (DAVID) software (http://david.abcc.ncifcrf.gov/home.jsp) based on the Kyoto Encyclopedia of Genes and Genomes database (KEGG, http://www.genome.jp/kegg/) for pathway enrichment analysis. We also used gene ontology (GO) analysis to conduct in-depth analysis of enrichment pathways. Finally, we found key pathways and potential therapeutic targets that would help further analog docking of target proteins and ginseng active ingredients. We used AutoDock 4.2 software to simulate molecular docking to determine the binding affinity of candidate targets with the active ingredients of ginseng.
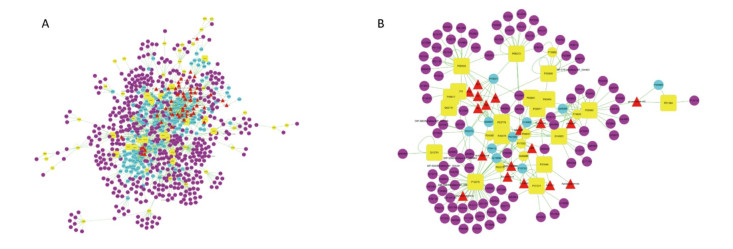
During the database search, we used oral bioavailability (OB ≥ 30%) as the active ingredient screening standard. We identified a total of 52 potential active constituents and 713 potential targets associated with the disease, and constructed an initial interaction network (Figure 1A).
Subsequently, we analyzed the three main topological parameters (Closeness Centrality, Betweenness Centrality, and Degree) of the nodes in the network; the medians of the three topological parameters were 0.25, 0.00, and 1.00, respectively. We re-screened the nodes with topological parameters greater than two times the degree median and greater than the medians of closeness centrality and betweenness centrality as the most relevant nodes. Ultimately, we obtained 52 potential targets that are usually considered targets of higher disease relevance, and constructed a new interaction network (Figure 1B). Figure 1B shows 19 yellow squares that represent the central targets for the direct influence of drugs and diseases. They are generally considered the most valuable potential targets (Supplementary Table S1, available in www.besjournal.com). The 33 circular nodes in the figure represent potential targets that drugs and diseases may affect (Supplementary Table S2, available in www.besjournal.com). Trigonal nodes represent the most relevant active ingredients, which are considered the most effective ingredients in the therapeutic treatment of hypoxia with ginseng. As shown in Figure 1B, a total of 18 active ingredients were considered to be the main effective components (Supplementary Table S3, available in www.besjournal.com).
Canonical Name Closeness Centrality Degree Betweenness Centrality P10275 0.32989691 44 0.11471502 P31645 0.25608195 6 0.000634 P37231 0.27100271 9 0.01100077 P29474 0.29059208 5 0.01292898 O14920 0.31007752 16 0.02883368 P09601 0.25764895 1 0 P09917 0.25764895 1 0 P06213 0.29175784 16 0.03970182 P25963 0.27118644 20 0.02882924 P01584 0.21768707 3 0.0025 P29475 0.19079418 1 0 Q12791 0.2230276 4 0.0025 P35968 0.26569246 12 0.03388688 P60484 0.24883359 3 0.00114724 P00533 0.28776978 23 0.051043 Q92731 0.24813896 6 0.00139398 P56817 0.22346369 2 0.00000279 O76074 0.25340513 5 0.00335647 P53779 0.27453672 4 0.00395467 Table Supplementary Table S1. Directly Acting Disease Target of Ginseng and its Topological Parameters
Canonical Name Closeness Centrality Degree Betweenness Centrality P04637 0.31683168 60 0.14615061 P07900 0.34334764 36 0.12746932 P19793 0.26195154 27 0.03007051 Q00987 0.3160806 25 0.06115837 P03372 0.29563932 23 0.04309506 Q15596 0.31570639 22 0.05120718 Q04206 0.28776978 21 0.03984164 P63279 0.26818639 17 0.03412236 P05412 0.30464585 15 0.03161197 P62988 0.2961866 14 0.06107175 O14965 0.2728513 13 0.02056573 Q96EB6 0.256246 12 0.02087995 P24385 0.28089888 10 0.01394337 P18031 0.28188865 8 0.0092714 P38398 0.28129395 6 0.01475217 O15111 0.27472527 6 0.00620379 Q00653 0.24821595 6 0.000159 Q9Y6K9 0.27350427 5 0.00460599 P32121 0.25982462 5 0.00197333 Q05086 0.26945099 4 0.00155382 Q15653 0.24821595 4 0.000159 Q03135 0.28070175 3 0.00817846 Q93009 0.26263953 3 0.000432 Q9Y265 0.26041667 3 0.000721 Q14643 0.25715204 3 0.000607 O75925 0.25125628 3 0.0000961 Q9Y6X2 0.25125628 3 0.0000961 Q13105 0.25125628 3 0.0000961 Q99558 0.26437541 2 0 Q92993 0.26385224 2 0.000402 Q99933 0.26024723 2 0.000430 Q9UBL3 0.2499219 2 0.000302 P35269 0.24829299 2 0.0025 P10275 0.32989691 44 0.11471502 P31645 0.25608195 6 0.000634 P37231 0.27100271 9 0.01100077 P29474 0.29059208 5 0.01292898 O14920 0.31007752 16 0.02883368 P09601 0.25764895 1 0 P09917 0.25764895 1 0 P06213 0.29175784 16 0.03970182 P25963 0.27118644 20 0.02882924 P01584 0.21768707 3 0.0025 P29475 0.19079418 1 0 Q12791 0.2230276 4 0.0025 P35968 0.26569246 12 0.03388688 P60484 0.24883359 3 0.00114724 P00533 0.28776978 23 0.051043 Q92731 0.24813896 6 0.00139398 P56817 0.22346369 2 0.00000279 O76074 0.25340513 5 0.00335647 P53779 0.27453672 4 0.00395467 Table Supplementary Table S2. Interaction Proteins Associated with Hypoxia and Their Topological Parameters
Compound Name Closeness Centrality Degree Betweenness Centrality paeonol 0.29304029 29 0.0455495 Rg1 0.32560033 55 0.14958158 Rb2 0.29563932 33 0.05028605 beta-sitosterol 0.31695721 37 0.09406137 Deoxyharringtonine 0.24976584 2 0.0000486 suchilactone 0.28673835 15 0.01855356 Inermin 0.24860162 16 0.00487428 Rc 0.27406646 15 0.00457764 Rb1 0.28348689 20 0.0100524 Fumarine 0.29122679 26 0.04537663 ginsenoside rh2 0.27758501 12 0.02548461 DBP 0.29186428 20 0.01846146 Aposiopolamine 0.2302821 8 0.0000611 Rg2 0.32180209 58 0.11351369 kaempferol 0.34692108 59 0.24413376 Frutinone A 0.29487652 16 0.01985047 Linoleic 0.25764895 14 0.00283966 Re 0.2862254 25 0.01329203 Table Supplementary Table S3. Ginseng Active Ingredients and Their Topological Parameters.
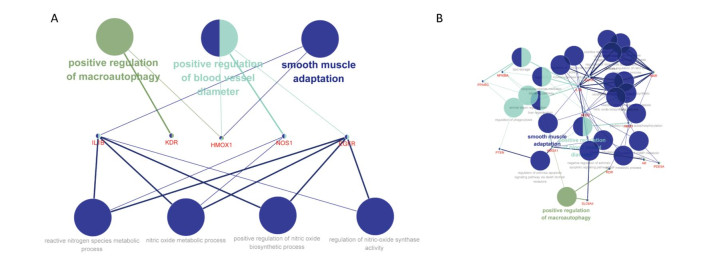
Based on the findings described above, we performed KEGG pathway enrichment for these 52 potential targets. We found that a total of nine pathways are involved in the therapeutic effects of ginseng on human hypoxia (Supplementary Table S4, available in www.besjournal.com). Supplementary Table S4 clearly shows that the P-values of those nine pathways are all less than 0.001, indicating that they are highly correlated with the therapeutic mechanism of ginseng in the treatment of hypoxia. However, the biological effects specifically controlled by these pathways during hypoxia remain unclear. To further clarify the biological effects of the major regulatory pathways involved in the treatment of hypoxia with ginseng, we performed GO analysis of drugs and diseases (Figure 2A). Ultimately, we discovered that ginseng exerts a therapeutic effect on human hypoxia, primarily through three biological pathways: smooth muscle adaptation, positive regulation of blood vessel diameters, and positive regulation of macroautophagy. Five targets directly regulate the three pathways listed above: interleukin-1 beta, heme oxygenase 1, vascular endothelial growth factor receptor 2, epidermal growth factor receptor, and nitric oxide synthase. When used as the main research subjects, these five the 18 potential targets that directly influence targets were found to be involved in the regulation of five other pathways, including the nitric oxide metabolic pathway, nitric oxide biosynthetic pathway, reactive nitrogen species metabolic pathway, nitric oxide synthase pathway, and reactive oxygen species biosynthetic pathway (Figure 2B).
Pathway Count Percent P-Vaule NOD-like receptor signaling pathway 9 17.3 3.90×10-9 PI3K-Akt signaling pathway 15 28.8 8.40×10-8 NF-kappa B signaling pathway 9 17.3 1.60×10-7 FoxO signaling pathway 10 19.2 3.80×10-7 TNF signaling pathway 9 17.3 7.40×10-7 MAPK signaling pathway 12 23.1 1.50×10-6 Toll-like receptor signaling pathway 8 15.4 9.50×10-6 RIG-I-like receptor signaling pathway 7 13.5 9.70×10-6 Neurotrophin signaling pathway 7 13.5 2.10×10-4 Table Supplementary Table S4. Enrichment of KEGG Pathway for Ginseng Treating Hypoxia

Figure 2. Results of the GO analysis of the regulatory pathway pertaining to ginseng hypoxia treatment.
We simulated the docking of 19 active ingredients, which were predicted by network pharmacology and five candidate targets-interleukin-1 beta; heme oxygenase 1; vascular endothelial growth factor receptor 2; epidermal growth factor receptor; and nitric oxide synthase-to investigate the docking activity. We selected the components with higher docking activity to provide a theoretical basis for the development of more targeted drugs. The molecular docking information pertaining to the components with strong binding power and the candidate targets is provided in Supplementary Table S5 (available in www.besjournal.com). The mechanism by which ginseng treatment ameliorates hypoxia can be explained more profoundly, as follows. Ki is often used as an indicator of joint strength; it represents the minimum concentration at which spontaneous reactions can occur, with smaller values indicating stronger binding forces. The combination of free energy can also characterize the strength of bonding from the perspective of total energy. A negative value indicates that the reaction is favored. The greater the absolute value, the stronger the bond. We used Ki < 1 μmol/L as a condition for further component screening. We displayed images of the docking processes with strong binding forces. We also demonstrated docking for potential targets with only one dockable component.
Compounds FE (kcal/mol) ki (μmol/L) T/K Epidermal growth factor receptor Rg2 -6.32 23.39 298.15 Heme oxygenase 1 Rh2 -7.41 3.69 298.15 Aposiopolamine -9.75 0.09592 298.15 Kaempferol -9.15 0.19568 298.15 Frutinone A -8.15 1.07 298.15 Inermin -6.68 12.7 298.15 Linoleic -7.14 5.81 298.15 Paeonol -5.72 63.68 298.15 Suchilactone -9.17 0.18889 298.15 Beta-sitosterol -7.03 7.03 298.15 Interleukin-1 beta Beta-sitosterol -6.97 7.75 298.15 Deoxyharringtonine -7.53 3.03 298.15 Frutinone A -9.12 0.20748 298.15 Inermin -7.87 1.69 298.15 kaempferol -6.36 21.85 298.15 Linoleic -6.01 39.13 298.15 Suchilactone -8.4 0.69197 298.15 Vascular endothelial growth factor receptor 2 Aposiopolamine -8.3 0.82603 298.15 Nitric oxide synthase Aposiopolamine -8.44 0.65516 298.15 Beta-sitosterol -9.29 0.1558 298.15 Deoxyharringtonine -6.26 25.96 298.15 Frutinone A -8.17 1.02 298.15 Inermin -8.64 0.46408 298.15 kaempferol -6.68 12.62 298.15 Linoleic -7.28 4.63 298.15 Suchilactone -9.42 0.12453 298.15 Table Supplementary Table S5. Molecular Docking Information for Components and Candidate Targets
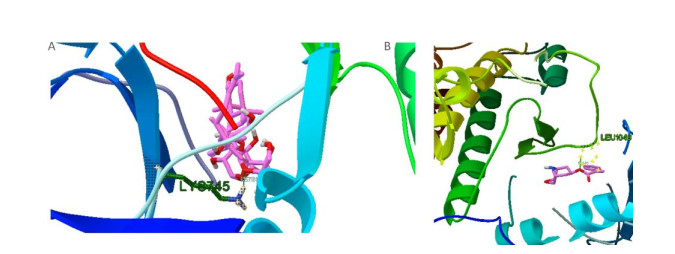
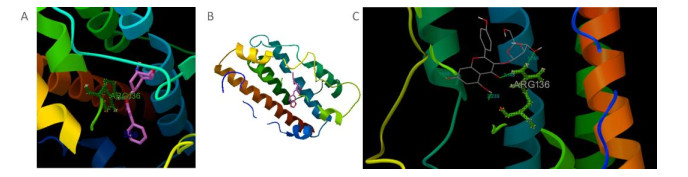
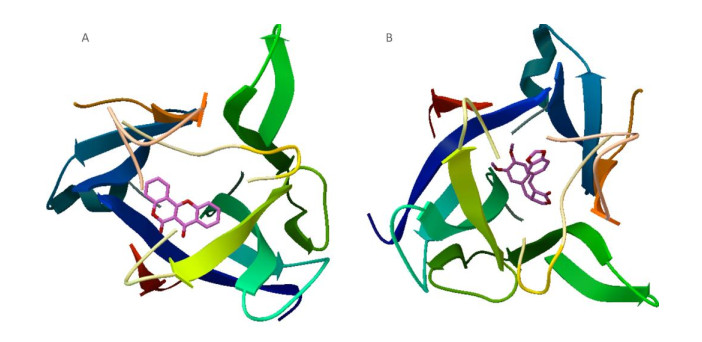
We found that only Rg2 and aposiopolamine were capable of binding to the potential targets of the epidermal growth factor receptor and vascular endothelial growth factor receptor 2, respectively (Supplementary Figure S1, available in www.besjournal.com). However, aposiopolamine, kaempferol, and suchilactone exhibited strong binding to heme oxygenase 1 (Supplementary Figure S2, available in www.besjournal.com). Furthermore, interleukin-1 beta bound strongly to various compounds such as frutinone A and suchilactone (Supplementary Figure S3, available in www.besjournal.com). Finally, we found that nitric oxide synthase also bound strongly to four active compounds: aposiopolamine, beta-sitosterol, inermin, and suchilactone (Supplementary Figure S4, available in www.besjournal.com).
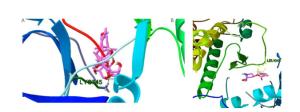
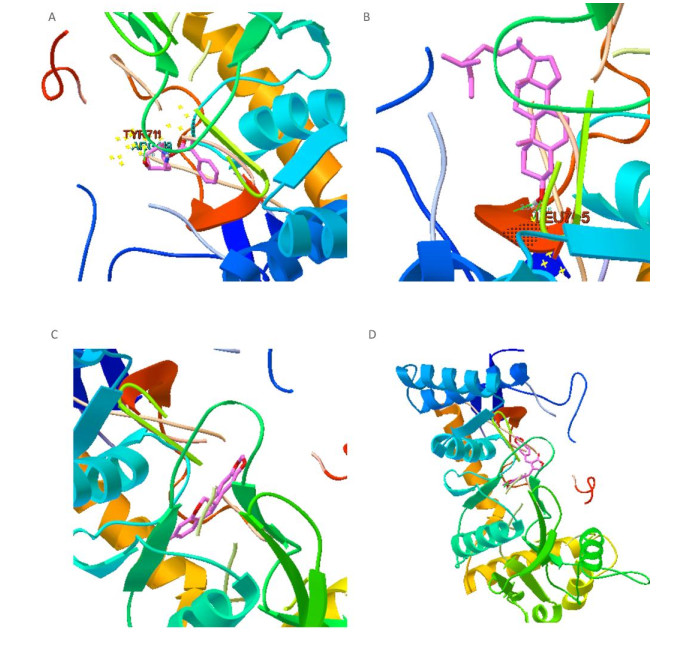
Figure Supplementary Figure S1. Simulated molecular docking, epidermal growth factor receptor and Rg2 (A), vascular endothelial growth factor receptor and aposiopolamine (B).
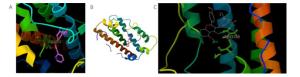
Figure Supplementary Figure S2. Simultaneous docking of analog molecules, aposiopolamine (A), suchilactone (B), and kaempferol (C) docking with Heme oxygenase 1.
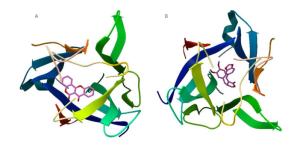
Figure Supplementary Figure S3. Simulated molecular docking, interleukin-1 beta with frutinone A (A) and suchilactone (B).
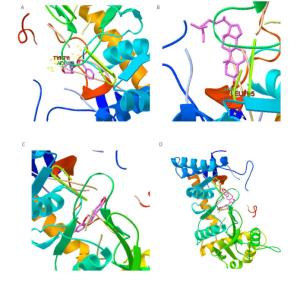
Figure Supplementary Figure S4. Simulation analysis docking, nitric oxide synthase with aposiopolamine (A), beta-sitosterol (B), inermin (C), and suchilactone (D), respectively.
Recent studies have shown that inadequate oxygen supply to tissue can trigger the activation of multiple inflammatory pathways, exacerbating tissue damage[5-8]. From a clinical perspective, neural hypoxia is more common than myocardial hypoxia, and neurotrophic factors, which are key regulators of neuronal survival and death, play an important role in neuron survival during hypoxia[9]. However, the present study revealed that ginseng has a multi-component effect, a multi-target effect, and multi-path control characteristics with regard to the treatment of human hypoxia.
The biological GO analysis revealed that five potential targets were closely related to the regulation of the biological pathways. Our results suggested that ginseng has a regulatory effect on these key proteins. Our molecular docking study revealed that Rg2, aposiopolamine, kaempferol, suchilactone, frutinone A, beta-sitosterol, and inermin-all of which are found in Ginseng-bind strongly to the five candidate targets mentioned above, and may be the most important components in ginseng for hypoxia treatment.
Use of Network Pharmacology and Molecular Docking to Investigate the Mechanism by Which Ginseng Ameliorates Hypoxia
doi: 10.3967/bes2018.114
The current study was financially supported by 1226 major project AWS16J018
- Received Date: 2018-08-09
- Accepted Date: 2018-11-05
| Citation: | WANG Tao, LI Hao Tian, WEI Shi Zhang, CAI Hua Dan, ZHU Yun, LIU Hong Hong, LI Yong Zhi, WANG Jia Ping, ZOU Wen Jun, ZHAO Yan Ling. Use of Network Pharmacology and Molecular Docking to Investigate the Mechanism by Which Ginseng Ameliorates Hypoxia[J]. Biomedical and Environmental Sciences, 2018, 31(11): 855-858. doi: 10.3967/bes2018.114 |








 Quick Links
Quick Links
 DownLoad:
DownLoad: