HTML
-
Phenolic acids play an important role in the prevention of human diseases including cancer[1, 2], inflammatory diseases[3, 4], and diabetes mellitus[5]. Wheat bran is the hard outer layer of wheat grains and is produced in enormous quantities as a major byproduct of the milling process in the production of white wheat flour, with an annual world estimated production of 90 million tons[6]. Wheat bran contains a variety of phenolic acids, including ferulic acid, vanillic acid, coumaric acid, caffeic acid, and chlorogenic acid[7]. Recently, it has been suggested that phenolic acids inhibit cancer and cardiovascular diseases by reducing the oxidative damage to cells and cell components[8]. A significant reduction in pro-inflammatory cytokine levels in humans was also attributed to phenolic compounds[9]. Studies have shown that phenolic acids function as free-radical scavengers, reducing agents, and quenchers of singlet oxygen formation[10-12]. Phenolic acids are among the most abundant and ubiquitous metabolites of cereal crops with health-related activities[13]. Many traditional chemistry methods and, recently, more advanced physicochemical methods have been used to release bound phenolic acids from the cell wall of cereal crops. These include ultrasound-assisted extraction, supercritical fluid extraction, microwave-assisted extraction, accelerated solvent extraction[14], and steam explosion-assisted extraction[15]. However, these methods mostly use highly concentrated alkaline and acid solvents to extract cell wall-bound phenolic compounds and have posed serious environmental risks. Fermentation by microorganisms has attracted much attention owing to its effectiveness and environment friendliness, and has been reported to effectively release phenolic acids from materials such as soybean products, rye bran, finger millet, and whole meal rye sourdoughs[16-18].
A few reports have indicated that solid-state fermentation by fungi can release phenolic compounds from wheat bran. Our previous results indicate that breakdown of wheat bran is an important prerequisite for the release of the free phenolic content. The enzymes degrading cell wall polysaccharides, and not the esterases breaking the ester linkages between phenolic acids and polysaccharides, were determined to play the most important role in the release of free phenolic content. We identified a strain of Aspergillus niger (A. niger) that could efficiently destroy the backbone of wheat bran. We determined that the strain of A. niger that we screened is a scientifically valuable strain for solid-state fermentation of wheat bran[19]. Molecular identification techniques based on PCR analysis of specific genomic sequences were used for the phylogenetic analysis of this A. niger strain (FA-WB).
Phenolic acids are usually good antioxidants and have potential application value in the functional food and nutraceutical field. There are many kinds of phenolic acids in wheat bran and fermentation with different microbes may release different phenolic acids with different biological activities. In this study we compared the antioxidant activity of FA-WB and ferulic acid standard, and used erythrocyte hemolysis to further evaluate the antioxidant activity of FA-WB. RAW264.7 cells were used as a cell model to evaluate the anti-inflammatory activity of FA-WB.
-
Defatted wheat bran was purchased from Guangzhou Runfon Flour Co. Ltd. (Guangzhou, China). 6-hydroxy-2, 5, 7, 8-tetramethylchroman-2-carboxylic acid (Trolox), 2, 2′-azobis-2-methyl-propanimidamide (AAPH), lipopolysaccharide (LPS), and 3-(4, 5-dime- thylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay reagents were procured from Aladdin Industrial Co. (Shanghai, China). Reagents for measuring malondialdehyde (MDA) content, reactive oxygen species level, enzymatic activity of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), as well as bicinchoninic acid (BCA) protein assay kits were acquired from Beyotime Institute of Biotechnology (Shanghai, China). A. niger was isolated from soil and was maintained in our laboratory. Sheep blood was purchased from Hongquan Biotech Ltd. (Guangzhou, China). The murine macrophage cell line RAW264.7, was obtained from the Traditional Chinese Medicine Hospital of Guangdong (Guangzhou, China). Rapid Yeast Genomic DNA Isolation Kit and lywallzyme were obtained from Tiangen Biotech Co. Ltd (Beijing, China).
-
The cultured fungus was scraped off and placed in a microcentrifuge tube with 1 mL of physiological saline. Centrifugation was performed for 1 min at 16, 000 ×g. The supernatant was discarded, and the pellet was subsequently resuspended in 600 μL of sorbitol buffer and 50 μL of lywallzyme. This was incubated at 30 ℃ for 120 min[7]. The remaining steps were performed following the instructions of the yeast genomic DNA extraction kit.
The PCR mixture was constituted as follows: 2.5 μL 10× Taq Buffer, 2 μL dNTPs (2.5 mmol/L), 0.25 μL ExTaq (5 U/μL), 16.25 μL ddH2O, 3 μL DNA, 0.5 μL 10 μmol/L universal ITS1 forward primer (27F) 5′-TCCGTAGGTGAACCTGCGG-3′, and 0.5 μL 10 μmol/L universal ITS4 reverse primer (1492R) 5′-TCCTCCGCTTATTGATATGC-3′.
PCR amplification was performed in a gradient thermal cycler, Amplitronyx™ (NYXTECHNIK, UK). The thermal cycling profile was as follows: 5 min at 94 ℃ for pre-denaturation, then 30 cycles of 30 s at 94 ℃ for denaturation, one minute at 55 ℃ for annealing, and 90 s at 72 ℃ for elongation, and then 10 min at 72 ℃ for final extension. The PCR products were sent to Majorbio, Shanghai for sequencing.
-
Wheat bran was sieved through a 100-mesh sieve to remove starch, dried in a cabinet dryer for 4 h at 50 ℃, and then ground for 20 s. The ground wheat bran (2 g) was transferred to a 100-mL shaking flask and autoclaved at 121 ℃ for 30 min. Aspergillus species were maintained on Czapek Dox agar slants. Fresh cultures were collected with an inoculation loop and added to sterile distilled water to prepare a spore suspension. Spores were counted with a hemocytometer and adjusted to 1 × 106 spores/mL. Autoclaved media were inoculated with 0.6 mL of the spore suspension and incubated at 28 ℃ for 7 days under 70% humidity.
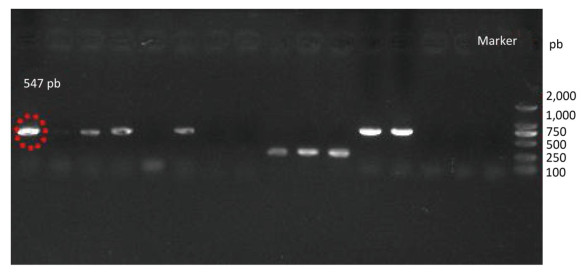
Four strains of A. niger were used to ferment the wheat bran. The production efficiency of ferulic acid was 62.37, 165.44, 117.65, and 358.72 μg/g respectively. The strain with the highest production efficiency was the target strain in this study.
-
The fermented slurries were cryogenically homogenized, extracted with methanol, and filtered through gauze. After centrifugation at 8, 000 ×g, methanol was removed by rotary evaporation. The pellets were frozen, vacuum dried, and dissolved in 0.25 mol/L NaOH; pH was subsequently adjusted to 7.0 using hydrochloric acid. The crude extracts were filtered through an organic phase membrane with a 5, 000-Da cutoff. Ferulic acid was concentrated by ultrafiltration using a 150-Da membrane and then vacuum dried.
-
Peroxyl radical scavenging activity was determined using the oxygen radical absorbance capacity (ORAC) assay, as reported previously[20, 21]. Filters for excitation and emission wavelengths were 485/20 nm and 528/20 nm, respectively. Five Trolox concentrations between 6.25 and 100.00 μmol/L were used for calibration. Ferulic acid was analyzed at different concentrations, and measured in triplicate. ORAC was calculated using the area under the curve and was expressed as the Trolox equivalent (TE) per gram of defatted wheat bran.
-
The intracellular antioxidant capacity of ferulic acid was assessed using the cellular antioxidant activity (CAA) assay as described previously[22], with some modifications. HepG2 cells were cultured in Dulbecco's modified eagle medium (DMEM) containing 10% (v/v) FBS, penicillin (100 U/mL), and streptomycin (100 U/mL) in a humidified incubator at 37 ℃ under 5% CO2. Cells in the logarithmic phase of growth were seeded into a 96-well microplate (6 × 104 cell/well) and incubated for 24 h. The culture medium was then removed, and cells were incubated for an additional 3 h with culture medium containing FA-WB (2.5, 5, 10 mmol/L) and 25 μmol/L 2′, 7′-dichlorofluorescein diacetate (DCFH-DA). The cells were then washed with PBS, after which 0.5 mmol/L AAPH was added to each well. Quercetin (10 μmol/L) was used as the positive control. Fluorescence was monitored every 10 min for 1 h on a Fluoroskan Ascent microplate fluorometer (Thermo Electron Corporation) at an excitation of 488 nm and emission of 525 nm. The fluorescence value was calculated using the method described by Liao et al.[22] All experiments were performed three times independently.
-
Red blood cells were isolated from sheep blood by centrifugation at 2, 000 rpm for 10 min. After washing three times with PBS, the RBCs were adjusted to a 20% suspension (hematocrit). The RBC suspension (0.2 mL) was pre-incubated with 0 μmol/L, 25 μmol/L, 50 μmol/L, and 100 μmol/L ferulic acid at 37 ℃ for 20 min, followed by adding 0.4 mL of 200 mmol/L AAPH. The reaction was stopped by adding 8 mL PBS after 2.5 h. After centrifugation, the OD540 of the supernatant was measured to determine hemolysis. The relative hemolysis of experimental groups was calculated by comparison with the complete hemolysis group treated with water. The pelleted RBCs were used for the measurement of membrane integrity and intracellular enzyme activity according to the method described by Liao et al.[22].
-
A reactive oxy gen species (ROS) assay kit with 2', 7'-Dichlorodihydro-fluorescein diacetate (DCFH-DA) as a fluorescent probe was used to determine the relative levels of intracellular ROS. Treated RBCs were collected by centrifugation at 1, 200 ×g for 10 min at 4 ℃, washed three times with PBS, and then incubated at 37 ℃ for 20 min in the presence of 10 μmol/L DCFH-DA, which is converted to the fluorescent compound 2′, 7′-dichlorofluorescin (DCF) within cells. The fluorescence intensity of the cells was then measured using a Fluoroskan Ascent microplate fluorometer (ex/em, 488/525 nm)[22].
-
The pelleted RBCs were lysed in deionized H2O and the supernatant was measured for MDA content, ROS level, and the enzymatic activity of SOD, CAT, and GPx, following the manufacturer's instructions (Beyotime Institute of Biotechnology, Shanghai, China).
-
To examine the morphological changes after FA-WB and AAPH treatments, RBCs were fixed in 2.5% glutaraldehyde at 4 ℃ for 2 h, followed by washing thrice with PBS. The cells were sequentially dehydrated using 50%, 70%, 80%, 90%, 95%, and 100% ethanol for 10 min. The cells were then cryogenically dried and coated with a thin layer of gold. The coated RBCs were viewed using a scanning electron microscope EM-3700[23].
-
RAW264.7 cells were grown in DMEM supplemented with 10% FCS at 37 ℃ in a 5% CO2 incubator. The cells were seeded onto a 96-well plate at 105 cells/well for 24 h prior to LPS stimulation. The inflammatory cytokines were measured as described previously[24].
To test the anti-inflammatory capacity of FA-WB, a range of FA-WB concentrations (0, 31.25, 62.50, 125.00, 250.00, and 500.00 μg/mL) supplemented with 20 μg/mL of LPS in 200 μL culture medium was added to each well. After treatment for 24 h, the supernatants were collected and the concentration of inflammatory cytokines TNF-α and IL-6 was determined by enzyme-linked immunosorbent assay (ELISA) using a kit from Neobioscience Technology Co. (Shenzhen, China). To assess cell viability, 10 μL of MTT was added to each well, and absorbance at 490 nm was measured using the Infinite M1000 Pro microplate reader (Tecan, Switzerland).
-
All experiments were performed at least three times. The data were expressed as mean ± SD from a representative experiment performed in triplicate. Statistical analysis was performed using SPSS 19.0 software (IBM, Chicago, USA); the data were subjected to one-way analysis of variance (ANOVA). Statistical significance (P < 0.05) was calculated by Duncan's multiple range test (DMRT). Otherwise, two-tailed, unequal variance Student's t-test was used to compare the significance between two groups as stated in the figure legends. To test the significance of the correlation coefficient, the t value was calculated using the formula t = r$ \sqrt {\left( {n - 2} \right) \div \left( {1 - {r^2}} \right)} $(r: correlation; n: sample size). The critical t-value (two-tailed) was ± 2.7764 with degrees of freedom at 4 and probability level at 0.05.
Materials
DNA Extraction and Sequence Analyses
Solid-state Fermentation
Ferulic Acid Extraction and Antioxidant Activity Measurement
ORAC Assay
CAA of Ferulic Acid Extraction from Fermented Wheat Bran (FA-WB)
Assay for Erythrocyte Hemolysis Mediated by Peroxyl Free Radicals
Determination of Intracellular ROS Level
MDA, ROS, SOD, CAT, and GPx Measurement
Scanning Electron Microscopy
Measurement of TNF-α and IL-6 Secretion by Murine Macrophages
Statistical Analysis
-
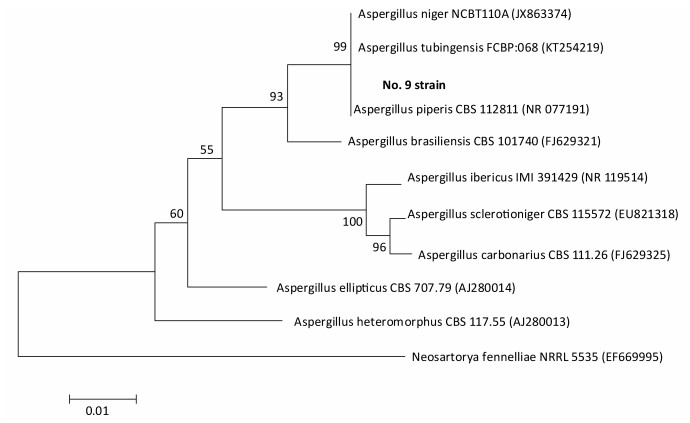
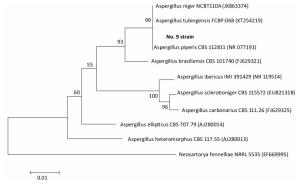
Species identification was carried out by performing sequence alignment using BLASTn (GenBank, USA) (Figures 1, 2). The filamentous fungal isolate was identified to be A. niger, showing a sequence identity of 98%. A phylogenetic tree was generated using the minimum evolution method (Figure 3) and the data were re-sampled 1, 000 times. The strain No. 9 was found to be in the same branch as A. niger, showing the closest evolutionary distance and a homology of 99.87%. Based on colony characteristics and the internally transcribed spacer (ITS) sequence analysis of the strain, the isolate was determined to be A. niger.
-
The ORAC assay has been extensively used to measure the antioxidant activity of many cereal and fruit extracts based on their peroxyl radical scavenging activities[25]. The antioxidant activities of FA-WB were evaluated in vitro using the ORAC assay. As shown in Table 1, a higher ORAC value (34.22 ± 1.76 mmol TE/g) was detected in FA-WB, which was considerably higher than that of ordinary ferulic acid (23.77 ± 2.03 mmol TE/g). It means that FA-WB showed higher in vitro antioxidant activity than ordinary ferulic acid. In addition, the CAA value of FA-WB was 79.6 ± 0.026 μmol QE/100 μmol ferulic acid, which was higher than that of the ferulic acid standard (59.8 ± 0.031 μmol of QE/100 μmol). This indicated that FA-WB exhibited stronger cellular antioxidant activity compared to ordinary ferulic acid.
Composition EC50 (μmol) ORAC (mmol TEa/g) CAA (μmol QEb/100 μmol ferulic acid) Quercetin 4.63 ± 0.27 FA-WB 6.74 ± 0.01 34.22 ± 1.76 79.61 ± 0.02 Ferulic acid standerd 7.91 ± 0.01 23.77 ± 2.03 59.82 ± 0.03 Note. aTE, tocopherol equivalent; bQE, quercetin equivalents. Table 1. ORAC Values, CAA Values, and EC50 of Cellular Antioxidant Activities of Two Different Ferulic Acid
-
The antioxidant ability of FA-WB was further examined using erythrocyte hemolysis and plasma oxidation assays. In the erythrocyte hemolysis assay, AAPH, which can decompose at 37 ℃ to generate an alkyl radical, was used as an initiator. In the presence of oxygen, these alkyl radicals are converted to peroxyl radicals that can cause lipid peroxidation and loss of erythrocyte membrane integrity, ultimately leading to hemolysis. Hemolysis of erythrocytes has been used extensively as an ex vivo model for studying the protective effect of antioxidants[26-28]. Compared with other phenolic acids, such as chlorogenic acid, ρ-coumaric acid, caffeic acid, sinapic acid, trans-ferulic acid, and syringic acid, ferulic acid exhibited the highest antioxidant activity[10, 29]. We thus examined the antioxidant activity of FA-WB. The purity of the ferulic acid was determined to be 93.8%.
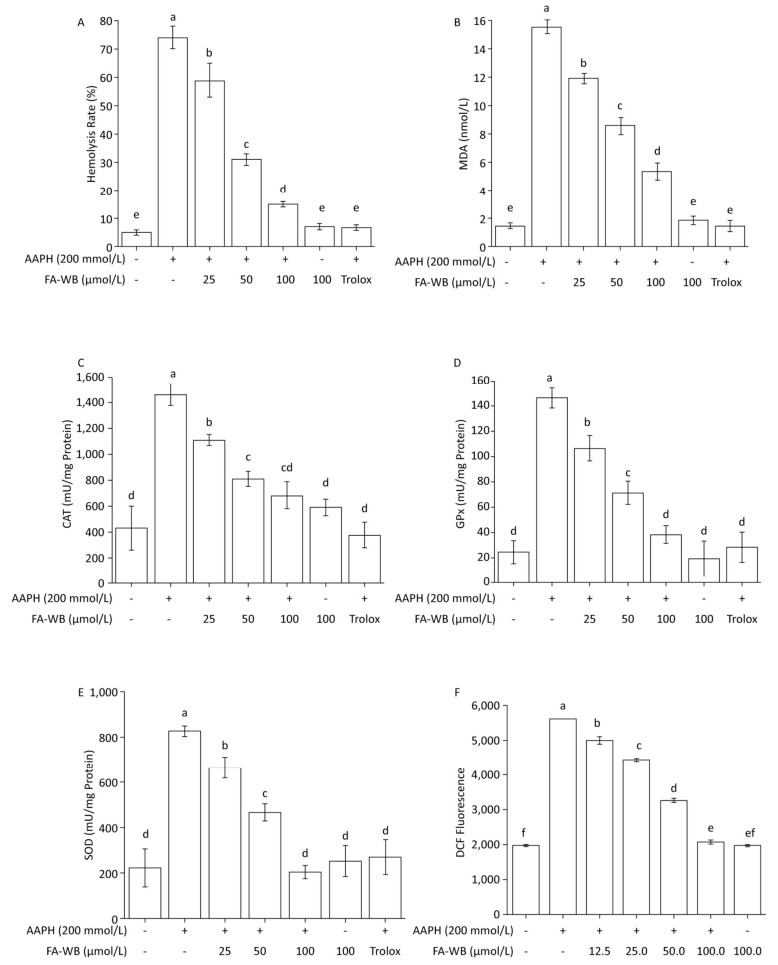
We used a well-established AAPH-induced red blood cell (RBC) damage model to evaluate the antioxidant activity of FA-WB by measuring erythrocyte hemolysis, MDA content, ROS level, and the enzymatic activity of SOD, CAT, and GPx.[30] As expected, direct addition of 200 mmol/L AAPH to the RBCs dramatically caused erythrocyte hemolysis (Figure 4A), triggered RBC membrane permeabilization (Figure 4B), induced ROS production (Figure 4F), and increased intracellular enzymatic activity of CAT, GPx, and SOD (Figure 4C-E) after 2.5 h of treatment. Pre-incubation of RBCs with at least 25 μmol/L FA-WB significantly reduced these adverse effects. Moreover, the increase in FA-WB content reversed the AAPH-induced RBC damage in a concentration-dependent manner (Figure 4A-F).
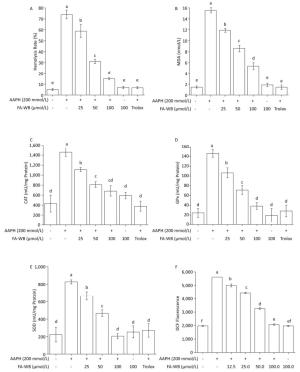
Figure 4. Protective Effects of FA-WB on AAPH-induced Erythrocyte Hemolysis Assay (A); Changes in MDA Content (B) and Enzyme Activities of CAT (C), GPx (D), and SOD (E) in Erythrocytes; Inhibitory Activity of Ferulic Acid on AAPH-induced ROS Overexpression in Sheep Erythrocytes (F). Different lower case letters indicate significant differences (P < 0.05) in multiple-range analysis among the groups.
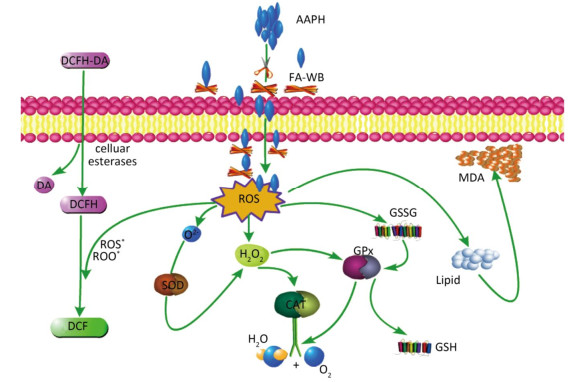
As shown in Figure 4A, erythrocyte hemolysis induced by AAPH was effectively attenuated by FA-WB in a dose-dependent manner, such that the inhibition rate was enhanced as the FA-WB concentration increased from 0 to 100 μg/mL. At a dose of 100 μg/mL, the hemolysis rate of FA-WB was 15.0%, which was comparable to that of vitamin C (6.7% at 1 mg/mL). AAPH-induced ROS generation can cause lipid peroxidation and result in the release of MDA. MDA is involved in tumor promotion, cellular metabolism disruption, and cell membrane dysfunction; excess MDA can ultimately lead to disruption of cellular metabolism. As seen in Figure 4B, the MDA level in erythrocytes was significantly increased from 1.5 to 15.5 nmol/L after treatment with 200 mmol/L AAPH for 2 h, indicating the occurrence of lipid peroxidation caused by AAPH-induced oxidative stress. For cells incubated with different concentrations of FA-WB, the level of MDA in erythrocytes was decreased, especially with the high concentration of FA-WB (100 μg/mL), wherein the MDA concentration declined to 5.4 nmol/L. For cells incubated with FA-WB without AAPH supplementation, the MDA level was comparable to that of the control, indicating that FA-WB itself does not induce MDA formation. Thus, lipid peroxidation caused by ROS generation was efficiently inhibited by FA-WB. The major radical scavenging antioxidant enzymes in the human body include SOD, GPx, and CAT. These enzymes constitute an intracellular defense system, which can coordinate the elimination of free radicals through a series of chain reactions. The activities of SOD, GPx, and CAT in erythrocytes were significantly increased after 200 mmol/L AAPH treatment for 2 h, showing that AAPH treatment activated the enzymatic antioxidant defense systems in erythrocytes. This was particularly the case for high concentration (100 μg/mL) FA-WB treatment, which maintained the enzyme activities at normal levels. Thus, FA-WB can effectively attenuate AAPH-induced oxidative stress in erythrocytes, mainly through the inhibition of ROS generation, as illustrated in Figure 5.
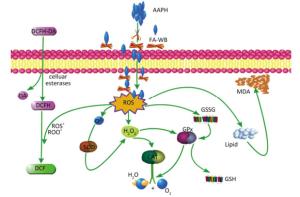
Figure 5. Possible intracellular antioxidant detoxifying mechanisms of FA-WB that attenuate aaph-induced oxidative stress through inhibition of ROS generation[22].
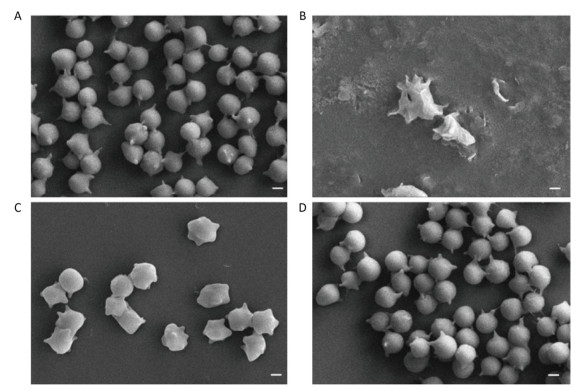
To further evaluate the protective effect of FA-WB on erythrocyte morphology after AAPH oxidation, we used scanning electron microscopy to image the RBCs pre-treated with FA-WB. As shown in Figure 6A, AAPH incubation alone caused irregular membrane blebbing and even membrane rupture in erythrocytes (Figure 6B). RBCs pre-treated with 50 μmol/L FA-WB displayed less stressed morphology (Figure 6C); with 100 μmol/L FA-WB pre-treatment, RBCs displayed almost normal morphology, similar to the group without AAPH treatment (Figure 6D). Ferulic acid possesses three distinctive structural motifs that can contribute to its free radical scavenging capability[31, 32]. Our data indicated that FA-WB possesses potent free radical-scavenging ability and can protect RBCs from oxidative damage.
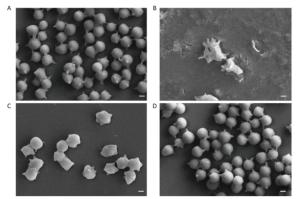
Figure 6. Scanning electron micrographs of erythrocytes after FA-WB and AAPH treatment. Human RBCs were Pre-treated with 0 μmol/L (AAPH only, B), 50 μmol/L (C) and 100 μmol/L (D) Ferulic acid prior to incubation with 120 mmol/L AAPH. The normal human RBCs were used as no treatment control (A). Scale bar, 10 μm.
-
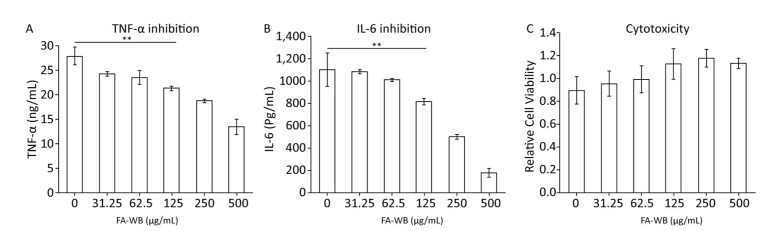
Pre-treatment of macrophages with ferulic acid reduced LPS-induced inflammation[33, 34]. We tested whether FA-WB can inhibit secretion of inflammatory cytokines TNF-α and IL-6 using supernatant collected from LPS-stimulated RAW264.7 cells. LPS alone induced significant amounts of TNF-α and IL-6 production. Addition of FA-WB gradually reduced TNF-α and IL-6 secretion in a concentration-dependent manner. There was no significant difference between FA-WB at a lower concentration and the control group. However, FA-WB at 125 μg/mL significantly suppressed TNF-α and IL-6 secretion by 23% and 26%, respectively, relative to LPS alone. FA-WB at 500 μg/mL inhibited TNF-α and IL-6 secretion by 51.4% and 83.9%, respectively (Figure 7A-B). This inhibitory effect was not caused by cell death as FA-WB does not show significant cytotoxicity to macrophages (Figure 7C).
Identification of A. niger
Determination of Oxygen Radical Absorbance Capacity (ORAC)
FA-WB Protects against AAPH-induced Oxidative Stress
FA-WB Suppresses Inflammatory Cytokine Secretion in Murine Macrophages
-
Wheat bran, an important by-product generated in large amounts during wheat flour milling, contains the highest amount of bioactive compounds, like ferulic acid. Ferulic acid is a ubiquitous plant constituent that arises from the metabolism of phenylalanine and tyrosine. It can exist as forms such as free ferulic acid, γ-oryzanol, a mixture of monoesters consisting of ferulic acid, and several kinds of triterpene alcohols, and is covalently linked to lignin and other biopolymers[35]. Due to its phenolic nucleus and an extended side chain conjugation, it readily forms a resonance stabilized phenoxy radical that accounts for its potent antioxidant potential. Such antioxidants are currently expected to prevent lipid oxidation in food as well as free-radical-induced diseases such as cancer and atherosclerosis or aging caused by oxidative tissue degeneration. Therefore, there is a possibility of developing an application of wheat bran pitch for human health[36]. However, most of the ferulic acid in wheat bran is bound. In this paper, we used solid-state fermentation of a new strain of A.niger to release the ferulic acid in wheat bran and extracted the ferulic acid in a fermentation product, namely FA-WB. We found that FA-WB was not free ferulic acid. Previous reports have suggested that bound ferulic acid dehydrodimers exist in the cell walls of plants[37-41]. However, we did not find dehydrodimers in FA-WB. This suggests that FA-WB is a new form of ferulic acid. We also measured the antioxidant activity of FA-WB using chemical experiments and cell models. The antioxidant mechanism of FA-WB was found to be through direct scavenging of free radicals and adjusting the activity of antioxidant enzyme (SOD, GPx, and CAT). However, the activity of FA-WB was higher than that of the ferulic acid standard, namely free ferulic acid. The anti-inflammatory activity of FA-WB was also higher than that of the ferulic acid standard. All these results indicated that the biological activity of FA-WB may be better than that of ferulic acid standard. There are also previous reports suggesting that the biological activities of other forms ferulic acid are better than those of free ferulic acid[37-41]. Although it is known that FA-WB is not free ferulic acid or a ferulic acid dehydrodimer, the structure of FA-WB is still unknown. Thus, it is difficult to analyze its structure-function relationship and the difference of activity between FA-WB and free ferulic acid. Determination of the structure of FA-WB is necessary for its further studies and applications. Thus, FA-WB still needs to be explored further.
-
We screened and identified a new strain of A. niger, and used it in solid-state fermentation to release the bound ferulic acid in wheat bran. A new form ferulic acid, FA-WB, was produced, and showed better antioxidant and anti-inflammatory activity than that of free ferulic acid. These results suggest the potential of FA-WB for application in the treatment of diseases in which ROS overproduction is the etiological factor and in inflammation-induced diseases, and provide proof that wheat bran has high added-value and is worth recycling.









 Quick Links
Quick Links
 DownLoad:
DownLoad: