-
Epstein-Barr virus (EBV) infection is closely associated with nasopharyngeal carcinoma (NPC) and considered one of the major risk factors[1]. A limited number of viral proteins, such as latent membrane proteins (LMP1, LMP2) and EB nuclear antigen1 (EBNA1), are expressed in NPC cells[2]. Recognition epitopes for CD8+ and CD4+ T cells were included in the LMP2 antigen and EBNA1 C-terminal region (amino acid No. 380-641), respectively[3, 4]. Both CD4+ and CD8+ memory T-cell responses were efficiently reactivated by EBNA1 and LMP2[5, 6]. However, little is known about whether EBNA1-specific CD4+ T-cell responses interfere with LMP2-specific CD8+ T-cell responses. Therefore, we constructed recombinant adenovirus (type 5, rAd) expressed EBNA1 and LMP2 antigens to investigate the immunological responses to combinations of EBNA1 and LMP2 in mice.
Lmp2 and ebna1 fragments were inserted into a replication-deficit recombinant adenoviral vector (rAd- lmp2, rAd-ebna1, respectively). rAd-lmp2 and rAd-ebna1 were injected alone or in combination into Balb/c mice at a dose of 2 × 107 VP per mouse each time, and phosphate-buffered saline (PBS) and null rAd (rAd-null) were injected into control group mice. Epitope-specific responses to LMP2 and EBNA1 were measured and compared at different time points after two intramuscular immunizations. The immunization schedule was shown in Table 1. Between-group differences were analyzed by using statistical software (GraphPad Prism V5.0).
Groups Ingredient Volume (mL) Doses (VP/Mouse) Immune Intervals (week) Detection Intervals (week) Immune Sections Numbers 1 PBS 0.1 _ 0, 2nd 1st, 2nd, 4th, 8th hind limb 20 2 rAd-null 0.1 107 0, 2nd 1st, 2nd, 4th, 8th hind limb 20 3 rAd-LMP2 0.1 107 0, 2nd 1st, 2nd, 4th, 8th hind limb 20 4 rAd-EBNA1 0.1 107 0, 2nd 1st, 2nd, 4th, 8th hind limb 20 5 rAd-LMP2 & rAd-EBNA1 mixture 0.1 107 0, 2nd 1st, 2nd, 4th, 8th hind limb 20 6 rAd-LMP2 (First) rAd-EBNA1 (Second) 0.1 107 0, 2nd 1st, 2nd, 4th, 8th hind limb 20 7 rAd-EBNA1 (First), rAd-LMP2 (Second) 0.1 107 0, 2nd 1st, 2nd, 4th, 8th hind limb 20 Table 1. Schedule for Single and Combined Immunization in Balb/c Mice
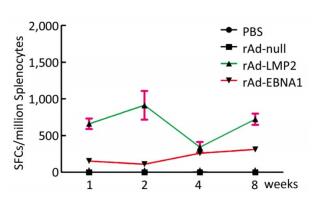
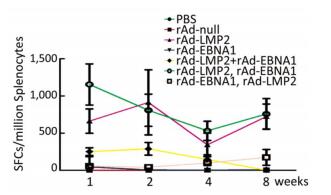
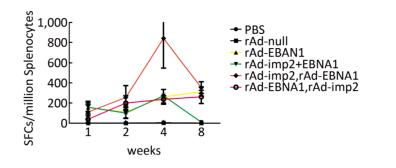
Both EBNA1-and LMP2-specific cellular responses were detected at the 1st, 2nd, 4th, and 8th weeks after mice were immunized by rAd-LMP2 or rAd-EBNA1 alone (Supplementary Figures S1-S2, available in www.besjournal.com). CD8+ T-cell responses activated by LMP2 were much higher than CD4+ T-cell responses activated by EBNA1 at each time point (Figure 1). There was a statistically significant difference between the CD8+ and CD4+ T-cell responses (Newman-Keuls test, P < 0.05; Figures 1, 2). LMP2-specific cellular immune responses were variable with different combinations of EBNA1 and LMP2 in mice (Supplementary Figure S3, available in www.besjournal.com). LMP2-specific cellular immune responses in the combination of Ad-LMP2 injected first and rAd-EBNA1 second were higher than those in other groups (Figure 2).
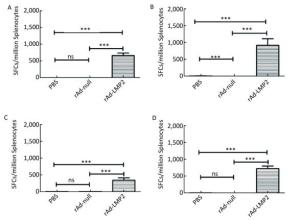
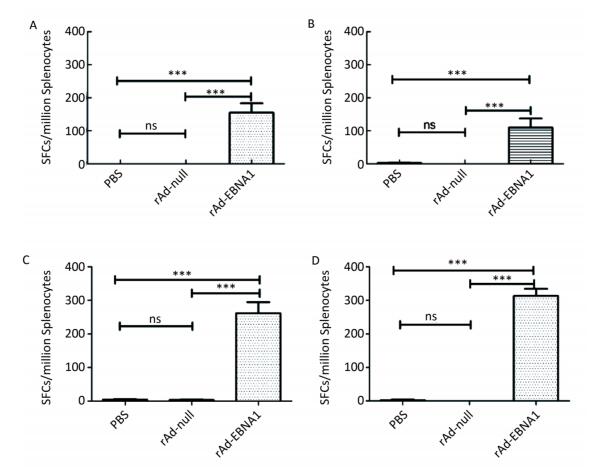
Figure Supplementary Figure S1. LMP2-specific cellular responses in Balb/c mice as determined by ELISOPT assay. (A) LMP2-specific cellular responses at the 1st week post-immunization. (B) LMP2-specific cellular responses at the 2nd week post-immunization. (C) LMP2-specific cellular responses at the 4th week post-immunization. (D) LMP2-specific cellular responses at the 8th week post-immunization. * * *represent that P < 0.001, ns represents no significant difference, the following is same.
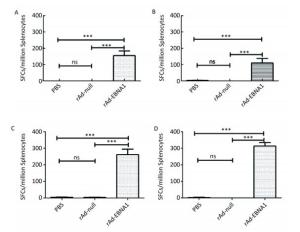
Figure Supplementary Figure S2. EBNA1-specific cellular responses in Balb/c mice as determined by ELISPOT assay. (A) EBNA1-specific cellular responses at the 1st week post-immunization. (B) EBNA1-specific cellular responses at the 2nd week post-immunization. (C) EBNA1-specific cellular responses at the 4th week post-immunization. (D) EBNA1-specific cellular responses at the 8th week post-immunization. * * *indicates P < 0.001, ns represents no significant difference, the following is same.
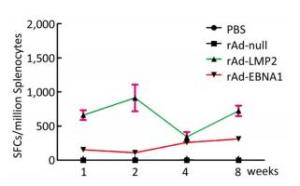
Figure 1. EBNA1- and LMP2-specific responses in Balb/c mice at different time points as determined by ELISPOT assay.
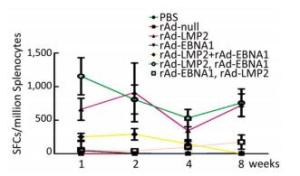
Figure 2. LMP2-specific responses in Balb/c mice at different time points as determined by ELISPOT assay.
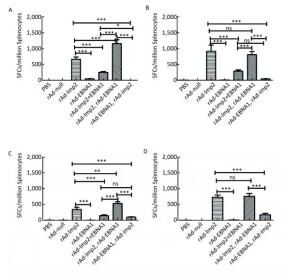
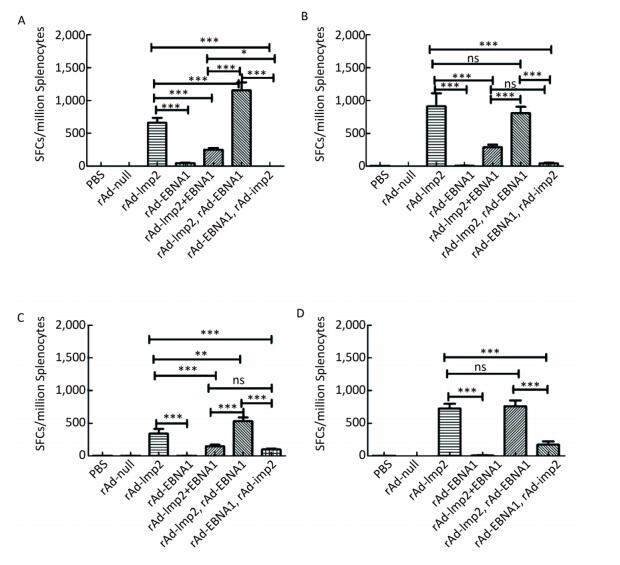
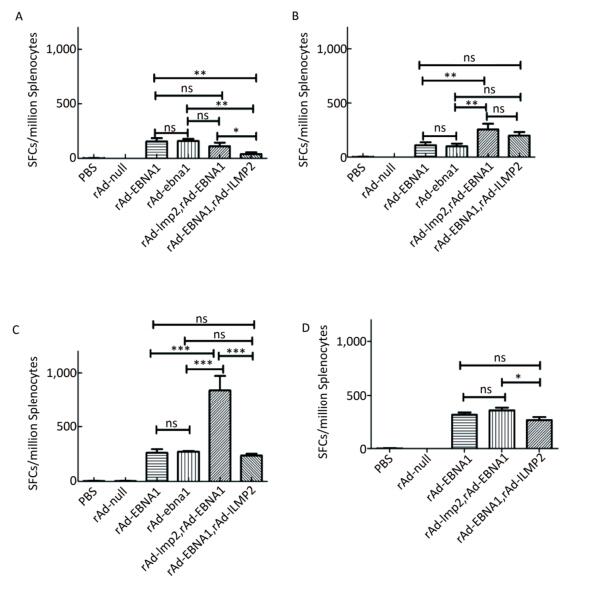
Figure Supplementary Figure S3. LMP2-specific cellular responses in Balb/c mice after immunization with a combination of rAd-LMP2 and rAd-EBNA1 as determined by ELISPOT assay. (A) LMP2-specific cellular immune responses at the 1st week after vaccination. (B) LMP2-specific cellular immune responses at the 2nd week after vaccination. (C) LMP2-specific cellular immune responses at the 4th week after vaccination. (D) LMP2-specific cellular immune responses at the 8th week after vaccination. *indicates P < 0.05, **indicates P < 0.01, * * *indicates P < 0.001, ns represents no significant difference, the following is same.
It has been reported that chimeric antigens of LMP2 and EBNA1 can activate the cellular immune responses of both CD4+ and CD8+ T cells[7]. However, it is unclear how CD8+ T-cell responses to LMP2 and CD4+ T-cell responses to EBNA1 interact. In this study, we designed the immunization schedule according to this objective with different time points and combinations of vaccines.
The cellular responses to rAd-LMP2 were much higher than those to MVA-LMP2[8, 9]; hence, we constructed individual recombinant adenoviral vectors containing either the EBNA1 fragment or the LMP2 gene to explore the immunological responses in mice after vaccination and investigate the interaction between EBNA1 and LMP2. Our results indicated that vaccination with rAd-LMP2 and rAd-EBNA1 alone can strongly induce the cellular responses in Balb/c mice, but the CD8+ T-cell responses activated by LMP2 were much stronger than the CD4+ T-cell responses activated by EBNA1.
It has been shown that combined immunization with multi-vector vaccines containing the same antigen could enhance the cellular immune responses[10]; however, it is still unknown whether combined immunization with vaccines made of the same vector with different antigens could also take such a role in activating the cellular immune response. We found that various immune responses were activated by different vaccine combinations. LMP2-specific responses activated by the combination of rAd-LMP2 followed by rAd-EBNA1 were stronger, whereas all combinations of rAd-EBNA1 and rAd-LMP2 produced weaker responses (Supplementary Figures S4-S5, available in www.besjournal.com). The interaction between LMP2 and EBNA1 plays a role in boosting specific T-cell responses in a timed, sequential manner. It was suggested that EBNA1 might play dual roles in activating T-cell responses.
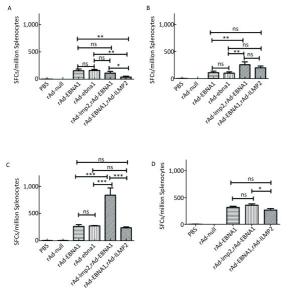
Figure Supplementary Figure S4. EBNA1-specific cellular responses in Balb/c mice after immunization with a combination of rAd-LMP2 and rAd-EBNA1 as determined by ELISPOT assay. (A) EBNA1-specific cellular immune responses at the 1st week after vaccination. (B) EBNA1-specific cellular immune responses at the 2nd week after vaccination. (C) EBNA1-specific cellular immune responses at the 4th week after vaccination. (D) EBNA1-specific cellular immune responses at the 8th week after vaccination. *indicates P < 0.05, **indicates P < 0.01, * * *indicates P < 0.001, ns represents no significant difference, the following is same.
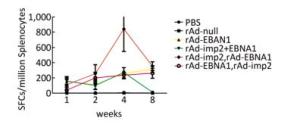
Figure Supplementary Figure S5. EBNA1-specific cellular responses in Balb/c mice at different time points as determined by ELISPOT assay.
In conclusion, through a methodical vaccination experiment, we developed a timed immunization sequence for activated T-cell-specific responses against EBV in mice. It was unclear that the cause of various immune responses activated by the immune combinations. It remains to be proven whether a similar pattern exists in primates, and further investigation in clinical trials is warranted.
HTML
-
DU Hai Jun and ZENG Yi conceived and designed experiment. TONG Yan Yan and ZHANG Li Xia performed experiment. DU Hai Jun and TONG Yan Yan collected and analyzed the data. DU Hai Jun and WANG Zhan offered reagents/ materials. DU Hai Jun drafted the manuscript.
-
We are grateful to Dr. TANG Min Zhong for revised the manuscript.







 Quick Links
Quick Links
 DownLoad:
DownLoad: