HTML
-
Microgravity is a basic physical factor in the space environment. Numerous studies have demonstrated that microgravity dramatically affects the physiological functions of human body inducing impairments in cardiovascular, musculoskeletal, endocrine and immune systems[1]. Besides, it was also suggested that cerebral malfunction occurred during or after spaceflight. For example, Roberts and Alperin reported the narrowing of central sulcus, the upward transfer of brain and periventricular white matter hyperintensity in astronauts[2-3]. Meanwhile, cerebral electrical activities and functional connectivity among brain regions were suggested to be disrupted by microgravity[4-6]. Moreover, many other investigations also revealed that microgravity strongly affected the structure and physiology of brain[7-10]. Considering the irreplaceable role of brain in human activities, it is of great importance to further explore the impacts of microgravity and underlying mechanism on brain.
Due to the high cost, low frequency and technical difficulties of space experiments, ground-based simulated microgravity (SM) models have been established. Rodent tail-suspension (TS) is a validated one to mimic multiple effects of real microgravity on organism and used to investigate the involved mechanism[11]. To date, the TS model is widely used in the study of microgravity-related impacts including those on bone[12-14], muscle[15-17], endocrine[18-20], and hepatic function[21-23]. Meanwhile, the TS model is well accepted for investigating the effects of microgravity on brain. For instance, Yoon found that 14-day TS up-regulated the expression of neuronal nitric oxide synthase in rat brain[24]. Several cerebral proteins involved in sleep/wake cycle, drinking behavior and endocrine were differentially regulated by 7-day TS[25, 26]. Other studies also suggested that transmitter metabolism, eletrical physiology and vascular tension in rat brain were significantly changed by TS[27-30]. However, most experiments were performed for single period; and investigations regarding the dynamic impacts of SM are relatively less. Moreover, the impacts of SM on individual vital brain regions, such as hippocampus, cerebral cortex and striatum have not been compared yet.
The aim of this study was to explore the dynamic impacts and underlying mechanism of SM on 3 vital brain regions. Rats were tail-suspended for 7 and 21 days, respectively, to mimic short and long-term microgravity. Histomorphology, oxidative stress markers, inflammatory cytokines and the expression of some key proteins were determined in hippocampus, cerebral cortex and striatum. The results might provide neurological insight of SM, which might help to understand the health risk and to prevent brain damage for astronauts in space travel.
-
Hematoxylin-eosin staining kit was purchased from Beyotime Biotechnology (Shanghai, China). Hydrogen peroxide (H2O2), malonaldehyde (MDA), superoxide dismutase (SOD) and glutathione (GSH) biochemical kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Interleukin-6 (IL-6), interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) ELISA kits were supplied by Neo Bioscience Technology Co., Ltd (Guangzhou, China). RIPA buffer and protein loading buffer were obtained from Solarbio Life Sciences (Beijing, China). Cocktail protease inhibitor and phosphatase inhibitor were from Roche Diagnostics (Indianapolis, USA). BCA Protein Assay Kit was purchased from Thermo Fisher Scientific Inc. (Waltham, USA). Primary antibodies of brain derived neurotrophic factor (BDNF), c-Jun, c-Fos and glyceraldehydes-3- phosphate dehydrogenase (GAPDH) were supplied by Abcam PLC (Cambridge, UK). HRP-conjugated secondary antibody was from ComWin Biotech Co., Ltd (Beijing, China). All other chemicals and solvents were of analytical grade.
-
The present study complied with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication no. 85-23, revised in 1985), and all the animal experiments were approved by Beijing Institute of Technology Animal Care and Use Committee. A total of 24 SD rats (male, SPF, 180-220 g) were obtained from Academy of Military Medical Sciences (Beijing, China). Rats were raised in a temperature and humidity controlled room (temperature 25 ℃, humidity 55%) with an artificial 12-h light-dark cycle and had free access to water and normal standard chow diet. All the animals were adapted to the environment for one week prior to the study.
The rats were randomly divided into 4 groups (6 rats per group): Group 1, 7-day control group, rats were kept normally without TS for 7 days; Group 2, 7-day SM group, rats were tail-suspended for 7 days; Group 3, 21-day control group, rats were kept normally without TS for 21 days; and Group 4, 21-day SM group, rats were tail-suspended for 21 days. TS was performed as previously described with little modification[11]. Briefly, sterilized rat tail was connected to a pulley using surgical tape and then suspened by a metal bar. The tilt angle was about -30 ° in relation to the horizontal. Rats could freely move in TS cages and had free access to water and food.
-
Sample collection was performed after TS for 7 and 21 days, respectively. Briefly, rats were sacrificed by heart perfusion with ice-cold saline under deep anesthesia with pentobarbital sodium. Brain was harvested and divided into 2 hemispheres: one was immediately immersed in 4% formaldehyde solution; hippocampus, cerebral cortex and striatum of the other hemisphere were collected and stored at -80 ℃.
-
The tissue preserved in 4% formaldehyde solution was taken, cropped and made into wax pattern, thereafter sliced up (5 μm) and dyed with hematoxylin-eosin. Tissue structure and cellular morphology were observed with high power field microscope.
-
Markers of oxidative stress (H2O2, MDA, SOD and GSH) and inflammatory cytokines (IL-6, IFN-γ and TNF-α) were detected using reagent kits according to the supplier's instructions.
-
Frozen brain tissue was put into 100 μL of ice-cold RIPA buffer (containing cocktail protease inhibitor and phosphatase inhibitor), homogenized and then centrifuged at 12, 000 × g for 10 min at 4 ℃ to extract total protein. Protein concentration was determined by BCA protein assay. Equal amount of total protein was seperated by 12% SDS-PAGE and transferred to a PVDF membrane. After blocking the non-specific binding sites for 2 h with 5% non-fat milk in TBST buffer at room temperature, the membranes were incubated with primary antibodies (antibodies were 1, 000 times diluted with TBST buffer) of BDNF, c-Jun, c-Fos and GAPDH overnight at 4 ℃. Later, the membranes were subjected to 4-time wash with TBST buffer and then incubated with HRP-conjugated secondary antibody for 2 h at room temperature. After another 4-time wash with TBST buffer, target proteins were visualized using ECL plus western blot detection system. GAPDH protein levels were used as a loading control. The relative expression levels of target proteins were expressed as the ratio of their band gray value to that of GAPDH (the gray values were analyzed by software ImageLab 4.0).
-
Statistical analysis was performed using SPSS 20.0 software (IBM, USA). Data was expressed as mean ± standard deviation (SD). Difference between groups was determined by independent-samples t test. P-values of less than 0.05 were considered statistically significant.
Reagents
Animals
Sample Collection
Hematoxylin and Eosin Staining
Biochemical Tests
Western Blot Assay
Statistical Analysis
-
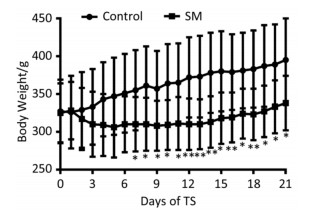
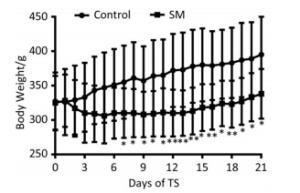
The body weight of 21-day groups was measured consecutively, which was illustrated in Figure 1. Though the 2 groups both gained weight at last, 21-day SM group experienced weight lose during the initiation of TS and thus presented significant lower body weight from day 7, as compared with 21-day control group (P < 0.05 or 0.01).
-
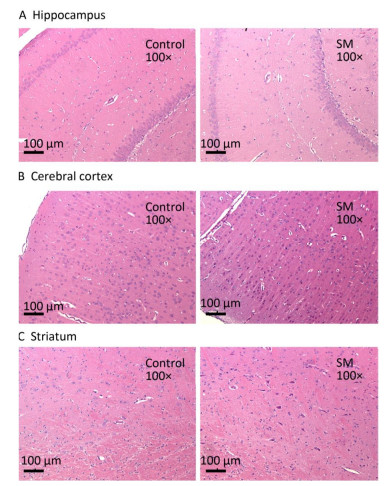
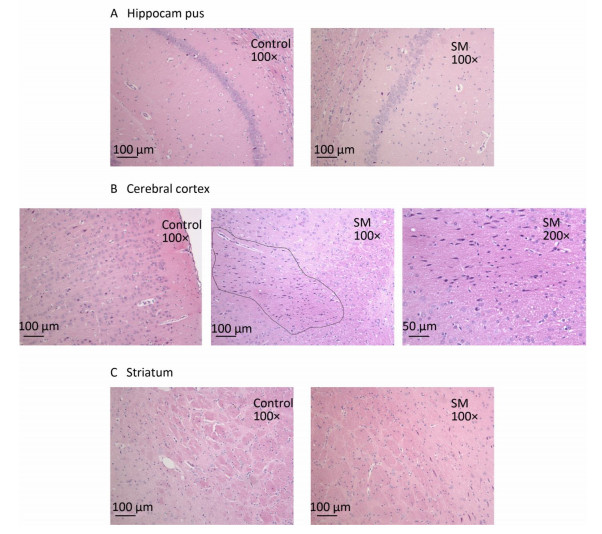
After 7-day TS, no obvious morphological alterations were observed in hippocampus, cerebral cortex or striatum. Cellular structures of brain tissue in both groups were normal (Figure 2). However, 21-day TS induced atrophy of regional neurons in cerebral cortex of 21-day SM group (3 out of 6 rats), as shown in Figure 3B.
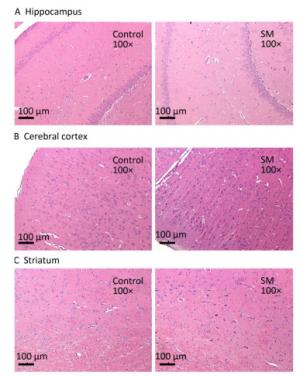
Figure 2. Histomorphology of rat brain regions after 7-day TS. Cellular structures of (A) hippocampus, (B) cerebral cortex, and (C) striatum in both groups were normal. Magnification was shown in each photo. SM: simulated microgravity. TS: tail-suspension.
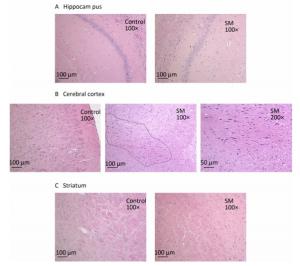
Figure 3. Histomorphology of rat brain regions after 21-day TS. Cellular structures of (A) hippocampus and (C) striatum in both groups were normal. The black loop in (B) illustrated the area where neuron atrophy in cerebral cortex was observed and 200× photo of atrophy was shown as well. magnification was shown in each photo. SM: simulated microgravity. TS: tail-suspension.
-
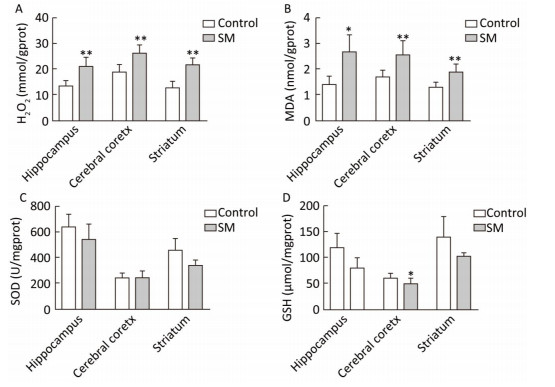
As shown in Figure 4, H2O2 concentration was marked 20.73, 26.00 and 21.25 mmol/gprot in hippocampus, cerebral cortex and striatum of 7-day SM group, respectively, showing sharp increase as compared with 7-day control group (P < 0.01, Figure 4A). MDA levels of 7-day SM group dramatically increased to 2.63, 2.53, and 1.87 nmol/mgprot in the 3 regions (compared with 7-day control group, P < 0.05 or 0.01, Figure 4B). However, few obvious changes were observed in SOD or GSH level of 7-day SM group (except GSH in cerebral cortex showing significant decrease, compared with 7-day control group, P < 0.05, Figure 4C and D).
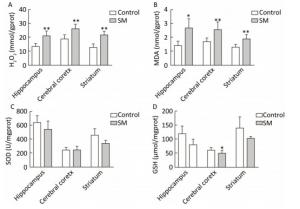
Figure 4. Content of oxidative stress markers in rat brain regions after 7-day TS. Excessive (A) H2O2 and (B) MDA were generated in all regions. However, endogenous antioxidants (C) SOD and (D) GSH were barely affected. Values represented the mean ± SD. *P < 0.05, **P < 0.01, compared with 7-day control group. SM: simulated microgravity. TS: tail-suspension.
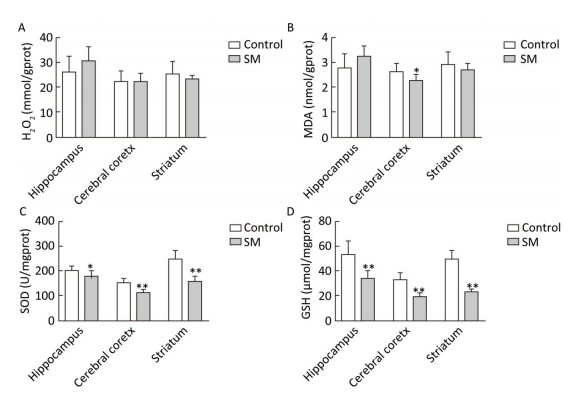
After 21-day TS, H2O2 and MDA in brain regions of 21-day SM group almost returned to physiological levels (MDA in cerebral cortex was even lower as compared with 21-day control group, P < 0.05, Figure 5B). However, remarkable down-regulation of SOD and GSH was observed (compared with 21-day control group, P < 0.05 or 0.01, Figure 5C and D).
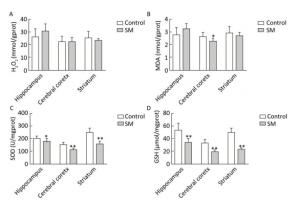
Figure 5. Content of oxidative stress markers in rat brain regions after 21-day TS. (A) H2O2 and (B) MDA returned to physiological levels. However, endogenous antioxidants (C) SOD and (D) GSH were sharply down-regulated. Values represented the mean ± SD. *P < 0.05, **P < 0.01, compared with 21-day control group. SM: simulated microgravity. TS: tail-suspension.
-
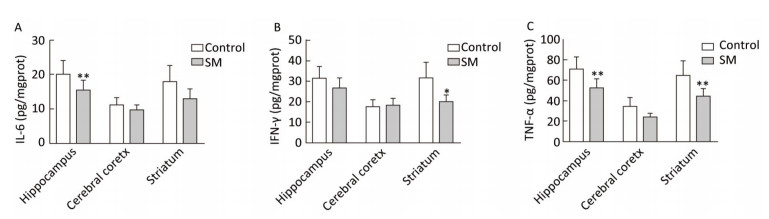
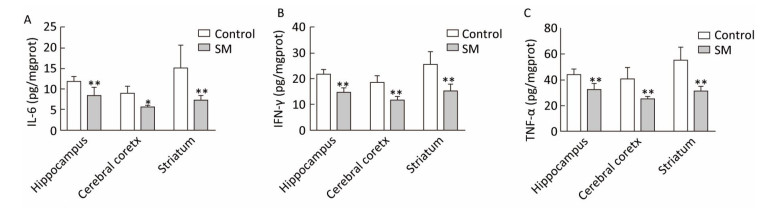
Expression of inflammatory cytokines was illustrated in Figures 6 and 7. 7-day TS induced dramatical decrease of TNF-α in hippocampus and striatum of 7-day SM group (compared with 7-day control group, P < 0.01, Figure 6C). Tests of IL-6 and IFN-γ showed similar results in hippocampus or striatum, respectively (compared with 7-day control group, P < 0.05 or 0.01, Figure 6A and B). Moreover, 21-day TS triggered comprehensive reduction of inflammatory cytokines in all regions of 21-day SM group (compared with 21-day control group, P < 0.05 or 0.01, Figure 7).

Figure 6. Content of inflammatory cytokines in rat brain regions after 7-day TS. Concentration of (A) IL-6, (B) IFN-γ and (C) TNF-α began to decrease in some of the regions. Values represented the mean ± SD. *P < 0.05, **P < 0.01, compared with 7-day control group. SM: simulated microgravity. TS: tail-suspension.
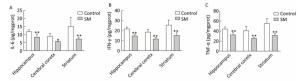
Figure 7. Content of inflammatory cytokines in rat brain regions after 21-day TS. Production of (A) IL-6, (B) IFN-γ, and (C) TNF-α was comprehensively suppressed. Values represented the mean ± SD. *P < 0.05, **P < 0.01, compared with 21-day control group. SM: simulated microgravity. TS: tail-suspension.
-
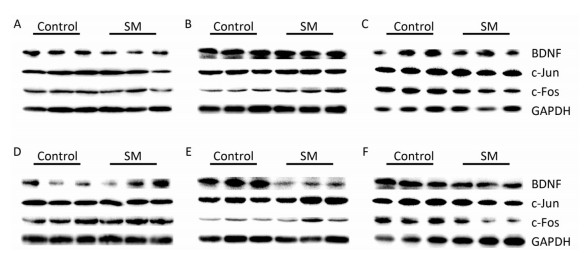
Representative bands of western blot assay were shown in Figure 8.
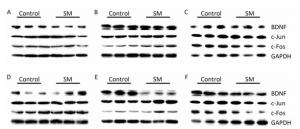
Figure 8. Representative bands of western blot assay. (A) hippocampus, (B) cerebral cortex, and (C) striatum were results after 7-day TS; while (D) hippocampus, (E) cerebral cortex, and (F) striatum were results after 21-day TS. SM: simulated microgravity. TS: tail-suspension.
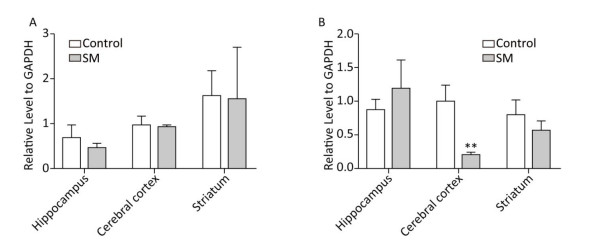
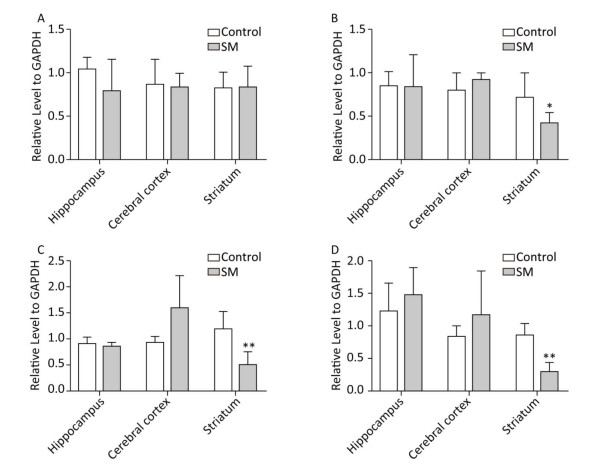
BDNF Relative level of BDNF was shown in Figure 9. Though 7-day TS caused no statistical variations of BDNF, 21-day TS significantly decreased the expression in cerebral cortex by about 81% (compared with 21-day control group, P < 0.01, Figure 9B).
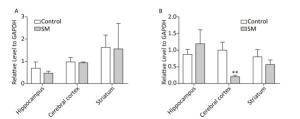
Figure 9. Relative level of BDNF in rat brain regions after (A) 7-day TS and (B) 21-day TS. Expression of BDNF was significantly inhibited by 21-day TS in cerebral cortex. **P < 0.01, compared with 21-day control group. SM: simulated microgravity. TS: tail-suspension.
c-Jun and c-Fos Figure 10 illustrated the relative level of c-Jun and c-Fos. The most remarkable changes of c-Jun/c-Fos expression occurred in striatum. 7-day TS significantly suppressed the expression of c-Fos in striatum of 7-day SM group (compared with 7-day control group, P < 0.05, Figure 10B). After 21-day TS, both c-Jun and c-Fos were sharply down-regulated in striatum of 21-day SM group (compared with 21-day control group, P < 0.01, Figure 10C and D).
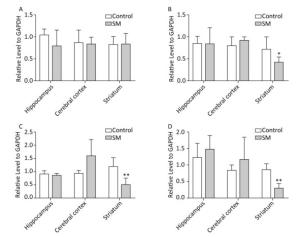
Figure 10. Relative level of (A) c-Jun and (B) c-Fos after 7-day TS; (C) c-Jun and (D) c-Fos after 21-day TS in rat brain regions. The most remarkable changes of c-Jun/c-Fos expression occurred in striatum. *P < 0.05, **P < 0.01, compared with respective control groups. SM: simulated microgravity. TS: tail-suspension.
General Data
Histomorphology
Oxidative Stress
Inflammatory Cytokines
Protein Expression
-
According to the duration of missions, astronauts may undergo short or long-term microgravity. Short-term microgravtiy usually induces acute stress response[31-34]. We tested the content of H2O2 and MDA in rat brain after 3, 5, 7 and 9-day TS, respectively, and found that 7 days was the best duration for oxidative stress induction. On the other hand, 21 days was the well-accepted duration for long-term SM[35-37]. Therefore, in the current study, 7 and 21-day TS were performed to mimic the two term types.
-
We first used hematoxylin-eosin staining to observe morphological alterations. Results revealed that cerebral cortex was the exclusive region where obvious neuron morphologic changes were induced only by long-term SM. A consistent pattern was also suggested in Bi's study: the ultrastructure of rat cerebral cortex was normal after 7-day SM, but showed significant changes after 14 and 21-day SM[38]. BDNF is a key neurotrophic factor supporting neurons in the nervous system[39]. The reduction of BDNF level is a common pathological factor in many neurodegenerative diseases and it was demonstrated that neuron necrosis was directly associated with the lack of BDNF[40, 41]. Therefore, the expression of BDNF was tested. Significant decrease of BDNF content was observed in cerebral cortex of 21-day SM group. Hence, we inferred that neuron atrophy was induced partially through the down-regulation of BDNF[42, 43].
The correlation between microgravity/SM and the expression of BDNF was seldom studied and results showed great contradiction. Data about the mRNA or protein level of BDNF in several regions of mouse brain after long-term spaceflight (30 or 91 days) indicated that BDNF was not sensitive to microgravity[44, 45]. However, using TS model, Wu reported notable down-regulation of BDNF in rat cerebral cortex after 48 days, which was in line with the present study[42]. Nevertheless, our data revealed that the impacts of SM depended on brain region and time course. Cerebral cortex is the superior center that governs the sensation, motion, learning and memory of body[46-51]; and vulnerable to chronic stress[42]. Therefore, impairments of cognitive[52-54] and motor[8, 10] functions in astronauts undergoing long-term microgravity might be partially caused by the dysregulation of BDNF in cerebral cortex and exogenous supplement of neurotrophins might be needed accordingly.
-
When the generation of reactive oxygen species (ROS), such as H2O2, exceeds the potential of anti-oxidative cascades, oxidative stress is triggered[55]. Under this condition, ROS can interact with metal ions producing highly reactive radicals, which can heavily oxidize biomacromolecules[56]. Lipids are the most sensitive cellular components to oxidative stress-associated damage and lipids peroxidation leads to the production of MDA, recognized as the marker of oxidative stress[57]. The sharp increase of H2O2 after 7-day SM indicated that excessive ROS was generated in rat brain tissue. Meanwhile, the accumulation of MDA verified the oxidative damage. Usually, H2O2 can be converted to H2O by catalase, but we did not find evident catalase activity in any of the brain regions. Therefore, SOD and GSH were selected as the representative enzymatic and non-enzymatic anti-oxidative molecules. Our data revealed that neither SOD nor GSH was affected by short-term SM and hence, oxidative stress observed in 7-day SM group might be simply attributed to the excessive ROS. On the contrary, long-term SM strongly down-regulated the expression of SOD and GSH. Interestingly, no excessive H2O2 was detected in any of the brain regions and MDA also returned to physiological level in 21-day SM group. These phenomena probably implied an adaptive mechanism of rat brain to long-term SM.
Brain is rich in lipids (especially polyunsaturated fatty acids), oxygen (due to its high metabolic activities), metal ions, but lack of anti-oxidative enzymes (especially catalase)[58, 59]. Therefore, brain is prone to oxidative stress, which is observed in several neurodegenerative diseases, such as Parkinson's disease, Huntington's disease, and Alzheimer's disease[60]. However, few studies have linked oxidative stress to cerebral impairments in microgravity with inconsistent and even contradictory outcomes. Wise demonstrated that increase of ROS and MDA, coupled with decrease of GSH were induced in mouse brain after 7-day SM[61]. Chowdhury and Soulsby reported the accumulation of MDA in rat brain after 14 and 21-day SM, respectively[62, 63]. Wang simply showed decrease of SOD level in rat hippocampus after 28-day SM[64]. Recently, a study regarding the dynamic impacts of SM on oxidative stress was conducted, in which increase of MDA and decrease of SOD and catalase were suggested to be significant only after 14-day SM[65]. Our data indicated an 'induction-attenuation' pattern of ROS and a 'stabilization-inhibition' pattern of endogenous antioxidants. In spite of the inconformity, oxidative stress is believed to be triggered during SM. Thus, anti-oxidative therapies should be taken into consideration for manned spaceflight.
-
The absence of gravity in the space would impair the function of immune system[66, 67] and lead to various infections during missions[68]. Studies about immune function under microgravity condition were mainly centered on plasma or immune organs, including thymus and spleen. For example, Kaur found that the ability of human innate immune system to phagocytise bacteria and to initiate oxidative burst was crippled after spaceflight[69, 70]. An in vitro study using RAW 264.7 cells indicated that SM suppressed the LPS-induced generation of TNF family proteins[71]. Novoselova also reported that microgravity lowered the content of IL-6 and IFN-γ in mouse plasma[72]. It might be the first time in the present study that immune molecules in SM brain tissue were evaluated. Our data doubtless suggested that SM suppressed the production of inflammatory cytokines in rat brain and this inhibitory effect strengthened with the extension of SM duration.
The regulation of inflammatory cytokines is very complicated and several kinds of transcription factors may participate in the pathway, including NF-κB, PI-3K/Akt, MEK/Erk and AP-1[73-75], among which AP-1 is the most studied one[76-79]. AP-1 consists of proteins from Jun/Fos family, such as c-Jun, JunB, c-Fos and FosB and functions as a dimer binding to enhancer sequence of target genes[76, 77, 79]. In a previous study of our group, AP-1 components c-Jun and c-Fos showed good positive correlation with inflammatory cytokines levels in rat brain[80]. Therefore, we analyzed the relative content of c-Jun and c-Fos using western blot method. However, only results acquired from striatum supported the hypothesis that SM inhibited the expression of IL-6, IFN-γ and TNF-α through AP-1 pathway. The dominant regulating mechanism in hippocampus and cerebral cortex of TS rat brain needed further investigations.
-
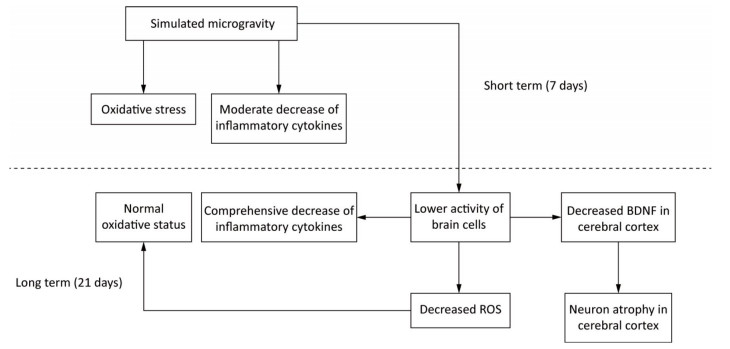
BDNF in brain is primarily synthesised by neurons and astrocytes[59]. Additionally, cerebral inflammatory cytokines are mainly secreted by activated microglia[73, 81, 82]. So the reduction of BDNF, inflammatory cytokines and antioxidants after long-term SM probably resulted from the decreased activity of brain cells. This repression initiated by SM might affect the physiological functions of brain indeed. However, the activated neurogliocytes can also exhibit cytotoxic effects through the release of ROS and nitric oxide[74]. Hence, the decreased activity might be an adaption mechanism to restrain the production of ROS and in this way restore the oxidative status in 21-day SM group (Figure 11).
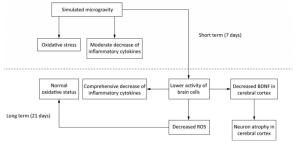
Figure 11. A possible mechanism of SM in affecting rat brain. Short-term SM triggered strong oxidative stress in all studied regions. Long-term SM inhibited the activity of brain cells. On one hand, the inhibition down-regulated the levels of inflammatory cytokines in all studied regions and BDNF in cerebral cortex, which resulted in neuron atrophy, but on the other, the inhibition attenuated the oxidative stress induced by SM. BDNF, brain derived neurotrophic factor. ROS, reactive oxygen species. SM: simulated microgravity.
The Decrease of BDNF might Contribute to Neuron Atrophy in Cerebral Cortex Induced by 21-day SM
Oxidative Stress was Triggered after Short-term SM, but was Quenched after Long-term SM
SM Suppressed the Production of Inflammatory Cytokines and in Striatum, the Suppression was Regulated Partially through c-Jun/c-Fos
A Proposed Mechanism of SM in Affecting Rat Brain
-
Collectively, SM showed obvious impacts on rat brain tissue in a duration and region-dependent manner. It induced atrophy of regional neurons, oxidative stress, together with repression of inflammatory cytokines. The results might provide further neurological insight of SM with a clue to assess the risk and to prevent brain damage for astronauts in space travel.
-
DENG Yu Lin and LI Yu Juan designed this work. CHEN Bo and ZHANG Yu Shi performed the experiments and analyzed the data. CHEN Bo, LI George and CHO Jun-Lae wrote the manuscript.
-
The authors declare that there are no conflicts of interest.







 Quick Links
Quick Links
 DownLoad:
DownLoad: