HTML
-
Recurrent outbreaks of influenza A virus cause respiratory disease in humans and animals, which significantly affect global health and the economy[1, 2]. Recent studies have demonstrated that influenza A (H3N2) virus significantly contribution to influenza morbidity and mortality worldwide[3, 4]. At present, influenza vaccines are considered the most effective strategy for the prevention and control of influenza[5, 6]. Currently, only three classes of influenza vaccines are licensed, these include inactivated, live attenuated and recombinant hemagglutinin (HA) vaccines[5, 7]. Although the respiratory mucosal surface is the main site of influenza virus infection and the resulting immune response, inactivated vaccines and HA vaccnines are intramuscularly or subcutaneously injected for immunization. These vaccines can induce a systemic response but no mucosal response, despite the mucosa being the site of entry and colonization for most pathogens[8]. Live attenuated influenza virus (LAIVs) vaccines are the only licensed mucosal vaccines. However, LAIVs can only be used in healthy people between the ages of 2 to 49 year old[9, 10]. In addition, recent studies have shown that mucosal vaccine elicits both systemic and mucosal immunity to induce IgG and secretory IgA (sIgA) production[8, 11]. The mucosal immune system is the first line of defense against influenza virus infections, and protective mucosal immune responses would be most effectively induced by mucosal immunization. Therefore, mucosal vaccines are a promising strategy for preventing pathogens that enter the host through the mucosal membrane.
Compared with traditional immunization routes, mucosal vaccination has a lot of advantages, including needle-free administration, reduced risk of cross-contamination and increased compliance[12-14]. However, no inactivated and recombinant HA mucosal vaccine has been licensed for humans, largely because of the lack of mucosal adjuvants. Hence, the development of mucosal adjuvants would significant in terms of nonliving mucosal vaccine development. Previous studies have proven that cholera toxin (CT) and Escherichia coli heat- labile toxin (LT) could significantly induce mucosal immunity when administered orally or intranasally[15]. However, they have not been developed commercially because of toxicity problems. Hence, a safe and effective mucosal adjuvant is needed for the delivery of mucosal vaccines.
CTA1-DD, which consists of the A1 subunit of cholera toxin (CTA1) linked to the dimer (DD) of Staphylococcus aureus protein A, is a well-known safe and effective mucosal adjuvant. In 1997, the adjuvanticity of CTA1-DD was first reported. In this study, systemic IgG and mucosal IgA responses were induced by nasal vaccination with CTA1-DD adjuvant, as well as CT. However, CTA1-DD was completely nontoxic[16]. Subsequenly, many studies focused on CTA1-DD as a mucosal adjuvant. Several studies have evaluated its efficacy in the context of vaccines aimed at preventing infectious diseases. In 2006, Akhiani found that intranasal immunization with CTA1-DD adjuvant could enhance protective immunity against Helicobacter pylori infection[17]. Andernsen also confirmed that this adjuvant could boost prior BCG immunity to Mycobacterium tuberculosis[18]. In addition, researchers demonstrated that it could also be used in HIV, HPV and rotavirus mocusal vaccines[14, 19, 20]. We have reported previously that nasal immunization with CTA1-DD could stimulate significant protection against Ebola virus and influenza A (H1N1) virus in mice[21, 22]. Based on these reports and suggestions, we believed it was also effective in other mucosal vaccines. Thus, this study was underta- ken to investigate whether nasal immunization with CTA1-DD and H3N2 split vaccine stimulated enhanced protective immunity against H3N2 influenza virus.
-
Pathogen-free female BALB/c mice aged 6-8 weeks were obtained from Beijing Vital River Laboratory Animal Technology (Beijing, China) and maintained under pathogen-free conditions. All animal experiments in this study were approved by the Animal Experimental Ethical Committee of the Nation Institute for Viral Disease Control and Prevention (No. 20170606019-2). The mice were euthanized by CO2 inhalation.
-
Influenza virus A/Hong Kong/1968 (H3N2 subtype) was used in the challenge infection. It was kindly supplied by Professor Earl Brown from the University of Ottawa. This virus was grown for 48 h in 9-11-day-old embryonated eggs at 37 ℃. Allantoic fluid was collected and stored at −80 ℃ until use.
H3N2 split vaccine (A/Hong Kong/4801/2014) (designated as sH3N2 vaccine) was donated by Yening Zou from Sinovac Biotech Ltd. Adjuvant of alum (aluminum hydroxide, 10 mg/mL) was purchased from Thermo ScientificTM (United States). Adjuvant of CTA1-DD was expressed in Escherichia coli (E. coil) BL21 (DE3) as described previously[22].
-
The formulations of vaccine and adjuvant were as previous reported[21].
-
Sixty mice were randomly assigned to five groups (Table 1). All animals were immunized twice, two weeks apart. Mice were anesthetized with CO2 before nasal immunization with 3 μg of H3N2 vaccine, either alone or with 5 μg of CTA1-DD in a final volume of 50 μL administered dropwise at 25 μL per nostril. Negative control mice were administered CTA1-DD alone or phosphate-buffered saline (PBS) via the i.n. route. Positive control mice were administered alum adjuvant plus H3N2 split vaccine via the intramuscular (i.m.) route. Two weeks later, mice received a second dose.
Groups Adjuvant Antigen Route PBS - PBS i.n. vaccine alone - H3N2 split vaccine i.n. vaccine+alum Al(OH)3 H3N2 split vaccine i.m. vaccine+CTA1-DD CTA1-DD H3N2 split vaccine i.n. CTA1-DD alone CTA1-DD - i.n. Table 1. Immunizations
On days 0, 14, 21, 28, and 35, blood samples were collected and serum were separated by centrifugation and stored at −20 ℃ prior to analysis. One week after boost, some mice were euthanized, and the spleens, bronchoalveolar lavages and vaginal lavages were collected.
For the challenge studies, at three weeks after boost, six mice from each group were anesthetized with CO2 and then infected with the minimal level of five times the 50% mouse lethal dose (5 × MLD50) of the mouse-adapted re-assortant influenza A H3N2 virus strain A/Hong Kong/1968 in PBS via the i.n. route. Body weight changes and mortality were monitored for 14 days after challenge.
-
HI assays were performed as described previously[23]. In this study, 4 HA units of the H3N2 A/Hong Kong/4801/2014 virus and 1% guinea pig red blood cells were used.
Antibody titers of influenza virus-specific IgM, IgG, IgG1, IgG2a, and IgA were determined using enzyme-linked immunosorbent assays (ELISAs). In brief, the assays were performed in 96-well plates coated at 4 ℃ for 12 h with 200 ng/well antigen protein, and then blocked with 3% BSA. Serially diluted serum and mucosal samples were incubated at 37 ℃ for 2 h in the plates. Antibody titers were measured with HRP-conjugated goat anti-mouse IgM, IgG, IgG1, IgG2a, and IgA secondary antibodies (Sigma-Aldrich, St. Louis, MO, USA) and TMB substrate (Solarbio, Beijing, China). When the reaction was stopped with 2 mol/L H2SO4, the values at 450 nm were detected by an ELISA reader (Thermo Scientific, Vantaa, Finland) and endpoint titers were defined as the highest dilution of sample for which the optical density (OD) was at least twice that of the negative control samples.
-
Two weeks after boost, the spleen samples were analyzed through interferon-γ (IFN-γ) and interleukin-4 (IL-4) ELISpot assays using an ELISpot kit (Dakewe, Beijing, China) according to the manufacturer's instructions.
-
One-way ANOVA with Tukey's multiple comparison tests (GraphPad Prism v.5) were used for the analysis of significance in all experiments groups. In addition, the Kaplan-Meier test was used to compare percent survival among groups of mice. Statistical significance is presented as P-values: P < 0.05 (*), P < 0.01 (**) and P < 0.001 (***).
Animals and Ethical Statements
Virus, Vaccine and Adjuvants
Vaccine Formulation
Animal Immunization, Sample Collection and Challenge
Hemagglutination Inhibition Assays and Antibody Responses
T Cell ELISpot
Data Analysis
-
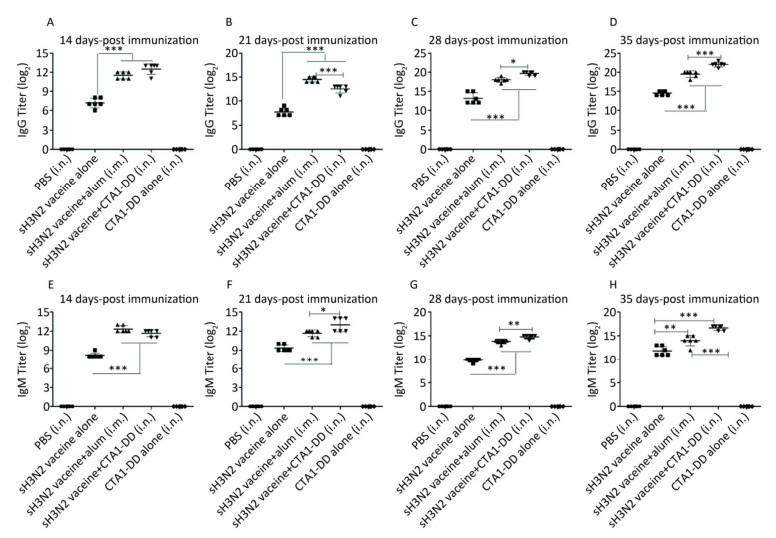
To characterize the mucosal adjuvant efficacy of CTA1-DD, mice were immunized as described in Table 1 and sera were collected on days 14, 21, 28, and 35 following primary immunization. For all of the samples, the antigen-specific IgG and IgM titers were determined (Figure 1). With increased immunization time, the IgG and IgM titers increased gradually, especially the H3N2 split vaccine plus CTA1-DD adjuvant. Compared with the sH3N2 vaccine group, mice in the sH3N2 vaccine combined with CTA1-DD or alum groups showed significantly increased antigen-specific IgG and IgM titers (P < 0.001; Figure 1). There was no significant difference in antibody titers between the alum adjuvant group and the CTA1-DD adjuvant group before booster vaccination (Figure 1A, E). Before challenge, the IgG and IgM titers of the CTA1-DD adjuvant group were significantly enhanced compared with the alum adjuvant group (P < 0.001; Figure 1D, H).
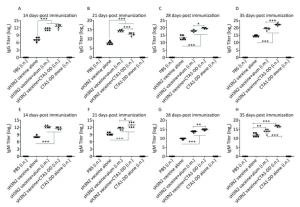
Figure 1. H3N2 HA-specific IgG and IgM are significantly enhanced by CTA1-DD adjuvant. Mice were immunized with PBS, CTA1-DD (5 μg/mouse), H3N2 split vaccine (3 μg/mouse) without or with CTA1-DD (5 μg/mouse)/alum adjuvant at days 0 and 14. Sera were collected on days 14, 21, 28, and 35. Then, H3N2 HA-specific IgG (A-D) and IgM (E-H) were determined by ELISA. Statistical analyses consisted of one-way ANOVA with Tukey's multiple comparison tests. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
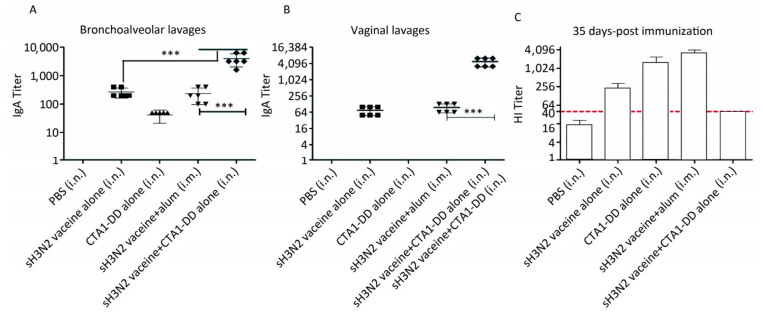
To test whether the CTA1-DD adjuvant was able to enhance the immunogenicity of influenza vaccines, we examined the HI titers (Figure 2C). Immunized mice did not induce detectable HI titers following primary immunization (data not shown). On day 35 post immunization, mice immunized with vaccine plus CTA1-DD developed significantly higher HI titers than the alum-adjuvant vaccine group (P < 0.05; Figure 2C). As shown in Figure 2C, the HI titer of the CTA1-DD-adjuvant vaccine group was approximately two-fold higher than that detected for the alum-adjuvant vaccine group.
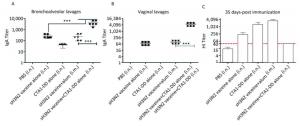
Figure 2. CTA1-DD increased the mucosal antibody responses and HI titers. Mice were immunized intranasally with PBS (negative control), or H3N2 split vaccine (3 μg/mouse) alone, or CTA1-DD (5 μg/mouse) alone, or H3N2 split vaccine (3 μg/mouse) plus CTA1-DD (5 μg/mouse). Positive control mice were immunized intramuscularly with H3N2 split vaccine (3 μg/mouse) and alum adjuvant. Three weeks after booster immunization, bronchoalveolar lavages (A) and vaginal lavages (B) were collected, and H3N2 HA-specific IgA was measured. Sera were also collected and HI titers (C) were assessed. The data are expressed as the mean ± SD (n = 6 mice per group). One-way ANOVA with Turkey's multiple test was used to analyze differences among groups. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
-
To investigate the mucosal antibody responses in mucosal lavages, we measured the sIgA titers in bronchoalveolar lavages and vaginal lavages. The results consistently demonstrated that CTA1-DD adjuvant could significantly improve the levels of sIgA in bronchoalveolar lavages and vaginal lavages (Figure 2A, B). However, sH3N2 vaccine alone or administered together with alum elicited extremely low sIgA titers (Figure 2A, B). These results highlighted the ability of CTA1-DD to induce strong mucosal responses, suggesting that it might be a promising mucosal adjuvant for the development of nasal vaccines.
-
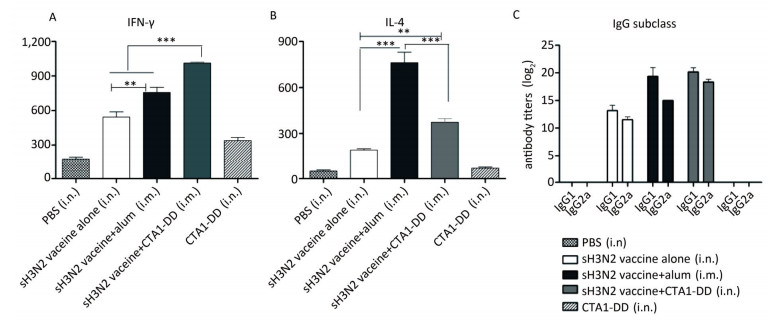
To evaluate the induced cellular responses, sera were analyzed by ELISA to determine the quantities of H3N2 HA-specific IgG1 and IgG2a, and the results are shown in Figure 3C. The two IgG subclasses, IgG1 and IgG2a, serve as markers for Th1 (IgG2a) and Th2 (IgG1) cells. Whether alum or CTA1-DD was used as adjuvant, the levels of IgG1 and IgG2a were significantly higher than those of the vaccine alone group (Figure 3C). Mice immunized with sH3N2 vaccine alone showed higher levels of IgG1 than IgG2a, and the IgG1/IgG2a ratio was 4/1. When alum was used as adjuvant, the IgG1/IgG2a ratio was increased to 19.69/1, indicating that alum adjuvant triggered a Th2-type response. However, using CTA1-DD as adjuvant reduced the IgG1/IgG2a ratio to 2.46/1 compared with vaccine alone, suggesting that CTA1-DD adjuvant enhanced the Th1-type response (Figure 3C).
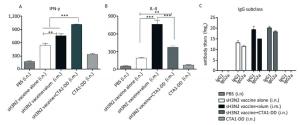
Figure 3. CTA1-DD enhanced the Th-1-type response. Animals (n = 6) were immunized twice (day 0 and day 14) with PBS, CTA1-DD (5 μg/mouse), H3N2 split vaccine (3 μg/mouse) without or with CTA1-DD (5 μg/mouse)/alum adjuvant. Cytokine production from splenocytes derived from immunized mice was determined. Three weeks after the booster, spleens were collected and splenic lymphocytes were cultured. The production of IFN-γ (A) and IL-4 (B) were measured by ELISpot. Sera were also collected and IgG1 and IgG2a (C) titers were detected. The data are expressed as the mean ± SD (n = 6 mice per group). One-way ANOVA with Turkey's multiple was used to analyze differences among groups. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To further assess the type of immune response evoked by CTA1-DD, we investigated the production of IFN-γ/Th-1 and IL-4/Th-2 by splenocytes (Figure 3A, B). Splenocytes from mice vaccinated with H3N2 split vaccine and alum showed a high level of IL-4 secretion compared with other groups (P < 0.0001; Figure 3B). Furthermore, the level of IFN-γ secretion elicited with the CTA1-DD-adjuvant vaccine was significantly higher than with other groups (P < 0.0001; Figure 3A). These data revealed that CTA1-DD enhanced a Th-1-type response.
-
Next, we asked whether CTA1-DD could be used as an adjuvant to produce protective immunity against H3N2 virus infection following i.n. vaccination. CTA1- DD or alum adjuvant were administered with H3N2 split vaccine and mice were immunized with two doses. Two weeks after the boost, mice were challenged with 5 × MLD50 mouse-adapted re-assortant influenza A H3N2 virus strain A/Hong Kong/1968, and the survival rates, body weight loss and clinical symptoms were recorded for 2 weeks.
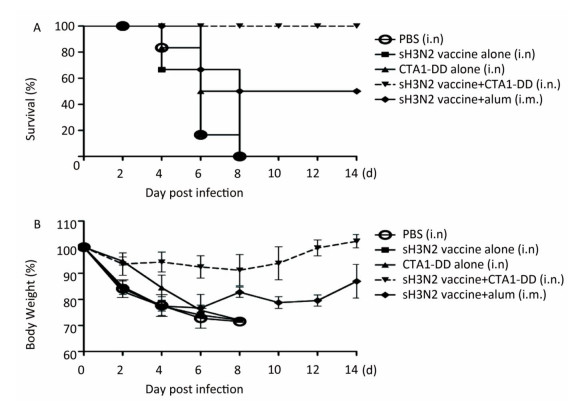
As expected, none of the mice in the PBS and CTA1-DD alone groups survived; all had died by days 4-8 (Figure 4A). In addition, no mice survived in the H3N2 split vaccine alone group. As shown in Figure 4A, only partial protection was provided when alum was used as an adjuvant, with the mice showing survival rates of 50%. Notably, the mice that received H3N2 split vaccine plus CTA1-DD exhibited 100% protection (Figure 4A). Furthermore, the body weights of this group barely changed, whereas the group immunized with vaccine plus alum exhibited body weight losses of approximately 20% (Figure 4B). Interestingly, except for the sH3N2 vaccine plus CTA1-DD group, the other groups developed symptoms of pilo-erection, anorexia and listlessness. Furthermore, 4 days after infection, clinical symptoms appeared in the sH3N2 vaccine plus alum group that had gradually disappeared by day 7.
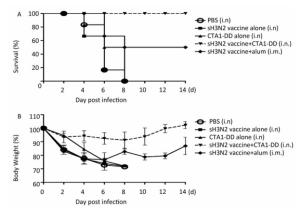
Figure 4. Protection of mice from lethal influenza virus. Mice were immunized with PBS, CTA1-DD (5 μg/mouse), H3N2 split vaccine (3 μg/mouse) without or with CTA1-DD (5 μg/mouse)/alum adjuvant at days 0 and 14. Three weeks after the last immunization, the immunized mice were challenged intranasally with 5 × MLD50 of the mouse-adapted re-assortant influenza A H3N2 virus strain A/Hong Kong/1968. Mice (n = 6) were monitored every two days for 14 days for body weight changes (A) and survival rates (B).
Taken together, i.n. vaccination with CTA1-DD as an adjuvant induced strong protective immunity against a lethal virus challenge infection in mice.
CTA1-DD Enhanced Humoral Immune Responses in Mice
CTA1-DD as Adjuvant Improved the Mucosal Antibody Responses in Mucosal Lavages
CTA1-DD Improved Cellular Responses to H3N2 Virus
CTA1-DD as Adjuvant Enhanced the Protective Efficacy of H3N2 Split Vaccine
-
Mucosal vaccination is more potent than traditional injection vaccination routes for the prevention and treatment of infectious diseases, particularly respiratory diseases. This immunization route can evoke both local and systemic immune responses to induce IgG and sIgA production[24, 25]. Because of the requirements for strong mucosal adjuvants, not only to argument immunogenicity, but also to avoid mucosal tolerance, and usually relatively large amounts of antigen[14, 26, 27], a limited number of mucosal vaccines have been approved for use in humans, and these are mainly live attenuated vaccines[28]. However, LAIV, which is a mucosal vaccine delivered via i.n. immunization, has been approved for use in healthy people aged 2-49[29]. As a result of the limitations of LAIV use, the development of an effective but safer mucosal vaccine for influenza is crucial. Compared with LAIV, split vaccine is a safer option. However, the antigenicity of the influenza split vaccine is low and i.n. immunization with this vaccine alone does not induce full mucosal immunity, such as sIgA[28]. Thus, it is necessary to combine the split vaccine with an effective mucosal adjuvant. In the present study, we evaluated the use of CTA1-DD-adjuvanted H3N2 split vaccine as a i.n. vaccine in mice.
CTA1-DD was first proposed as a mucosal adjuvant in 1997 and was shown to effectively augment B and T cells to produce antibodies or interferons and interleukin cytokines[16, 30]. Subsequent studies showed that it could provide effective protection against HIV, HPV, Helicobacter pylori and Chlamydia trachomatis by i.n. immunization as a mucosal vaccine[14, 17, 20, 31]. Based on our previous studies on i.n. immunization with CTA1-DD-adjuvanted H1N1 and EBOV vaccines[21, 22], we speculated that CTA1-DD might be an ideal safe mucosal adjuvant for intranasal vaccination of an influenza H3N2 vaccine.
Mucosal routes of immunization mainly include oral, nasal, rectal and vaginal delivery[32, 33]. Previous studies have demonstrated that intranasal vaccination could induce effective local and systemic immune responses in the respiratory and genitourinary tracts[34, 35]. Influenza H3N2 virus infection and transmission sites are generally imited to the upper respiratory tract mucosal epithelium[36]. In addition, Erilsson et al. showed that immunization via the i.n. route required less antigen and adjuvant than oral immunization[37]. Therefore, in our study i.n. vaccination was performed to ssess the effectiveness of CTA1-DD as a mucosal adjuvant.
Our results demonstrated that CTA1-DD is a highly efficient and safe mucosal adjuvant that can significantly enhance B and T cell responses following i.n. immunization. To determine the level of the B cell immune response, the levels of IgM and IgG in serum and IgA in mucous were measured. We found that both alum adjuvant and CTA1-DD mucosal adjuvant could induce higher levels of serum IgM and IgG compared with the H3N2 split vaccine alone (Figure 1). Alum adjuvant was more effective than CTA1-DD mucosal adjuvant after primary immunization, that is, the titers of serum antibodies were slightly higher than those induced by mucosal adjuvant (Figure 1A and E). However, the effect of CTA1-DD adjuvant was significantly higher than that of alum adjuvant after secondary immunization (Figure 1C, D and Figure 1F-H). These results indicated that CTA1-DD-adjuvanted H3N2 split vaccine administered by i.n. immunization could stimulate B cells to induce a significant humoral response in the serum. Recent studies have demonstrated that CTA1-DD as a mucosal adjuvant could significantly increase antigen-specific IgA titers[17, 20, 31]. Consistent with this finding, we found a significant increase in H3N2 HA-specific IgA titers in bronchoalveolar lavages and vaginal lavages following the use of CTA1-DD as a nasal adjuvant. By contrast, only low IgA titers were measured in the mucosal lavage fluids of the other groups (Figure 2A and B). We confirmed that i.n. immunization with CTA1-DD adjuvant and H3N2 split vaccine was more effective at preventing influenza H3N2 virus infection than intramuscular immunization with alum adjuvant.
At present, the serum HI titer is regarded as the criterion for evaluating the effectiveness of influenza vaccine[38]. In this study, no effective HI titer was measured after the primary vaccination in all groups (data not shown). Interestingly, 21 days after the booster vaccination (35 days-post immunization), the other groups showed an effective protective response, with the exception of the PBS group and the split vaccine alone group (Figure 2C). The definition of a 'protective' response in this case is a post-immunization serum HI titer of ≥ 1:40[39]. For all we know, all adults and children aged 3 years old or over are given single dose of the influenza vaccine[40]. But in our study, the vaccine must be given twice for mice. We inferred that the reason for this phenomenon was that human have been infected influenza viruses in nature, while the mice in the experiment were SPF mice that had not been infected with any pathogen, and the mice had no memory response.
Studies have shown that cell-mediated immunity is related to the development of immunity against influenza infection[41-43]. Our results revealed that the H3N2 split vaccine formulated with CTA1-DD as an adjuvant significantly promoted the proliferation and secretion of IFN-γ, indicating that this mucosal adjuvant predominantly induces a Th1-type immune response (Figure 3A). By contrast, alum adjuvant elicited higher secretion of IL-4 and triggered a predominant Th2-type immune response (Figure 3B). Studies have suggested that the production of IgG1 was associated with a Th2-type immune response, whereas IgG2a production was associated with a Th1-type immune response[44]. Consistent with the cytokine profiles, the mice nasally immunized with the CTA1-DD-adjuvanted H3N2 split vaccine showed a reduced ratio of IgG1/IgG2a (2.46/1), whereas alum improved the ratio to 19.69/1 (Figure 3C).
Lastly, we also examined the efficacy of H3N2 split vaccine plus CTA1-DD in protecting against mouse-adapted H3N2 virus challenge. In the present study, alum and CTA1-DD, when used as adjuvants in the H3N2 split vaccine, induced strong systemic responses. However, following virus challenge, the survival rate of mice immunized with alum as an adjuvant by intramuscular immunization was only 50% and these mice displayed various clinical symptoms, such as pilo-erection, anorexia and listlessness, which had disappeared by day 8 after challenge (Figure 4). Notably, when the mice were nasally administered CTA1-DD as adjuvant, a survival rate of 100% was observed along with no detectable clinical symptoms. The other groups all developed serious clinical symptoms and died. There may be several reasons for these differences. First, the H3N2 split vaccine has poor immunogenicity and cannot induce a protective immune response. When adjuvant was added, both alum and the mucosal adjuvant CTA1-DD improved the immunogenicity and stimulated an effective humoral response. In our study, H3N2 split vaccine plus alum immunized via intramuscular injection successfully induced IgG and IgM antibodies. These antibodies can prevent the severity of symptoms and the progression of disease following challenge[26], but cannot protect against the virus. In addition, this immunization method induced lower sIgA in the local upper respiratory tract, whereas an extremely low titer of sIgA was elicited when CTA1-DD was used as a nasal mucosal adjuvant. sIgA antibodies can neutralize the virus at the respiratory surface, prior to virus entry into cells. Studies have indicated that sIgA antibodies, which were secreted in the upper respiratory tract, were considered the first line of defense to prevent viral infection[45, 46]. This evidence highlights the role of sIgA in preventing influenza H3N2 virus infection. Finally, influenza is cleared by cytotoxic T lymphocytes that can kill virus-infected host cells by a granule-mediated method. Cytotoxic T lymphocytes are recruited to influenza-infected lungs by a Th-1-type response, specifically due to the production of IFN-γ[41, 47]. Our study confirmed that CTA1-DD-adjuvanted H3N2 split vaccine activated IFN-γ secretion causing a predominant Th-1-type response. Collectively, the enhanced protection offered by the CTA1-DD-adjuvanted H3N2 split vaccine could be due to the effect of higher levels of serum IgG and IgA, the induction of mucosal IgA and serum HI titers, as well as the activation of a Th-1-type response.
In conclusion, we provide a novel nasal split vaccine for seasonal influenza caused by H3N2 virus, using CTA1-DD as a mucosal adjuvant. This nasal vaccine induced stronger mucosal responses suggesting that CTA1-DD is a promising mucosal adjuvant for the development of mucosal influenza vaccines.
-
We thank Professor Earl Brown (Faculty of Medicine, University of Ottawa, Ottawa, Canada) for kindly providing the mouse adapted influenza A H3N2 virus strain A/Hong Kong/1968. We also thank Dr. Jing Tang (National Institute for Viral Disease Control and Prevention, Beijing, China) for excellent technical assistance.







 Quick Links
Quick Links
 DownLoad:
DownLoad: