-
Industrialization and urbanization have increased air pollution in developing countries. Haze pollution is now a severe environmental concern in China; it is mainly characterized by PM2.5, a particulate matter having an aerodynamic diameter of 2.5 μm or less. Northern China experiences persistent haze pollution that affects many cities and millions of people, every winter and spring. According to the World Health Organization (WHO), persistent atmospheric PM2.5 concentrations exceeding 10 μg/m3, increase the risk of death. The elderly, children, and patients with heart and lung conditions are most susceptible to PM2.5 contamination. Long-term PM2.5 exposure can lead to cough, dyspnea, pulmonary disorders, asthma, and can cause increased premature death in patients with chronic bronchitis, arrhythmias, non-fatal heart disease, or cardiopulmonary disease[1].
Xi'an, the biggest city in northwest China with 8 million residents and 2 million visitors yearly, suffers severe haze events every winter and spring with the daily average PM2.5 concentration ranging from 130-351 μg/m3; these values exceed that of the Chinese pollution standard (75 μg/m3) by 2-5 fold. PM2.5 poses a significant health challenge for the residents of Xi'an, with 1, 071, 338, 438, 273, and 1, 019, 503 disease cases reported in 2014, 2015, and 2016, respectively[2]. Besides the negative effects on health, PM2.5 can also attach to pathogens, and PM2.5-bound bacteria could exist freely as dividing and vegetative cells or spores in the atmosphere[3]. Although PM2.5-attached microorganisms have been considered a major cause of various allergies and respiratory diseases, little attention has been paid to analyzing the microbes associated with PM2.5. To comprehensively understand the effect of haze events on public health and to put forward proper prevention and control measures, it is necessary to study the microbial aspects associated with haze. Therefore, the present study investigated the culturable bacterial density and composition in haze events in the spring of 2017 in Xi'an.
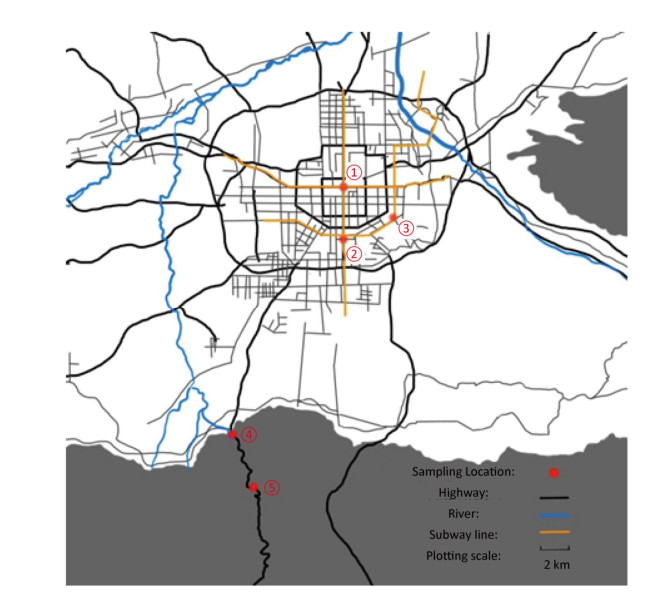
A simple syringe-filter device equipped with a 0.22 µm cellulose acetate microporous membrane (Xinya, China) and 200 mL sterile polypropylene filter (Zhengkang, China) was used for collecting air samples (Supplementary Figure S1 available in www.besjournal.com). Between 12:00 and 13:00 on 11th March, 2017, 200 L of air was collected simultaneously at 5 different sites with different visitor flow rates on the ground; this collected air had an average PM2.5 of 121 μg/m3 (Supplementary Figure S2 and Supplementary Table S1 available in www.besjournal.com, repeated 3 times at each sample site).
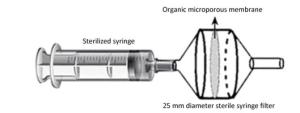
Figure Supplementary Figure S1. The air sample collection device composed of sterile polypropylene filter (25 mm), cellulose acetate microporous membrane (0.22 µm), and sterile polyvinyl chloride syringe (200 mL).
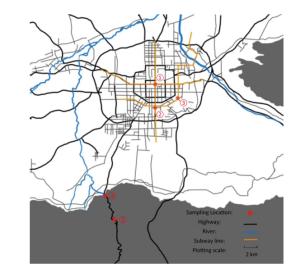
Figure Supplementary Figure S2. Sampling sites. Site 1. Municipal center, highest traffic, and human activity; Site 2. Commercial district south of the city, crowded shopping malls, and highest human activity; Site 3.Suburbs southeast of the city, urban-rural fringe area, and moderate human activity; Site 4. Exit of the valley and low human activity; Site 5. Deep in the valley and hardly any human activity.
Sampling Site Characteristics Approximate Visitors Flow Rate 1. Beidajie subway station Transfer station of subway 3 hospitals nearby 200, 000/day 2. Xiaozhai south road Business district nearby 100, 000/day 3. Shapo village Suburbs, 1 university nearby 30, 000/day 4. Jingye temple Entrance of the valley Nature tourist attractions < 1, 000/day 5. Jiulong pond Deep in the valley Hardly any Table Supplementary Table S1. Sampling Site Details
A high-rise building with busy traffic, industries, and other activities emitting particulate matter was selected in sampling site 2. Air samples (200 L), 1 m outside the window on the 1st, 11th, and 21st floors (0, 30, and 60 m above the ground) of the high-rise building, were also collected from 12:00-13:00 on 11th March, 2017, (repeated 3 times at each sample site).
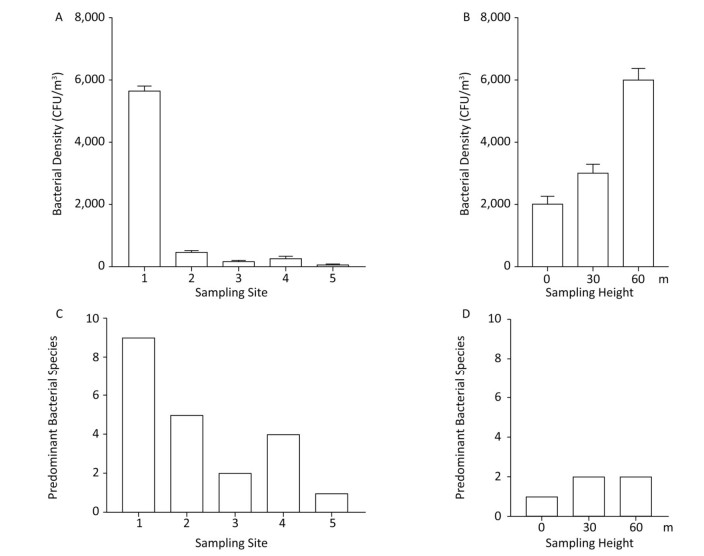
Samples collected were immediately taken to the Xi'an Jiaotong University, and the microporous membrane was sheared and placed in 2 mL of sterile PBS (pH 7.0), and vortexed for 5 min. The suspension was then serially 10-fold diluted with PBS and 20 μL of the dilution was spread on Columbia blood agar plates. The plates were incubated at 37 ℃ for 72 h, following which, the Colony Forming Units (CFU) were counted. Bacterial density at site 1 was approximately 5, 000 CFU/m3 and contained 9 prominent bacterial species, the highest among the 5 sites (Figure 1). The bacterial density at sites 2 and 3 was about 450 and 150 CFU/m3, with 5 and 2 predominant bacterial species, respectively. In the suburb (sites 4 and 5) the bacterial density was about 250 and 50 CFU/m3, with 4 and 1 predominant bacterial species, respectively. These data suggested that compared to sparsely populated areas, highly populated urban sites have a higher bacterial density. Site 5 had the lowest bacterial density and had the lowest number of predominant bacterial species, indicating a relationship between the bacteria in haze events, population density, and human activity. The bacterial densities at the 1st, 11th, and 21st floors were about 2, 000, 3, 000, and 6, 000 CFU/m3, respectively, indicating that bacterial density increased with height. The numbers of predominant bacterial species were 1, 2, and 2, respectively (Figure 1).

Figure 1. Bacterial density and predominant bacterial species in air samples from different sites and heights.
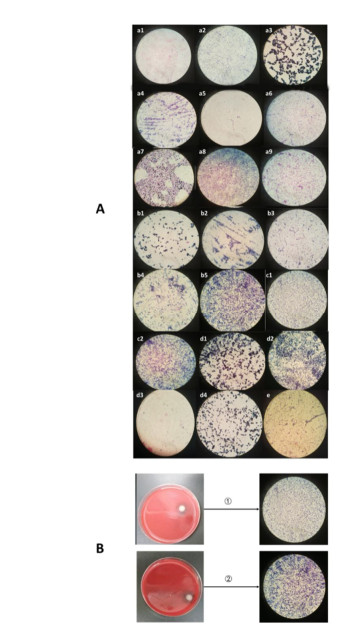
Gram stain was used for preliminarily analysis of the bacterial species at each site. Bacteria in sites 1, 3, 4, and 5 were mainly gram-positive coccus, and site 2 had mainly gram-negative bacterium (Supplementary Figure S3 available in www.besjournal.com). Genomic DNA was extracted from the culturable bacteria using a Bacteria DNA Kit (Tian Gen Biotech, Beijing, China). The V3 region of the 16S rRNA gene was PCR-amplified with the 341F primers (with GC-clamps). PCR reaction mixes comprised 2 μL of Tris-HCl (pH 8.3), 10 μL of ddH2O, 2 μL of 15 mmol/L MgCl2, 1.5 μL of 2.5 mmol/L dNTP, 2.5 U of Taq DNA polymerase, 1 μL of 200 mmol/L primers (V3F+GC:5'-CGCCCGCCGCGCGCGGCGGGC GGGGCGGGGGCACGGGGGGACTCCTACGGGAGGCAGCAG-3' and V3R: 5'-ATTACCGCGGCTGCTGG-3'), and 2 μL of DNA template. PCR was performed using the Golden Easy PCR System, following the thermal cycling parameters: 96 ℃ for 3 min, followed by 40 cycles of 96 ℃ for 1 min, 55 ℃ for 1 min, 72 ℃ for 1 min, and 72 ℃ for 10 min. The PCR products were mixed with 6 x loading buffer (40 mmol/L of Tris, pH 8.0, 40% sucrose, 1.15% acetic acid, 1 mmol/L of EDTA, and 0.25% bromophenol-blue), and analyzed by 2% agarose gel electrophoresis in TAE buffer (40 mmol/L of Tris, 20 mmol/L of acetic acid, 1 mmol/L of EDTA; pH 8.3) at 80 V for 45 min. Ethidium bromide (50 μg/mL) was incorporated into the agarose gel and visualized by UV illumination using the Syngene automatic gel imaging analysis system.
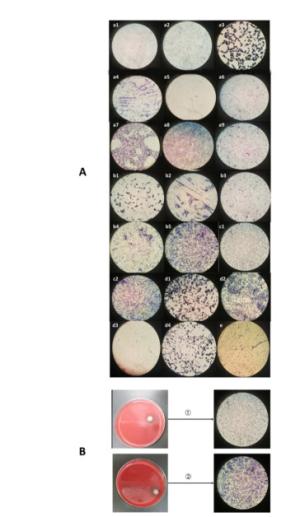
Figure Supplementary Figure S3. (A) Gram stain images of bacteria from air samples. a1-9: site 1; b1-5: site 2; c1-2: site 3; d1-4: site 4; e: site 5. (B) Large colonieswith hemolytic rings and their gram stain images. 1: site 2; 2: site 3.
PCR amplicons were further analyzed by denaturing gradient gel electrophoresis (DGGE) and sequencing. DGGE gels were prepared using 120 μL of 10% ammonium persulfate (APS), 50 μL of tetramethylethylenediamine (TEMED), 17.5 mL of 30%, and 65% denaturant solution. Electrophoresis was performed in TAE buffer for 30 min at 150 V. The sample was then loaded, and electrophoresis was continued for 2 h at 60 V and then 13 h at 90 V. Finally, the gel was stained with ethidium bromide (50 μg/mL) for 20 min and analyzed using the gel imaging analysis system. The selected gel bands were excised and placed into 50 μL of sterilized water at 37 ℃. Next, 8 μL of the sample was re-amplified using the same primers (without GC-clamps). Finally, the re-amplified PCR products were sequenced (Sangon, Shanghai, China) and analyzed by BLAST. Independent t-test using the SPSS software (SPSS 22.0 for windows, IBM, Inc, Chicago, IL, USA) was used to validate the significant differences, and P values < 0.01 were considered statistically significant.
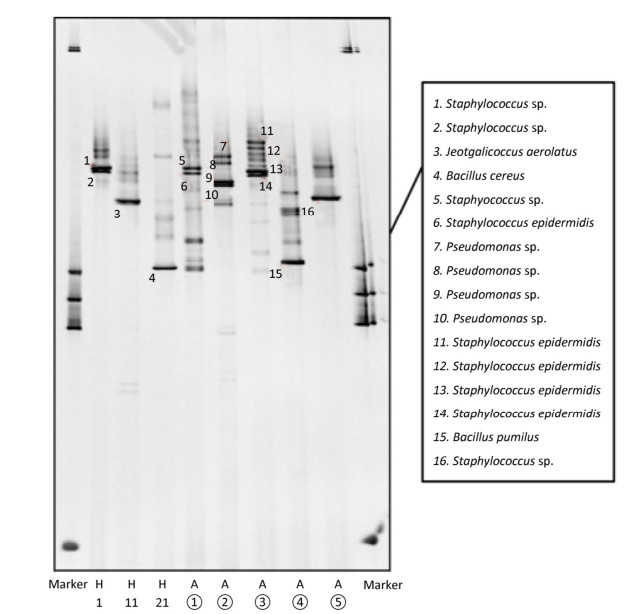
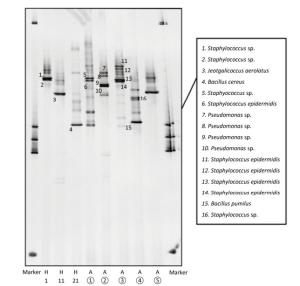
The predominant bacterial species identified in urban central areas (sites 1 and 2) were Staphylococcus epidermidis and Pseudomonas spp.; at sites 4 and 5, the predominant bacterial species were S. epidermidis and Bacillus pumilus (Figure 2 and Table 1). We also observed large colonies with hemolytic rings in case of samples from sites 2 and 3. Morphological examination revealed that they were gram-positive cocci and gram-negative bacteria, respectively (Supplementary Figure S3). The DGGE results suggested that they were S. epidermidis and Pseudomonas spp. The bacterial species at 0, 30, and 60 m above the ground were S. epidermidis and Streptococcus caprae, Jeotgalicoccus aerolatus, and Bacillus cereus, respectively (Figure 2).
Samples Description Sequence Length Similarity Value (%) Accession ① Staphylococcus sp. strain 42 16S ribosomal RNA gene, partial sequence 289 99 MG162644.1 ① Staphylococcus epidermidis strain DH20 16S ribosomal RNA gene, partial sequence 292 99 KX648542.1 ② Pseudomonas sp. WB17-07 16S ribosomal RNA gene, partial sequence 279 99 GU595346.1 ② Pseudomonas sp. strain AL138 16S ribosomal RNA gene, partial sequence 289 99 MG819238.1 ② Pseudomonas sp. strain AL138 16S ribosomal RNA gene, partial sequence 276 99 MG819238.1 ② Pseudomonas sp. CB10 16S ribosomal RNA gene, partial sequence 283 98 EU482914.1 ③ Staphylococcus epidermidis strain DH20 16S ribosomal RNA gene, partial sequence 289 99 KX648542.1 ③ Staphylococcus epidermidis strain DH20 16S ribosomal RNA gene, partial sequence 289 98 KX648542.1 ③ Staphylococcus epidermidis strain DH20 16S ribosomal RNA gene, partial sequence 289 98% KX648542.1 ③ Staphylococcus epidermidis strain HD14.1Nin 16S ribosomal RNA gene, partial sequence 292 100 KX170747.1 ④ Bacillus pumilus strain MB-15 16S ribosomal RNA gene, partial sequence 279 98 HM055957.1 ⑤ Staphylococcus sp. strain 107 16S ribosomal RNA gene, partial sequence 283 98 MG162688.1 1st floor Staphylococcus sp. strain 42 16S ribosomal RNA gene, partial sequence 296 100 MG162644.1 1st floor Staphylococcus sp. strain 42 16S ribosomal RNA gene, partial sequence 285 99 MG162644.1 11th floor Jeotgalicoccus aerolatus strain D56 16S ribosomal RNA gene, partial sequence 285 99 KU922214.1 21st floor Bacillus cereus strain MB-40 16S ribosomal RNA gene, partial sequence 287 99 HM055982.1 Table 1. Sequence Length, Accession Number, and Similarity Values from BLAST Analysis of Samples
The chemical composition and characteristics of PM2.5 have been well studied. However, relatively less information exists regarding its biological components (bioaerosols), especially the bacterial components. As microbial carriers, PM2.5 are considered to harbor airborne microorganisms, and rising concentrations could provide surfaces for more airborne bacteria to adhere to. Aerosol, soot, dust, and smoke particles, etc. could serve as bacterial carriers in haze events. Aerosols containing sulfate and nitrate could supply nitrogen and sulfur as nutrients for bacterial growth[4], and bacteria-containing aerosols can be dispersed to areas far from their sources[5]. Soot particles can induce structural changes in the biofilm architecture and function of bacteria[6]. Dust particles can provide nutrition and more favorable conditions for microbial proliferation and growth[5].
Using epifluorescence microscopy, Dong et al. found that the total microbial concentration on hazy days was higher than that on non-hazy days in Qingdao, China[4]. However, a pyrosequencing-based study by Wei et al. showed no significant difference in the abundance and community structures for the top 13 bacterial genera between hazy and sunny days in Beijing, China, but the authors indicated that airborne bacterial structures varied with studies and locations[7]. The present study investigated the culturable bacterial density in haze events in Xi'an, and found that the bacterial density in highly populated urban sites was higher than in sparsely populated sites, suggesting a possible relationship between bacterial concentration and human activity in haze events.
Fan et al, used a high throughput sequencing method to discover many opportunistic pathogenic bacteria that exist in the winter haze events in Xi'an, similar to the results obtained in this study. They speculated that opportunistic airborne pathogenic bacteria might mainly originate from soil, leaf surfaces, and the surrounding environment[6]. Using DGGE analysis, we further identified that S. epidermidis and Pseudomonas spp. were the predominant bacterial species in haze events at urban sites, and S. epidermidis, Bacillus pumilus, and Firmicutes spp. were the most common bacterial species in haze events at suburban sites in Xi'an. In a previous study, Ramakreshnan et al. indicated that the proportion of opportunistic pathogenic bacteria, including S. epidermidis and Pseudomonas increased significantly in areas with severe air pollution, and that higher PM2.5 contamination could lead to a higher proportion of Pseudomonas spp.[8].
The dominant bacterial genera in air samples collected from sites 1 and 3 (a subway station and a surrounding area of a university campus, respectively) was Staphylococcus spp. Pseudomonas spp. was isolated from site 2 (a commercial center). These bacterial species might be related to human activity. The same results were also obtained in a previous study by Fan et al., who investigated the bacterial aerosols at Beijing subway during peak hours, providing a direct connection between human activity and subway bioaerosols[9]. The main virulence factor of S. epidermidis is the biofilm, which allows other pathogenic bacteria to come together to form multiple biofilms, consequently making the pathogenic bacteria more resistant. S. epidermidis can also induce endocarditis that is common in patients with heart valve defects. Pseudomonas spp., especially P. aeruginosa, can cause sepsis, pneumonia, endocarditis, urinary tract infection, and meningitis or brain abscess.
Xiao et al. investigated the vertical distribution of PM2.5 in Tianjing, China, during a heavy pollution period. They found that vertical PM2.5 concentration distribution was closely associated with mixed-layer heights. PM2.5 concentrations below the mixed layer were higher with insignificant vertical variations, forming an obvious pollution layer; and PM2.5 concentrations were rapidly decreased and maintained at a low level above the mixed layer[10]. We collected air samples at 0, 30, and 60 m from the ground (below the mixed layer) and found that bacterial density increased with height, suggesting that bacterial density might be proportional to PM2.5 concentration. DGGE analysis indicated that the predominant bacterial species at 0, 30, and 60 m were S. epidermidis and S. caprae, J. aerolatus, and B. cereus, respectively. S. caprae has a stronger resistance and survival capability in chemical aerosol pollution, and is associated with bone, joint, blood stream, and urinary tract infections. The pathogenicity of J. aerolatus has been less studied and its infectious ability needs to be further investigated. B. pumilus can cause food poisoning by producing a complex lipopeptide, pumilacidin that is toxic to the epithelial cells.
In conclusion, this study investigated the culturable bacterial density and composition in haze events in Xi'an. The results indicated that bacterial density was higher in densely populated areas than in sparsely populated areas, and increased with height. S. epidermidis and Pseudomonas spp. were the main bacterial species found in urban central areas. S. epidermidis and B. pumilus were mainly found in the suburbs. The bacterial species present at 0, 30, and 60 m above the ground were S. epidermidis and Streptococcus caprea, Jeotgalicoccus aerolatus, and Bacillus cereus, respectively. The results of this study suggest that the abundance of opportunistic pathogens in haze events may affect the health of Xi'an's residents and visitors.
Culturable Bacterial Density and Composition in Haze Events in Xi'an, China
doi: 10.3967/bes2019.081
the National Natural Science Foundation of China 81401710
- Received Date: 2019-02-11
- Accepted Date: 2019-07-16
| Citation: | WANG Xiao Hui, WEI Yun Peng, LIU Cheng Cheng, WANG Yu Han, LI Huan, JI Lin, OTIENO Woodvine, XU Ji Ru. Culturable Bacterial Density and Composition in Haze Events in Xi'an, China[J]. Biomedical and Environmental Sciences, 2019, 32(8): 628-632. doi: 10.3967/bes2019.081 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: