-
Cypermethrin, a kind of type II synthetic pyrethroid insecticide, is introduced into the environment for the purpose of controlling field and indoor pests that are toxic to humans and animals[1, 2]. Cypermethrin may induce reproductive damage in males, such as testicular lesions, decreased sperm count, motility changes, and morphologic abnormalities[3]. Our previous studies also have shown that cypermethrin can bring about impairments in the structure of seminiferous tubules and spermatogenesis in male rats[3-5].
Cypermethrin has been shown to act as an endocrine-disrupting chemical with anti-androgenic activity responsible for male reproductive impairments. The androgen receptor (AR) is one of the targets of cypermethrin[4]. Androgens determine the expression of the male phenotype, as well as initiating and safeguarding spermatogenesis. The functions of androgens are primarily mediated by the AR. Upon binding to the AR, androgens can affect the AR signaling pathway to play a major role in male sex organ development and the maintenance of male reproductive function[6, 7]. Therefore, anti-androgenic actions of cypermethrin may be involved in cypermethrin-induced male reproductive toxicity by targeting the AR signaling pathway to inhibit AR transcriptional activity[8-11]. However, the mechanisms of actions are not well studied.
It is well known that the AR regulates AR target genes by binding to androgen response elements, followed by recruitment of coregulators. The transcriptional activity of the AR is modulated by AR coregulators, including coactivators and corepressors[12]. AR coactivators may interact with the AR to enhance transcriptional activation of target genes[13]. The transcriptional activity of AR can be inhibited by directly blocking the AR-coactivator interaction with anti-androgens[14]. We have demonstrated that cypermethrin inhibits AR transcription by disrupting the interaction between the AR and the coactivator steroid receptor coactivator-1 (SRC-1)[15]. However, few other coactivators have been tested for potential involvement in the anti-androgenic activity of cypermethrin.
Androgen receptor-associated protein 70 (ARA70), also known as nuclear receptor coactivator 4 (NcoA4), is one of the coactivators of the AR. In 1996, ARA70 was identified in a yeast two-hybrid screen as an AR-interacting protein and was shown to potentiate AR transcriptional activity[16]. The interaction between the AR and ARA70 may play an important role in the modulation of AR transcription. Human ARA70 has both LXXLL and FXXLF motifs that mediate p160 coactivator and nuclear receptor interactions. The FXXLF motif located at amino acids 328–332 is involved in the interaction with the ligand binding domain of AR[17, 18]. Furthermore, ARA70 has been reported to enhance AR transcription by increasing AR expression, protein stability, and nuclear translocation[19, 20]. ARA70 protein has been discovered in the reproductive tissues of male murines, including the prostate, testis, seminal vesicles, and epididymis[21]. It is suggested that ARA70 may play a role in reproductive health and that this role is associated with the AR–ARA70 interaction. However, the mechanisms of male reproductive toxicity of cypermethrin involving the AR–ARA70 interaction have not been characterized.
Androgen receptor-associated protein 55 (ARA55), the ligand-dependent AR-associated protein[22], was cloned and characterized using a yeast two-hybrid system in 1999. ARA55 may function as a specific coactivator to enhance the transcriptional activity of the AR[23, 24]. There is no homology between ARA55 and ARA70, and ARA55 lacks some common domains, such as the LXXLL and FXXLF motifs, compared with ARA70. ARA55 belongs to a group 3 subfamily of LIM domain proteins and contains three separate LIM domains in the COOH-terminal region. Some data suggest that the LIM motif may be involved in protein–protein interaction with the AR[25, 26]. The interaction between the AR and ARA55 may be a key target of anti-androgenic therapy. Interruption of the AR–ARA55 interaction may inhibit AR transcriptional activity[27] However, the effects of cypermethrin on the AR–ARA55 interaction have not been studied.
Cypermethrin shows an inhibitory effect on AR transcriptional activity. However, the possible mechanisms still need further clarification. The molecular mechanisms of the anti-androgenic activity of cypermethrin may involve changes in the interactions of the AR with the coactivators ARA70 and ARA55. In this study, mammalian two-hybrid assays were used to evaluate whether cypermethrin affected DHT-induced AR–ARA70 and AR–ARA55 interactions. The study shows that cypermethrin inhibits AR–ARA70 and AR–ARA55 interactions in a ligand-dependent manner.
-
Double hydrogen testosterone (DHT) and cypermethrin, both with 99% purity, were purchased from Sigma-Aldrich (St. Louis, MO, USA). Both of them were dissolved in absolute ethanol and diluted with culture medium to the desired concentrations in phenol red-free RPMI1640 medium (Life Technologies, Carlsbad, California, USA) before use. The ESCORT V Transfection Reagent was purchased from Sigma Chemical Co. (St. Louis, MO, USA), and the chloramphenicol acetyl transferase enzyme-linked immunosorbent assay (CAT-ELISA) kit was from Roche Molecular Biochemicals (Mannheim, Germany). The β-galactosidase (β-Gal) Enzyme Assay System with reporter lysis buffer was purchased from Promega (Madison, WI, USA).
-
The plasmid fusion vectors pVP16-AR, pM-ARA70, and pM-ARA55 were constructed. Amino acid residues 1–991 of the AR were subcloned into the pVP16 vector for expression as a fusion with the VP16 transactivation domain, yielding the fusion vector pVP16-AR. Amino acid residues 1–401 of the ARA70 N-terminal domain were subcloned into the pM vector for expression as a fusion protein with the GAL4 DNA binding domain, to yield the fusion vector pM-ARA70. Amino acid residues 1–444 of ARA55 were subcloned into the pM vector for expression as a fusion protein with the GAL4 DNA binding domain, to yield the fusion vector pM-ARA55. The Mammalian Matchmaker™ Two-Hybrid Assay Kit was purchased from Clontech Laboratories, Inc. (Palo Alto, CA, USA). The pG5CAT was used as the reporter vector. The β-Gal expression plasmid, pCMVβ, was purchased from Clontech Laboratories, Inc. (Palo Alto, CA, USA) and used as an internal control for transfection efficiency.
-
The African monkey kidney CV-1 (AR negative) cell line was purchased from the Institute of Biochemistry and Cell Biology in Shanghai, Chinese Academy of Science (Shanghai, China), and the cells were routinely maintained in phenol red-free RPMI1640 medium supplemented with 10% fetal bovine serum (FBS; Life Technologies, USA). We incubated the cells at 37 ˚C in a humidified atmosphere with 5% CO2. The CV-1 cells were dispensed in six-well microtiter plates (Corning Incorporated, USA) at a density of about 1.0 × 105 cells per well with 2.5 mL RPMI1640 medium containing 10% charcoal-dextran-stripped FBS and incubated at 37 °C.
-
Mammalian two-hybrid interaction assays were carried out to analyze the interaction of the AR with the coactivators ARA70 and ARA55, in the presence of DHT alone or with cypermethrin. CV-1 cells were incubated in six-well microtiter plates for 24 h before the reporter vector pG5CAT, pVP16-AR, pM-ARA70, or pM-ARA55, together with the internal control vector pCMVβ, were co-transfected into the cells with the ESCORT V Transfection Reagent. Following 24 h of transfection, cells were then treated with various concentrations of DHT (0 mol/L, 10−9 mol/L, 10−8 mol/L, 10−7 mol/L, and 10−6 mol/L) or a concentration of DHT (10−8 mol/L) mixed with various concentrations of cypermethrin (0 mol/L, 10−8 mol/L, 10−7 mol/L, 10−6 mol/L, or 10−5 mol/L). The cells were harvested 24 h after chemical addition, and the CAT values were measured with the CAT-ELISA kit according to the manufacturer’s instructions. The CAT amounts were normalized to the β-Gal amounts, which were determined by using the β-Gal Enzyme Assay System with Reporter Lysis Buffer. The relative CAT amounts are presented as fold induction, which is calculated relative to the untreated control vector.
-
All experiments were carried out in triplicate wells and repeated at least three times. For the mammalian two-hybrid assays, the values are shown as mean ± standard deviation (SD) from three parallel wells for each dose. One-way analysis of variance was used for multiple comparisons, followed by Dunnett’s post hoc test. The level of significance was set at 0.05. Data were analyzed using SPSS version 16.0 for windows (StatSoft, Tulsa, OK).
-
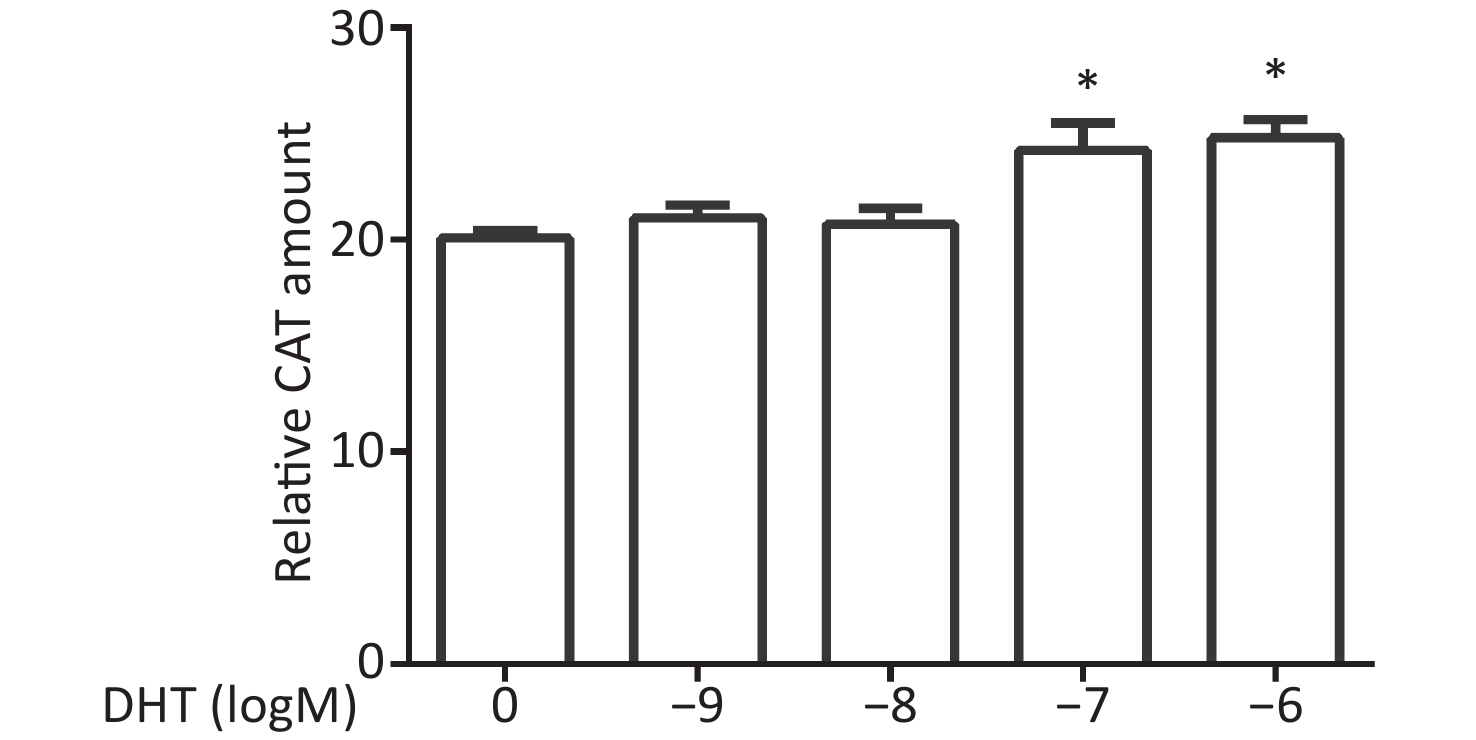
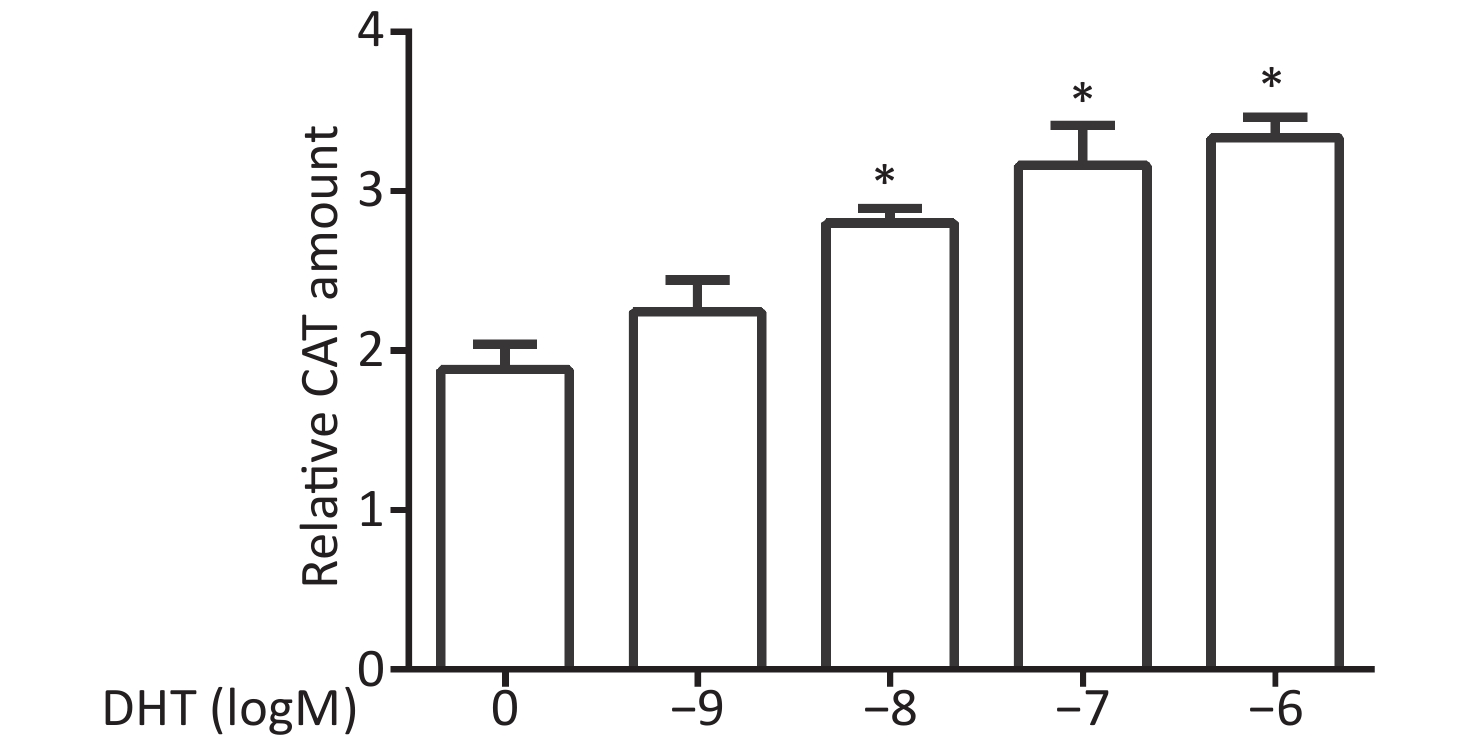
CV-1 cells were co-transfected with each of the vectors pG5CAT, pVP16-AR, and pM-ARA70, in combination with the internal control vector pCMVβ and then dosed with DHT at various concentrations. The value of the CAT reporter was tested after 24 h. The relative CAT amounts increased in response to DHT treatment compared with the untreated control values. Significant enhancement of reporter activity was detected at concentrations of 10−8 mol/L, 10−7 mol/L, and 10−6 mol/L (P ≤ 0.05, Figure 1).
-
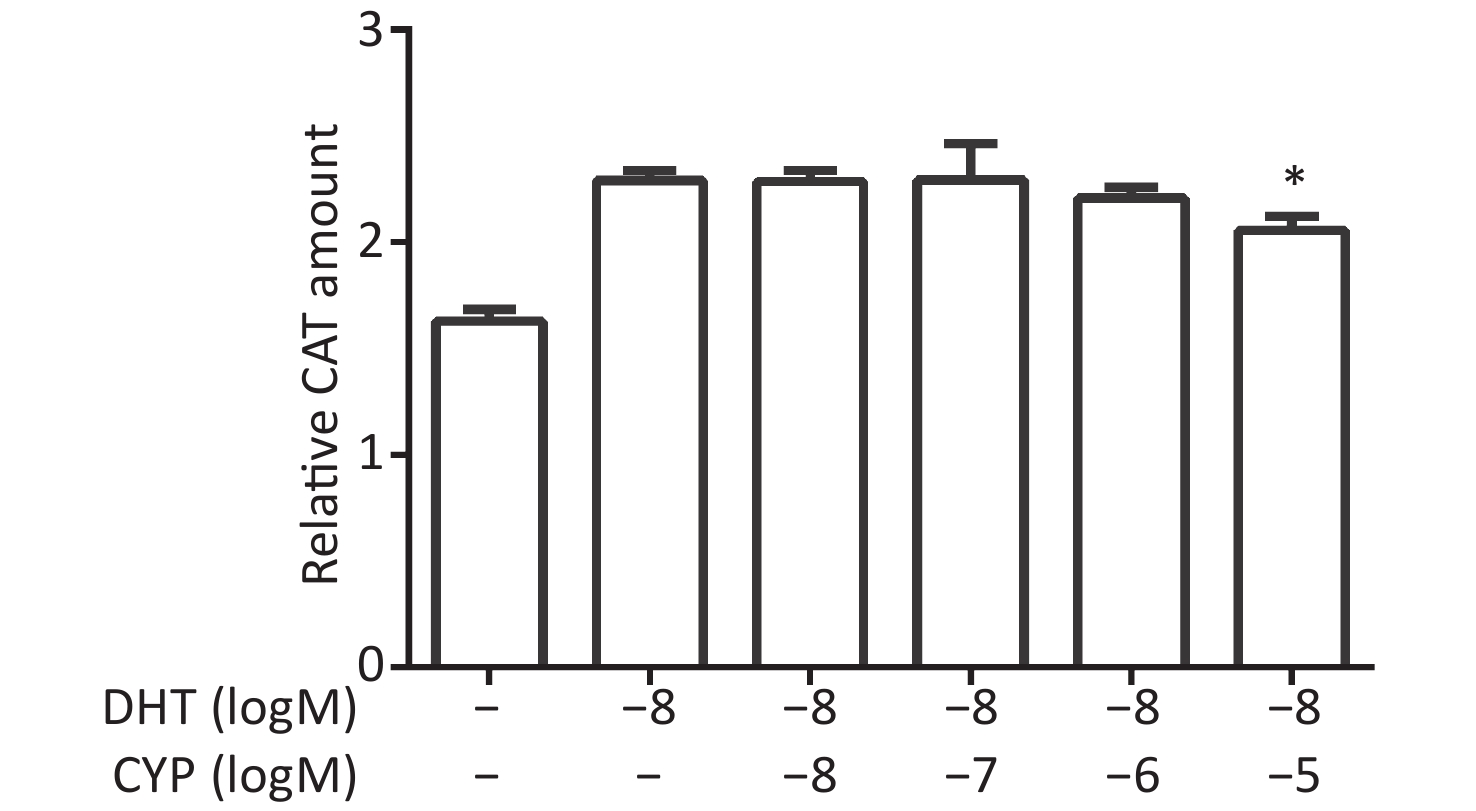
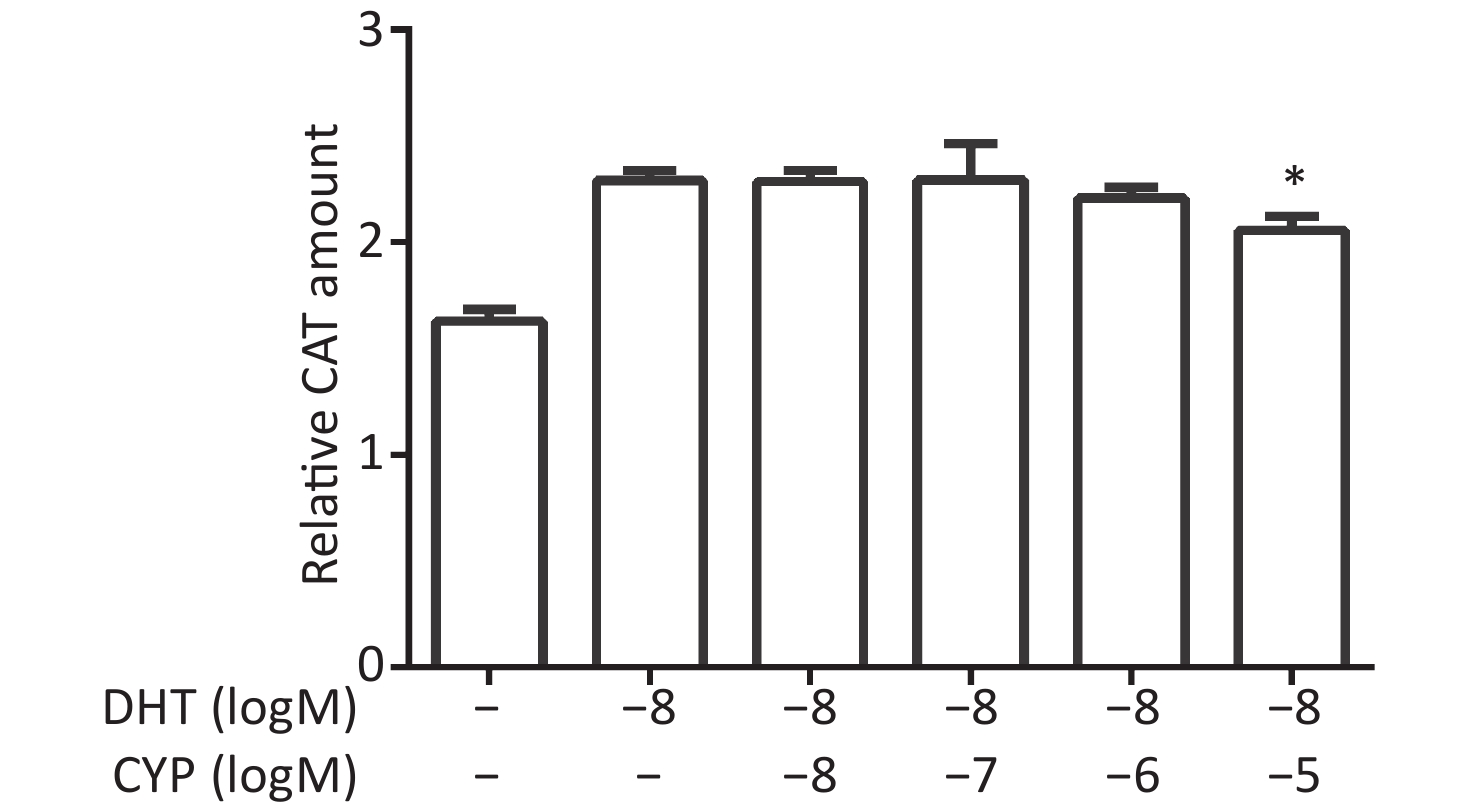
CV-1 cells were co-transfected with each of the vectors pG5CAT, pVP16-AR, and pM-ARA70, in combination with pCMVβ, and then dosed with a certain concentration of DHT and various concentrations of cypermethrin. The value of the CAT reporter was tested after 24 h. Cypermethrin suppressed the interaction of AR–ARA70 induced by DHT with maximal inhibition at a concentration of 10−5 mol/L compared with the relative CAT amount in the presence of DHT (P ≤ 0.05, Figure 2).
-
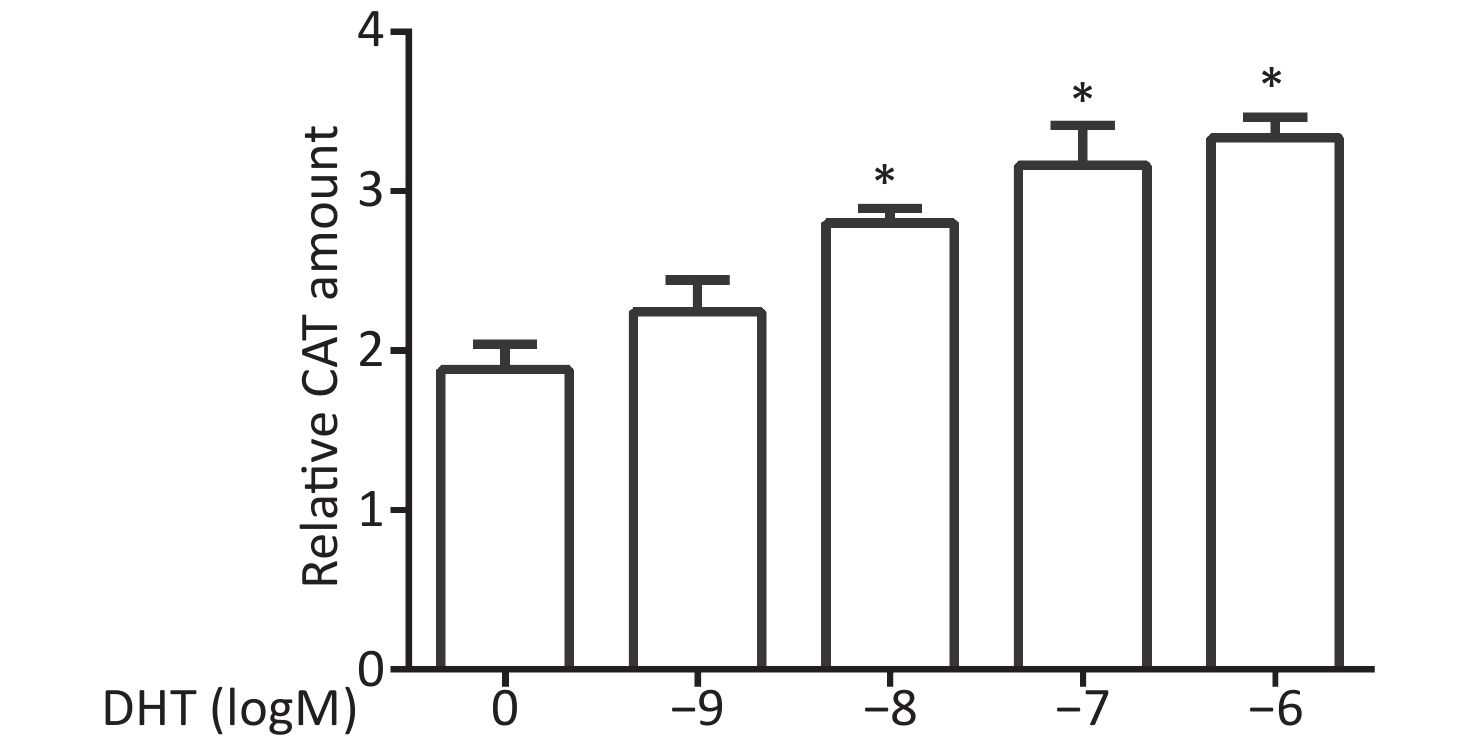
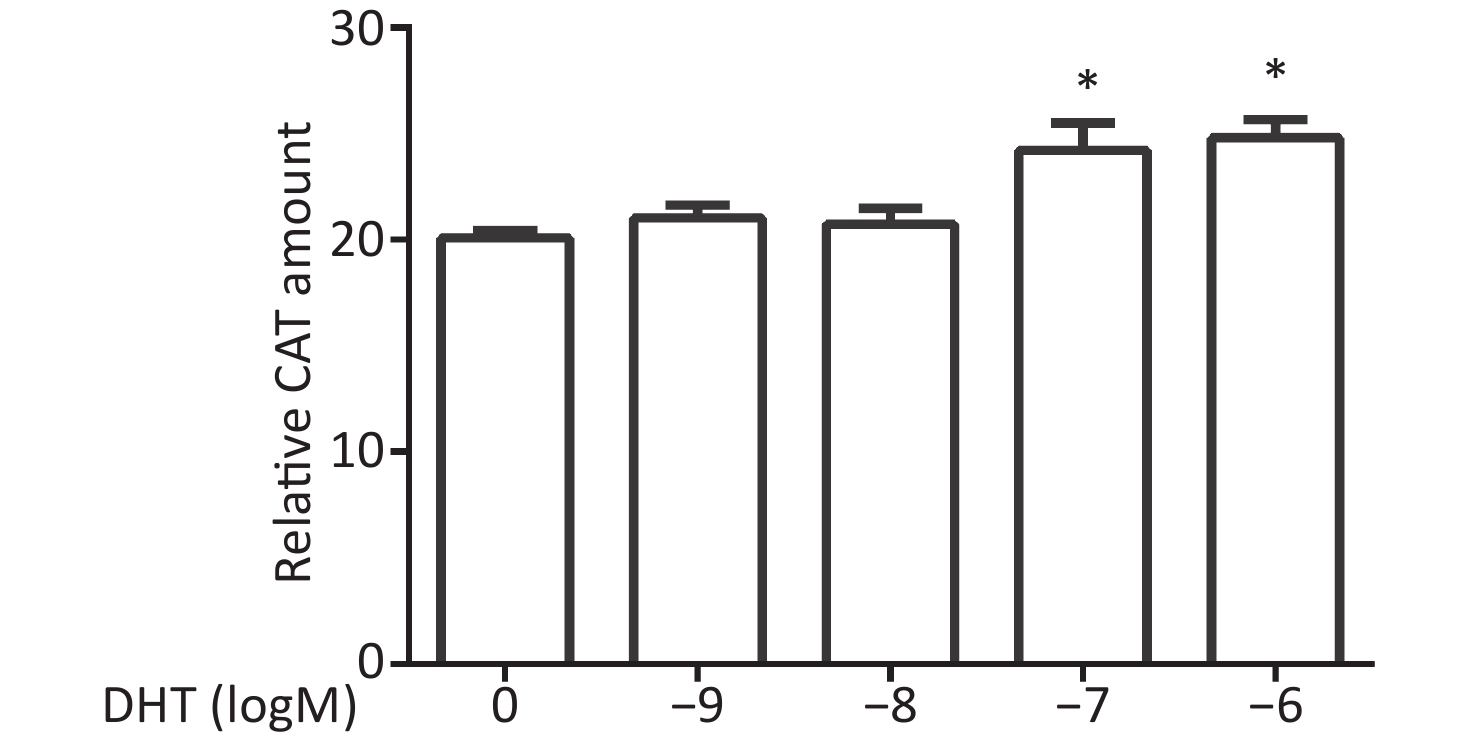
CV-1 cells were co-transfected with each of the vectors pG5CAT, pVP16-AR, and pM-ARA55, in combination with pCMVβ, and then dosed with DHT at various concentrations. Finally, the amounts of the CAT reporter were tested after 24 h. We observed that the relative CAT amounts increased, and significant enhancements were detected at concentrations of 10−7 mol/L and 10−6 mol/L (P ≤ 0.05, Figure 3).
-
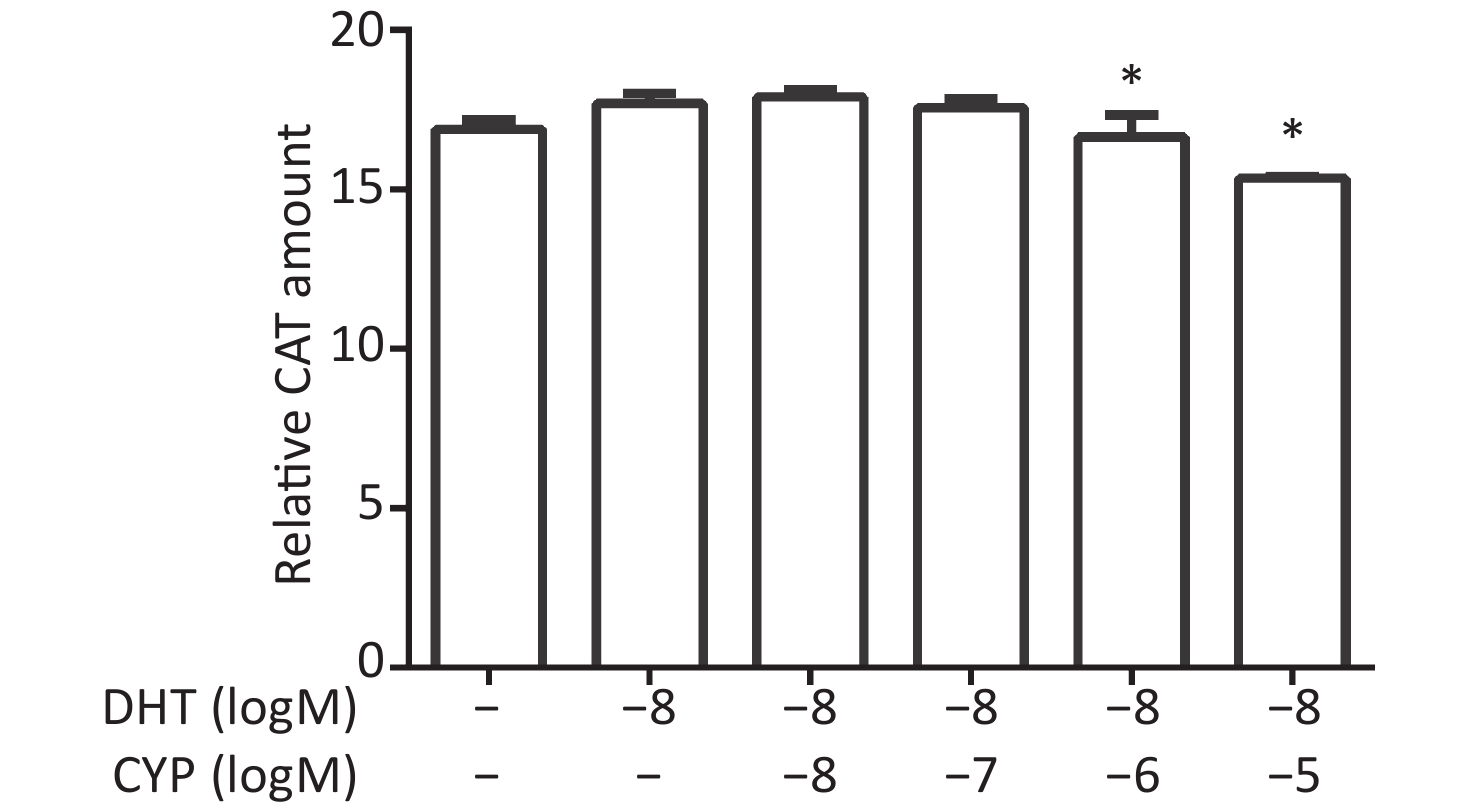
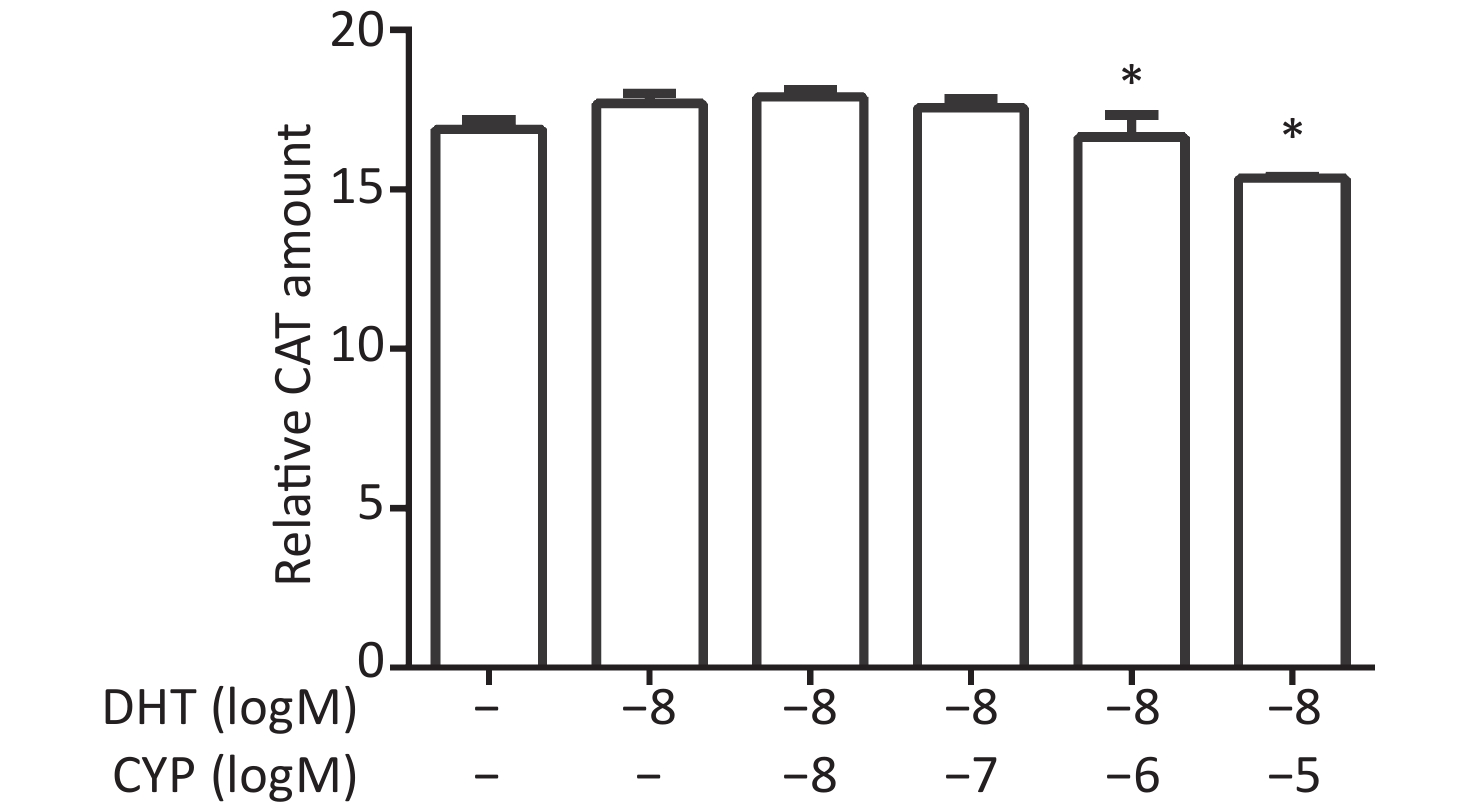
CV-1 cells were co-transfected with each of the vectors pG5CAT, pVP16-AR, and pM-ARA55, in combination with pCMVβ, and then dosed with a certain concentration of DHT and various concentrations of cypermethrin. The value of CAT reporter expression was tested after 24 h. The DHT-induced CAT amount decreased, and a significant inhibitory effect caused by cypermethrin was detected at 10−6 mol/L and 10−5 mol/L compared with positive control values for DHT (P ≤ 0.05, Figure 4).
-
The present study aims to investigate whether the molecular mechanisms of anti-androgenic activity caused by cypermethrin involve AR–ARA70 and AR–ARA55 interactions, by using mammalian two-hybrid assays. We obtained evidence that cypermethrin functions as an anti-androgen by inhibiting the interactions of the AR with ARA70 and ARA55 induced by DHT. The study provides new insight into the anti-androgenic activity of cypermethrin associated with the novel roles of the coactivators ARA70 and ARA55.
Coactivators can interact with the AR to enhance AR function. In this study, we developed mammalian two-hybrid assays to investigate AR–ARA70 and AR–ARA55 interactions. The results indicated that region 1–401 of ARA70 was sufficient for interaction with the AR, and region 1–444 of ARA55 was crucial for interaction with the AR. We provided evidence to determine the region responsible for ARA70 and ARA55 binding. The mammalian two-hybrid assays developed in this study will be valuable for detecting the interactions of the AR with the coactivators ARA70 and ARA55.
In our study, DHT-dependent AR–ARA70 and AR–ARA55 interactions were confirmed by the mammalian two-hybrid assays developed in this study. The results revealed that the AR interacted with ARA70 and ARA55 in the presence of DHT. Our observations are consistent with earlier reports that the coactivators ARA70 and ARA55 are recruited to the AR in the presence of ligands[17, 18, 24]. The interaction of ARA70 with DHT-bound AR enhances AR protein stability and increases the AR protein amount[ 28]. The LIM domain of ARA55 is more likely to serve as a platform for interactions between the AR and other transcription factors, besides direct interaction with the AR[24, 26, 27]. These coactivators may be conducive to AR protein stability in the presence of agonistic ligands or may influence the subcellular distribution of the AR, resulting in an overall influence on AR transcriptional activity in the presence of ligands. Therefore, ARA70 and ARA55 bind to the AR in a ligand-dependent manner and enhance its ability to promote the transcriptional activity of AR target genes.
Inhibition of DHT-induced AR–ARA70 and AR–ARA55 interactions has been suggested as one of the mechanisms of repression of AR transcription. As an environmental anti-androgen, we speculate that cypermethrin may suppress the interactions of the AR with coactivators ARA70 and ARA55. In this study, we have investigated the effects of cypermethrin on the interactions of AR–ARA70 and AR–ARA55. We found that AR–ARA70 and AR–ARA55 interactions are inhibited by cypermethrin. Then, cypermethrin can target AR–ARA70 and AR–ARA55 interactions to inhibit activation of AR signaling. These results agree with studies showing that interruptions of AR–ARA70 and AR–ARA55 result in repression of AR transcription. ARA70 protein expression is inhibited, and the interaction between AR and ARA70 is also disrupted by sesquiterpenoids[29]. Disruption of the normal function of ARA55 causes loss of the interaction with the AR, which may be able to suppress AR transcriptional activity[27]. Cypermethrin inhibits recruitment of ARA70 and ARA55 to the AR during co-exposure with DHT. Then, the interactions of AR–ARA70 and AR–ARA55 are suppressed by cypermethrin. This study provides evidence that cypermethrin may exert anti-androgenic actions via inhibition of AR–ARA70 and AR–ARA55 interactions.
In the presence of DHT, the AR may recruit coactivators such as ARA70 and ARA55 by increasing its binding affinity to these coactivators, which stabilize ligand-bound AR[30]. Cypermethrin prevents recruitment of coactivators ARA70 and ARA55 to AR, which eventually results in repression of gene expression. Inhibition of AR activation will impair the normal structure and function of the male reproductive system; therefore, blocking AR–ARA70 and AR–ARA55 interactions may be one important mechanism involved in male reproductive toxicity caused by cypermethrin. The present findings agree with previous research, which found that bicalutamide functions as an AR antagonist by causing ineffective recruitment of coactivators[31]. In future investigations, evaluation of the influences of cypermethrin on more coregulators in DHT-induced AR pathway is needed.
In addition, studies have shown mixed or joint toxicity studies for pesticides on the male reproductive system. For example, cypermethrin can synergize with malathion and prochloraz to cause toxic effects on zebrafish larvae[32]. The pesticides phoxim and fenvalerate cause sperm motility changes and testicular lesions in male rats, and the action may be additive[33]. Overall, the mixed or joint toxicity of cypermethrin requires further study.
-
In this study, we have shown that cypermethrin acts as an environmental anti-androgen, via repression of ligand-dependent AR–ARA70 and AR–ARA55 interactions. Our results have provided a novel mechanism involved in the anti-androgenic action of cypermethrin, which is associated with inhibiting the recruitment of ARA70 and ARA55 to the AR. This study reveals a novel aspect of toxicological mechanisms responsible for the contribution of the anti-androgenic activity of cypermethrin to male reproductive disorders.
-
The authors declare that there are no conflicts of interest.
-
DING Zhen was the principal investigator, who drafted the manuscript; SHEN Jun Yu and HONG Jia Wei were responsible for mammalian two-hybrid Interaction Assays; ZHANG Rui took the responsibled for cell culture; LI Zheng worked on doing plasmid extraction; WANG Qi participated in plasmid construction; ZHANG Jin Peng took the responsibled for Reagent purchase; ZHANG Mei Rong collated and analyzed the experimental data; XU Li Chun provided spell and grammar guidance and polished the whole article.
Inhibitory Effects of Cypermethrin on Interactions of the Androgen Receptor with Coactivators ARA70 and ARA55
doi: 10.3967/bes2020.022
- Received Date: 2019-06-20
- Accepted Date: 2019-10-17
-
Key words:
- Cypermethrin /
- Androgen receptor signaling /
- Androgen receptor-associated protein 70 /
- Androgen receptor-associated protein 55
Abstract:
| Citation: | DING Zhen, SHEN Jun Yu, HONG Jia Wei, ZHANG Rui, LI Zheng, WANG Qi, ZHANG Jin Peng, ZHANG Mei Rong, XU Li Chun. Inhibitory Effects of Cypermethrin on Interactions of the Androgen Receptor with Coactivators ARA70 and ARA55[J]. Biomedical and Environmental Sciences, 2020, 33(3): 158-164. doi: 10.3967/bes2020.022 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: