-
The principle for the selection of sedative or analgesic agents are to achieve appropriate levels of sedation and pain relief, and to consider the pharmacological properties of the different drugs and the characteristics and needs of the individual patient[1]. The optimal sedative agents should have favorable kinetics and clinical effects, a tolerable side effects profile besides adequately addressing sedation and pain[2].
Dexmedetomidine (Dex), a selective alpha-2 receptor agonist, has sedative, analgesic, anxiolytic and sympatholytic effects[3, 4]. It is reported that long-term sedation with Dex in critically ill patients might reduce the duration of mechanical ventilation and the length of Intensive Care Unit (ICU) stay. Moreover, it is shown that Dex does not depress the respiratory system unlike other sedative agents[5]. In addition, the combined use of Dex with anaesthetics, or other sedatives is likely to enhance pharmacological effects and, consequently, a reduced dosage of Dex or the concomitant drug may be necessary[6]. 94% of the Dex metabolites are to be excreted through kidney, however the results of pharmokinetics of Dex indicates that the dose of Dex needs no adjustment for the patients with kidney problems[7].
Previous research indicates that Dex can ameliorate oxidative stress to protect against ischemia/reperfusion injury through PI3K/AKT pathway in heart, liver, and kidney[8-10]. It is known that diabetic condition could elevate oxidative stress[11], moreover, elevated level of intracellular reactive oxygen species (ROS) is suggested to play an important role in the development of tubular epithelial-mesenchymal transition (EMT) and subsequent renal fibrosis[12]. Increasing research reports suggest the involvement of EMT in renal tubular epithelial cells in the irreversible progression of tubulointerstitial fibrosis[13]. However, the effects of Dex on EMT in renal tubular epithelial cells caused by high level of glucose remain unclear. Therefore, we used human renal tubular epithelial cell line (HK-2 cells) exposed to high level of glucose to explore the effects of Dex on EMT in HK-2 cells and underlying mechanisms, which will offer theoretical foundation and guidance for the selection of sedative agent for patients with diabetes mellitus.
-
Human renal tubular epithelial cells (HK-2) were purchased from the American Type Culture Collection (ATCC). HK-2 cell was incubated with Dulbecco's Modified Eagle Media- Nutrient Mixture F-12 (DMEM-F12) (Bioind) supplemented with 10% Fetal Bovine Serum (FBS, Bioind) at 37 °C with 5% CO2. When the confluence of HK-2 cells was 80%, the cells were cultured for 24 h in incubation medium without FBS, then the cells were randomly assigned to different groups with each group 2 flasks of cells, Normal group, Glucose group (40 mmol/L glucose), Dex (0.01 nmol/L Dex, Jiangsu Hengrui Pharmaceutical Corporation Limited, China) + Glucose (40 mmol/L), and the cells were to incubate in DMEM-F12 with 0.05% FBS for 6 h.
-
Cell Counting Kit-8 (CCK-8) was used to determine the cell viability of HK-2 cells co-cultured with Dex at different concentrations. HK-2 cells were seeded into a 96-well plate with each well of 8 × 103 cells. The cells were allowed to grow for 24 h and then glucose and Dex were added to the different wells at the final concentration of glucose, 0, 10 mmol/L, 20 mmol/L, 30 mmol/L, 40 mmol/L, 50 mmol/L, 60 mmol/L; final concentration of Dex, 0, 0.002 nmol/L, 0.01 nmol/L, 0.05 nmol/L, 0.25 nmol/L, 1.25 nmol/L. Five duplicate wells were assigned to one group. After the addition of glucose and Dex to the cells, the cells were further cultured for 6 h. The medium was removed and the cells were resuspened with 100 μL DMEM-F12 medium without FBS and 10 μL CCK-8 (Fuyuan Biotechnology Corporation Limited, Shanghai), then the mixture of cells and CCK-8 were incubated at 37 °C for 2 h. The adsorbance value of the cells were determined by BioTek- ELX-800 at the wavelength of 450 nm.
-
After the cells were treated with Dex or Dex+glucose for 6 h, the cells were collected and counted. 106 cells were put into one tube and the cells were washed by cold Phosphate Buffered Saline (PBS) twice, then the cells were resuspended by 1.5 mL 70% ethanol and stored at −20 °C overnight. After the cells were centrifuged at 4 °C, the cells were washed by cold PBS once and then the cells were resuspended by 200 μL propidium iodide/Rnase (BD, USA) and incubated for 30 min at room temperature. The cells were filtered through 300 mesh net and cell cycle, G1, G2, and S were detected by flow cytometry (BD, USA).
-
8 × 103 HK-2 cells were plated in a 96-well plate and incubated with DMEM-F12 (Bioind) supplemented with 10% FBS (Bioind) at 37 °C with 5% CO2 for 24 h. After 24 h, the medium was removed and the cells were washed by PBS, then the cells were treated with glucose or glucose+Dex for 6 hours. After 6 h, the medium was removed and the cells were washed by PBS. Then, 100 μL 10 μmol/L general oxidative stress indicator (CM-H2DCFDA) was added to the cells and the cells were incubated at 37 °C for 20 min in darkness. Then, the fluorescence intensity was detected by Cytation 3 Cell Imaging Multi-Mode Reader (BioTek, USA). The excitation wavelength and emission wavelength were 488 nm and 525 nm respectively.
-
PI3K/AKT inhibitor LY294002 and ERK inhibitor U0126 were purchased from Med Chem Express, USA. The concentration used in the present study was determined by CCK-8 and Western blot. HK-2 cells were exposed to either high level of glucose, Dex + glucose, LY294002 (final concentration 20 μmol/L) + glucose, or U0126 (final concentration 10 μmol/L) + glucose for 6 h.
-
Determination of the expression of PI3K, AKT, p-Akt, ERK, p-ERK, E-Cadherin, Claudin-1 and α-SMA was conducted according to the procedure of our previous study[14]. The antibody information was AKT Rabbit mAb (CST USA, 1:1,000), phospho-AKT (Ser473) Rabbit mAb (CST USA, 1:1,000), PI3K Rabbit mAb (CST USA, 1:1,000), ERK Rabbit mAb (CST USA, 1:1,000), phospho-ERK Rabbit mAb (CST USA, 1:1,000), E-Cadherin (CST USA, 1:1,000), Claudin-1 (CST USA, 1:1,000) and α-SMA (CST USA, 1:1,000), GAPDH mouse mAb (CST USA, 1:1,000). The images were obtained by Chemiluminescence imaging analysis system (ECL from Tanon 5200, Shanghai, China) and band intensity were analyzed by Tanon Gis analytical software (Shanghai, China).
-
The data in the present study was analysed by SPSS 21.0 software and expressed as measurement mean ± standard deviation (SD). Differences between two groups were compared with an independent sample t test while P < 0.05 was considered statistical difference. Our results were repeated independently at least three times apart of the indicated experiments.
-
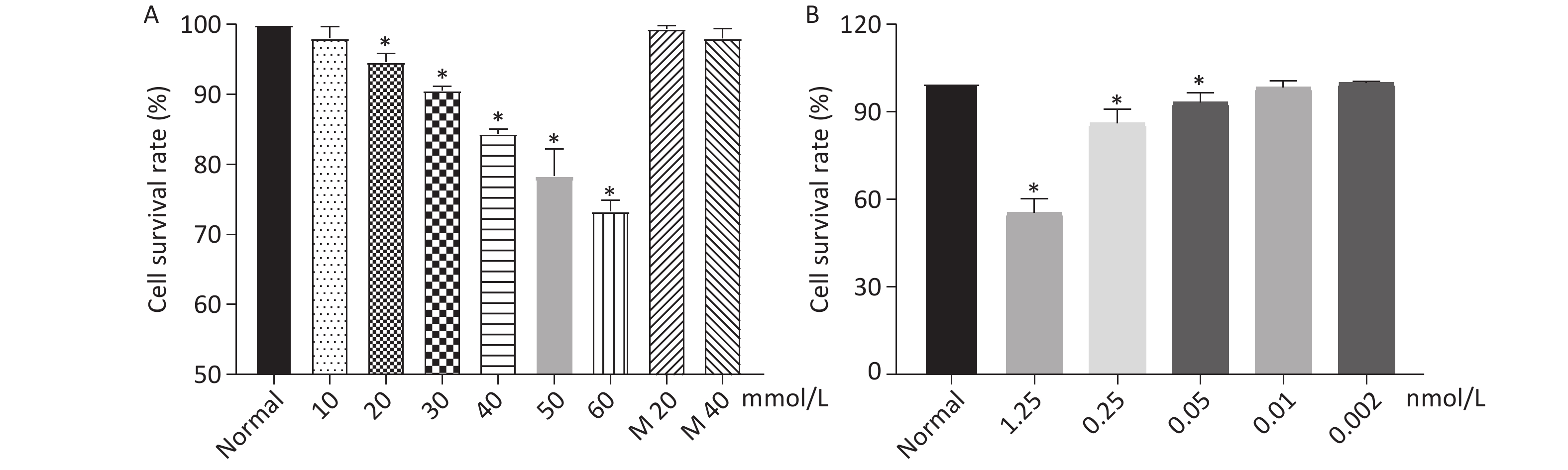
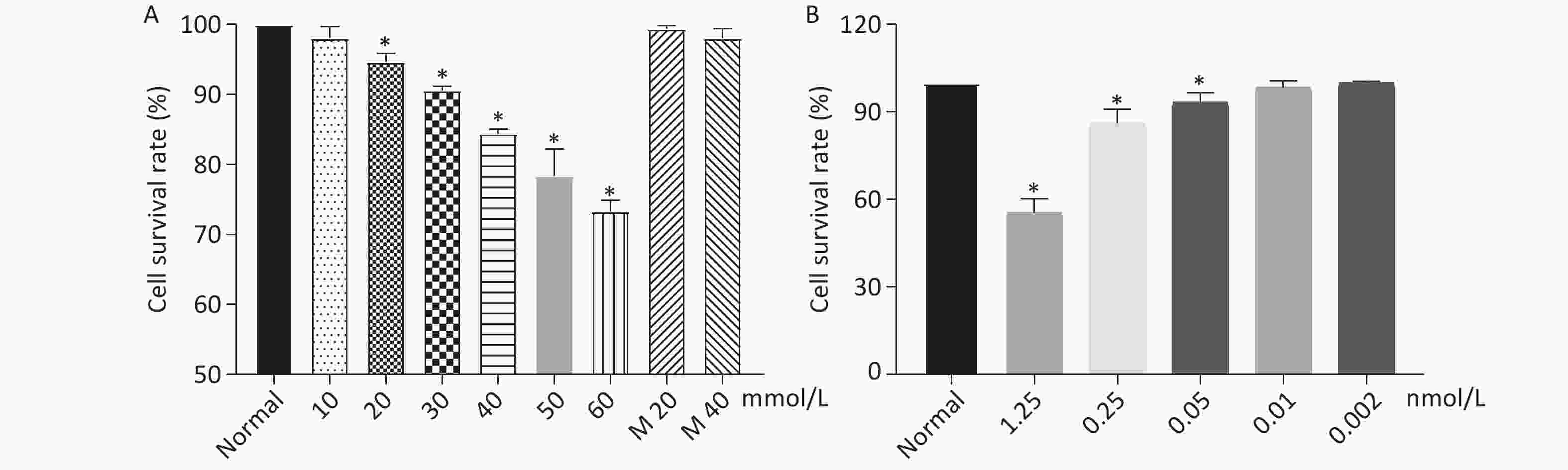
As shown in Figure 1, when the concentration of glucose and Dex was at 40 mmol/L and 0.01 nmol/L respectively, the cell survival rate was 84%, 98%. The further experiments were performed using glucose at 40 mmol/L and Dex at 0.01 nmol/L.
-
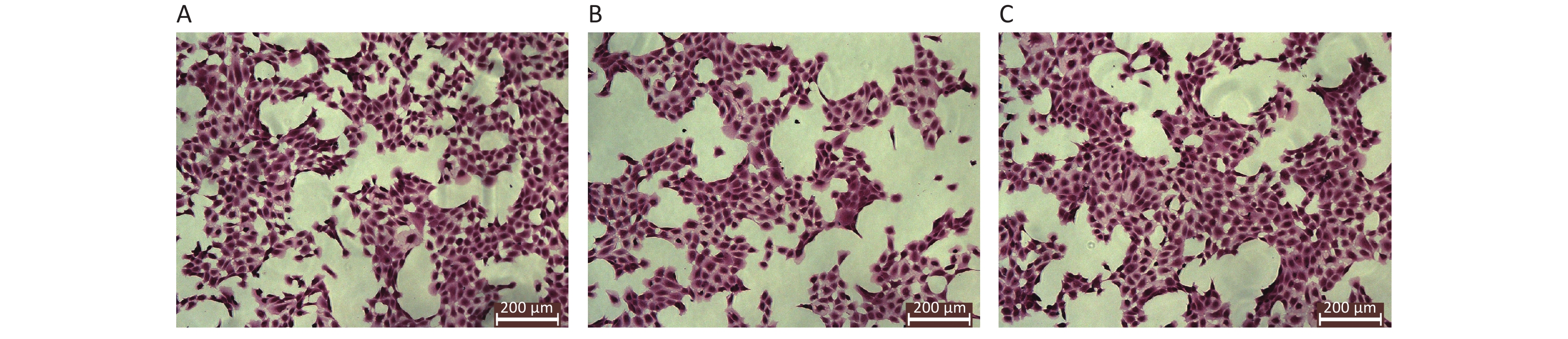
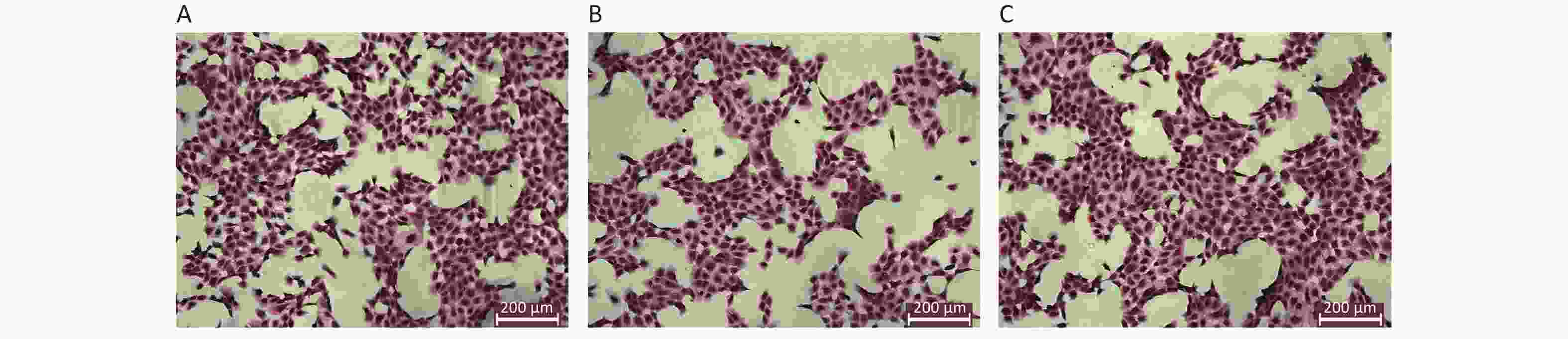
The results of HE staining of HK-2 cells demonstrated that the density of HK-2 cells exposed to high level of glucose was significantly decreased, the membrane of HK-2 was not complete. In contrast, HK-2 cells exposed to both glucose and Dex, the density of HK-2 cells was dense, the morphology was well preserved. The morphology of HK-2 at different groups was shown in Figure 2.
-
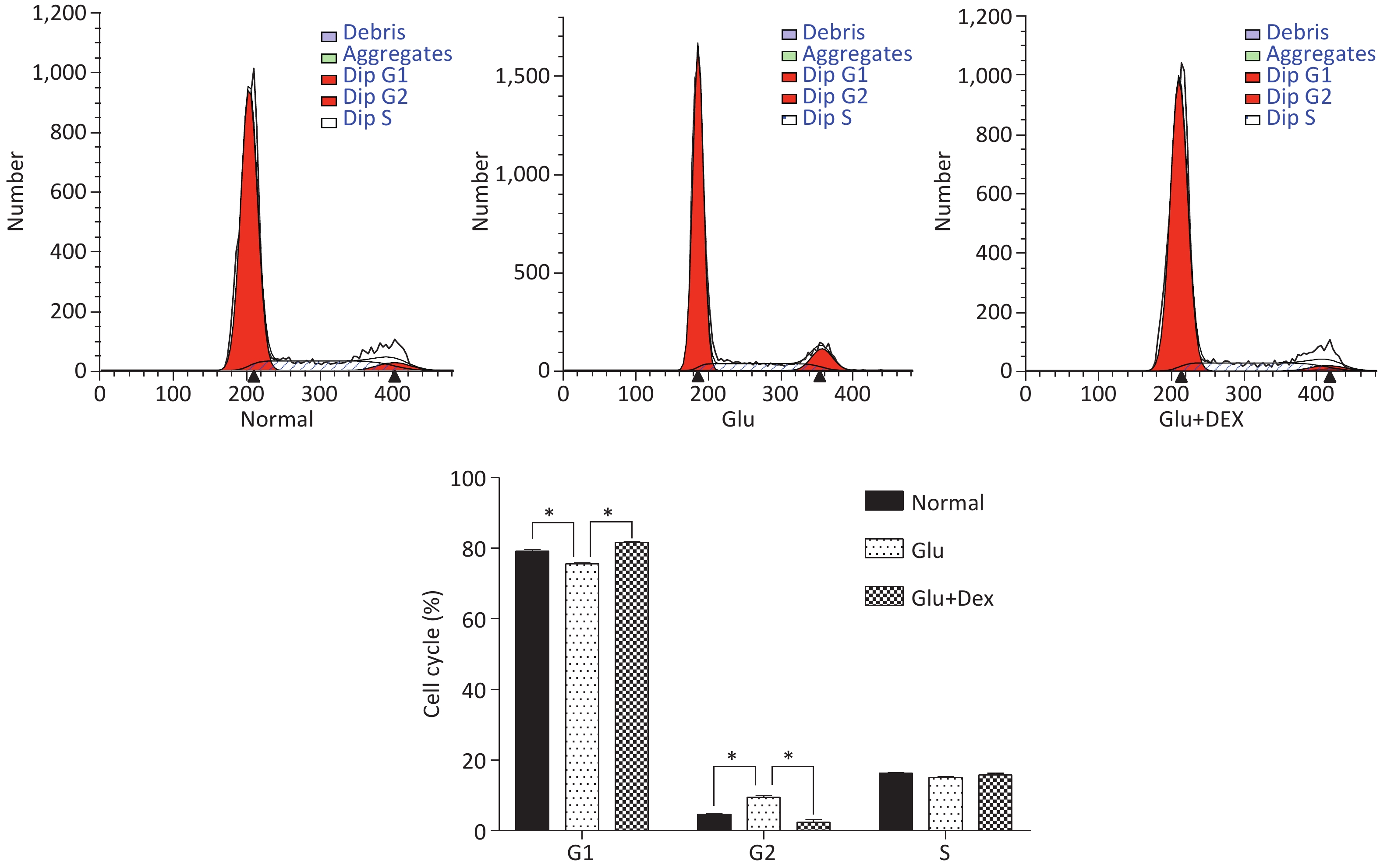
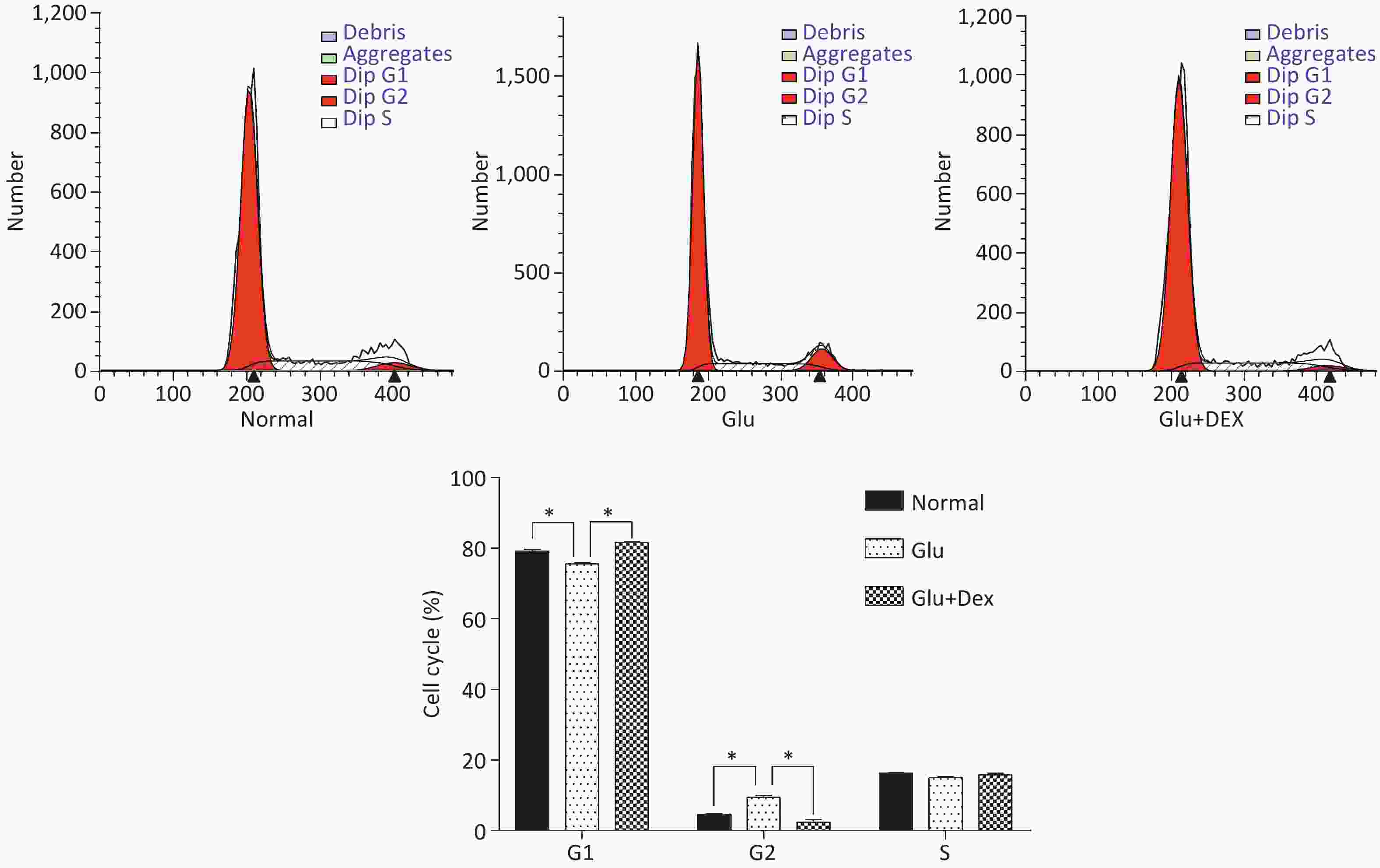
As demonstrated in Figure 3, HK-2 cells exposed to high level of glucose were stagnant at G2 phase, the percentage of HK-2 cells in G1 phase was significantly decreased. In contrast, HK-2 cells exposed to both glucose and Dex, they had higher percentage of cells in G1 phase.
-
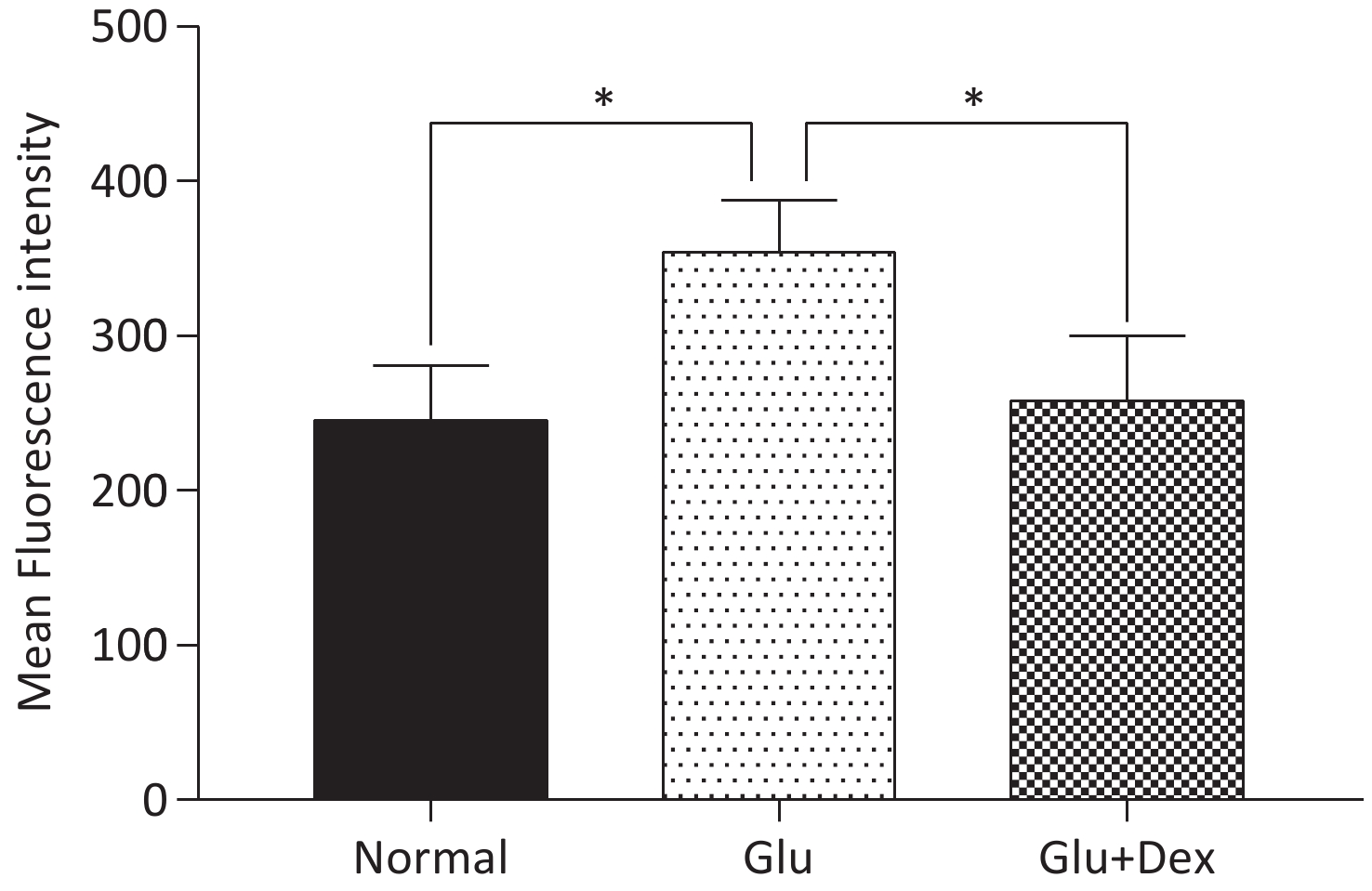
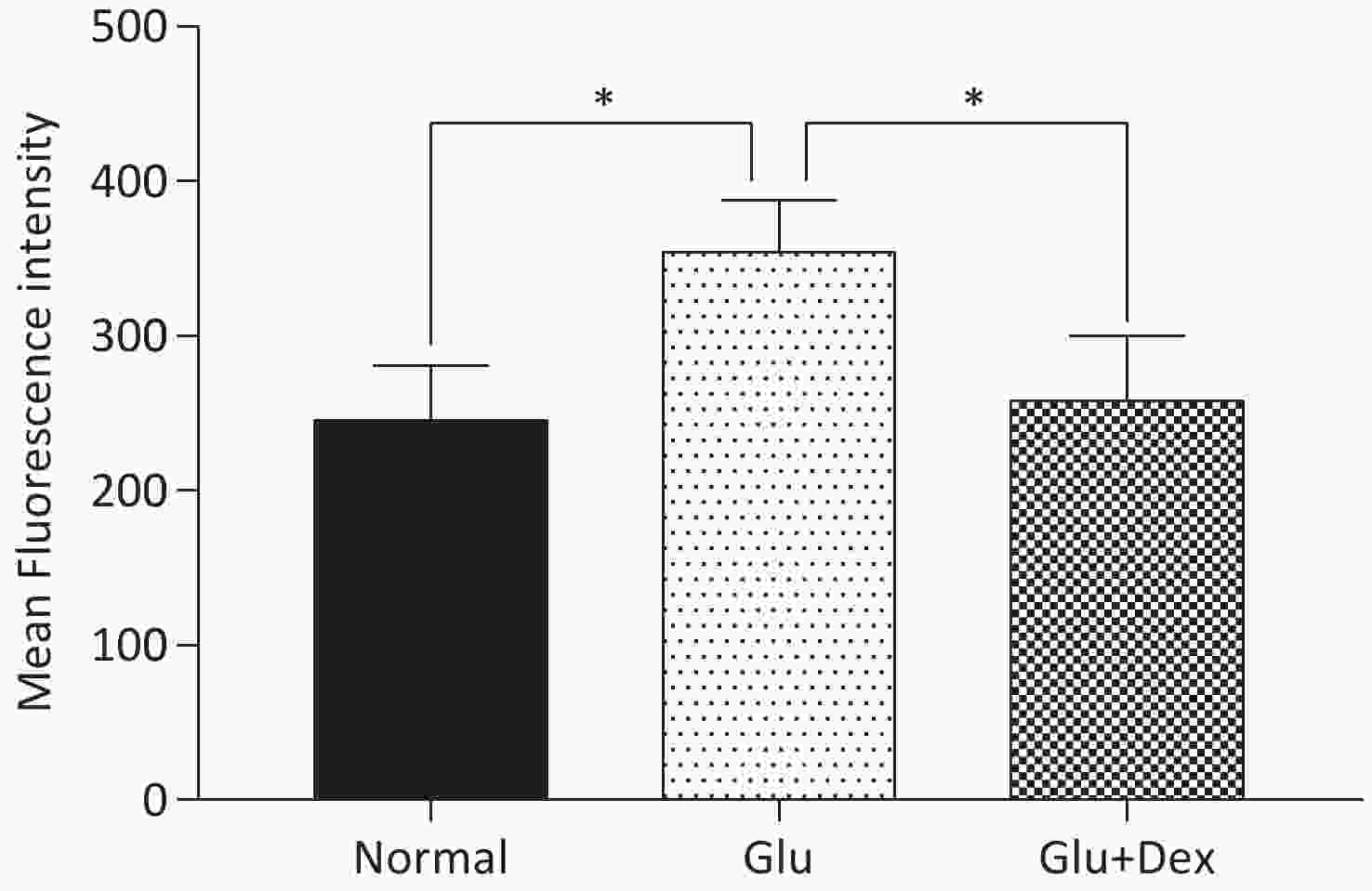
ROS production in HK-2 cells exposed to both Dex and glucose was significantly decreased than that in those exposed to glucose. The comparison of ROS production among different groups was shown in Figure 4.
-
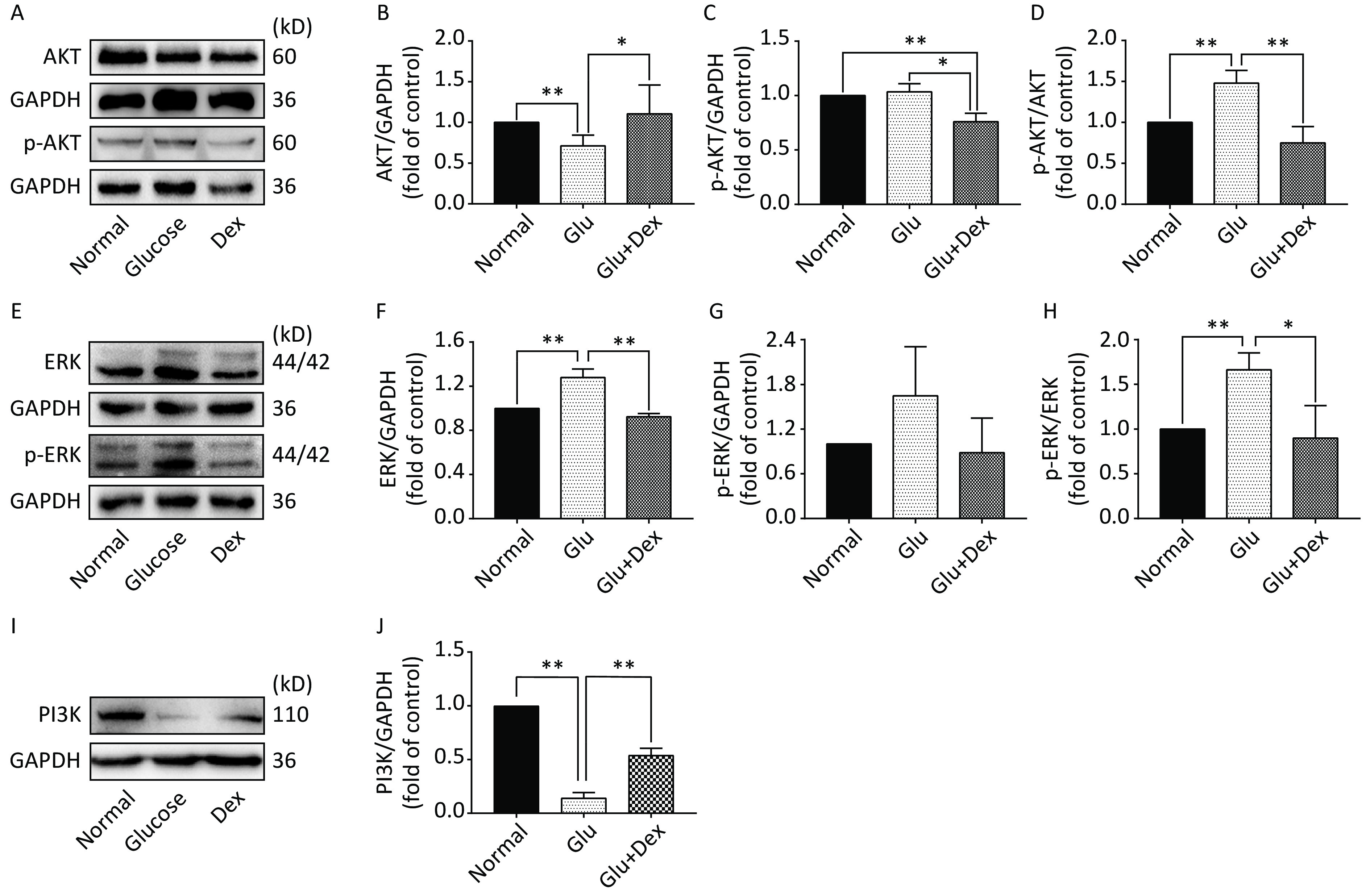
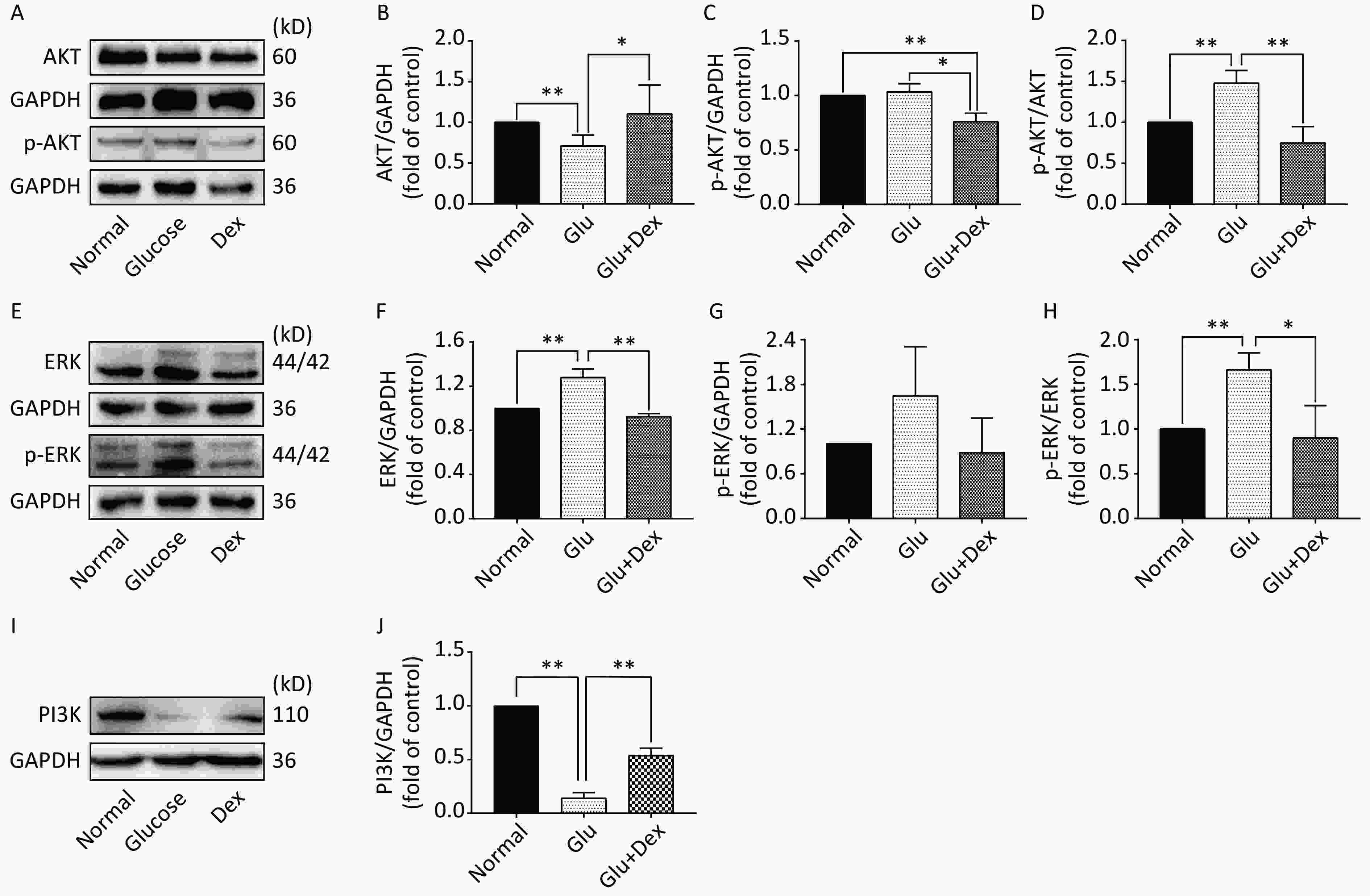
As demonstrated in Figure 5, the expression of AKT and ERK was significantly decreased in HK-2 cells exposed to both glucose and Dex compared with those exposed to glucose (P < 0.05). The expression of PI3K was significantly increased in HK-2 cells exposed to both glucose and Dex (P < 0.05).
-
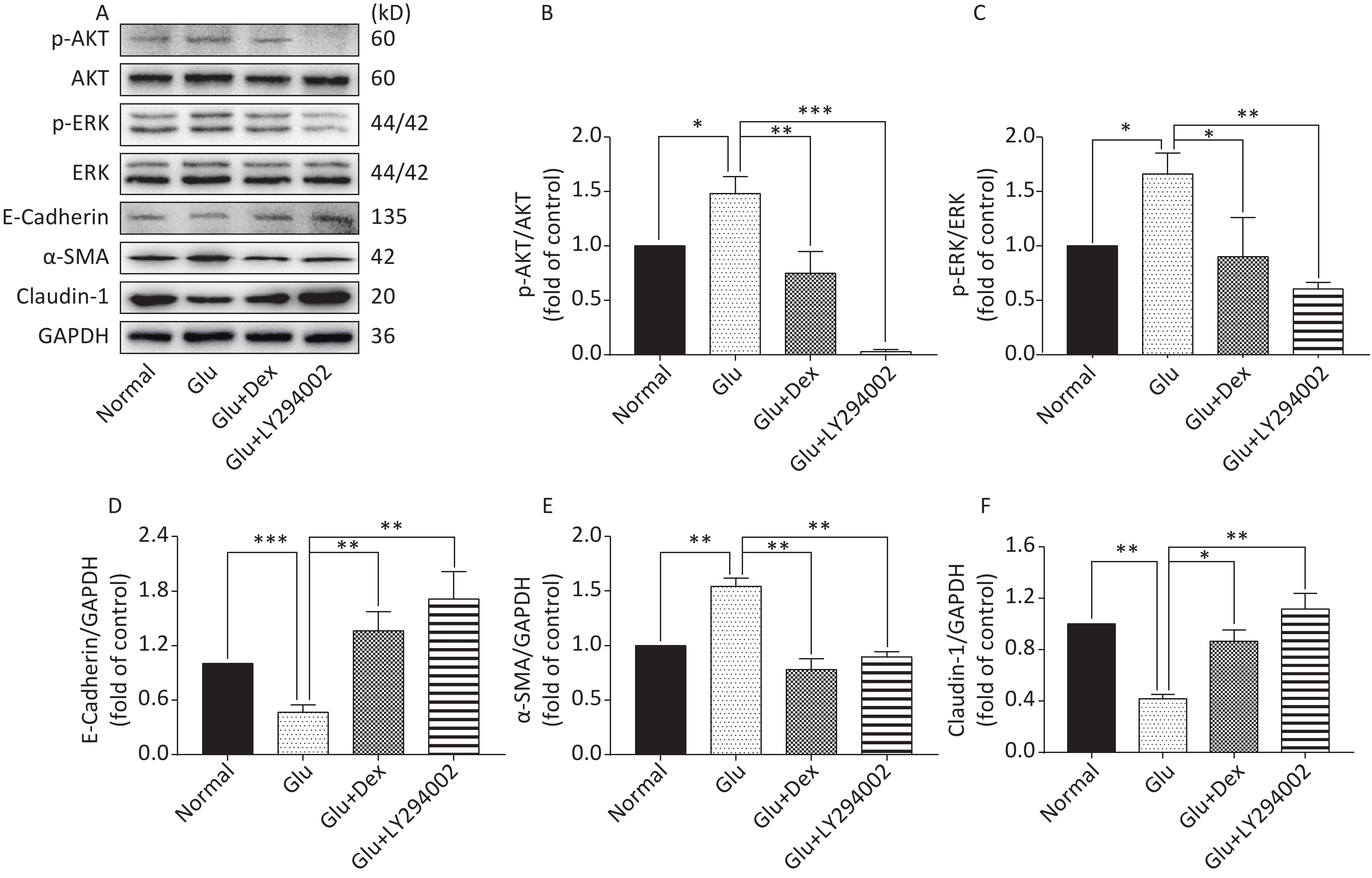
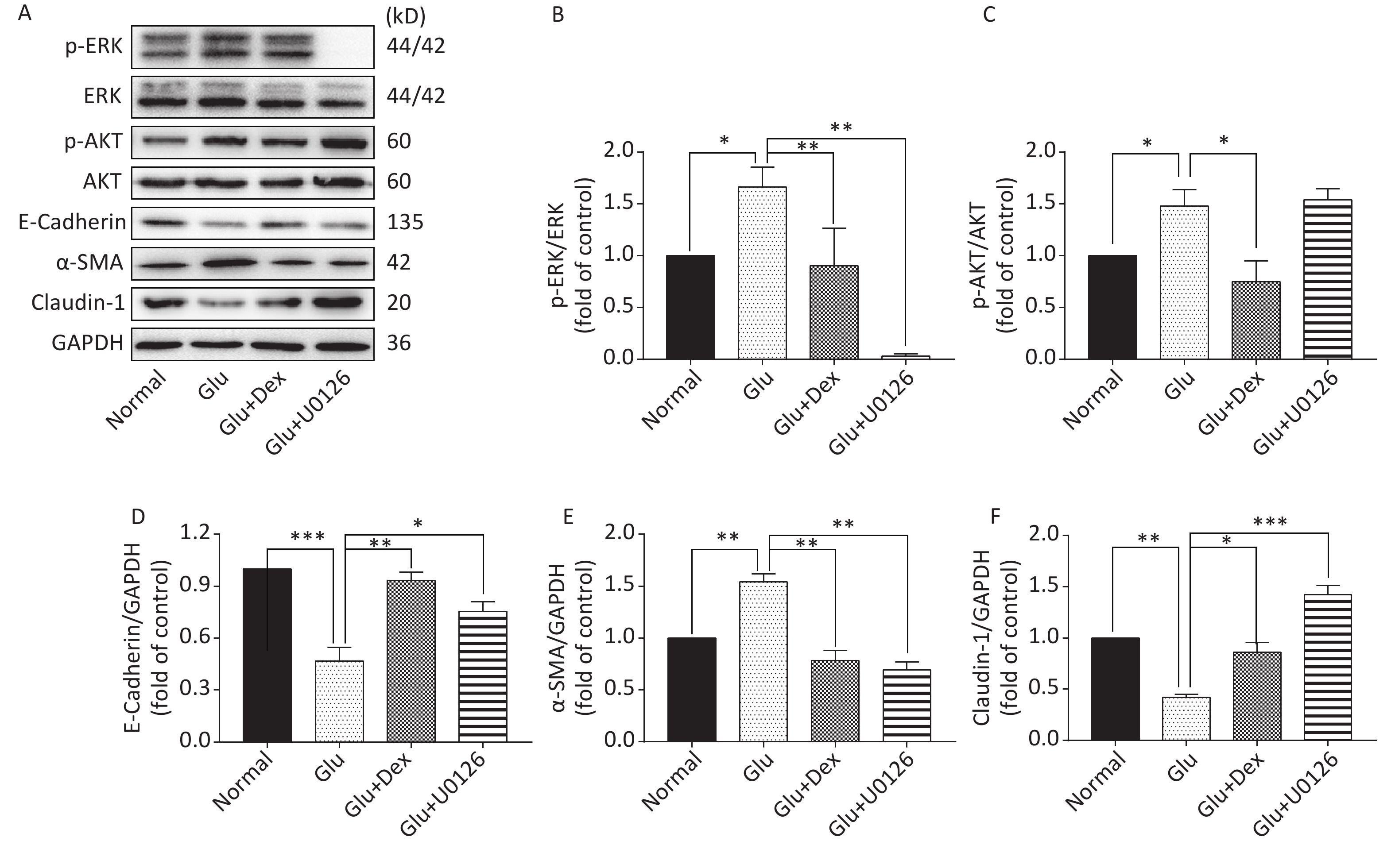
As shown in Figure 6 and Figure 7, the expression of p-AKT, p-ERK, and α-SMA was increased while the expression of E-Cadherin and Claudin-1 was decreased in HK-2 cells exposed to high level of glucose. When HK-2 cells were exposed to both Dex and glucose, or PI3K/AKT inhibitor LY294002 and glucose, or ERK inhibitor U0126 and glucose, the expression of p-AKT, p-ERK, and α-SMA was decreased while the expression of E-Cadherin and Claudin-1 was increased compared with HK-2 cells exposed only to glucose. Moreover, PI3K/AKT inhibitor LY294002 can significantly inhibit the activation of ERK besides inhibiting of AKT activation, while ERK inhibitor U0126, in contrast, cannot significantly inhibit the activation of PI3K/AKT.
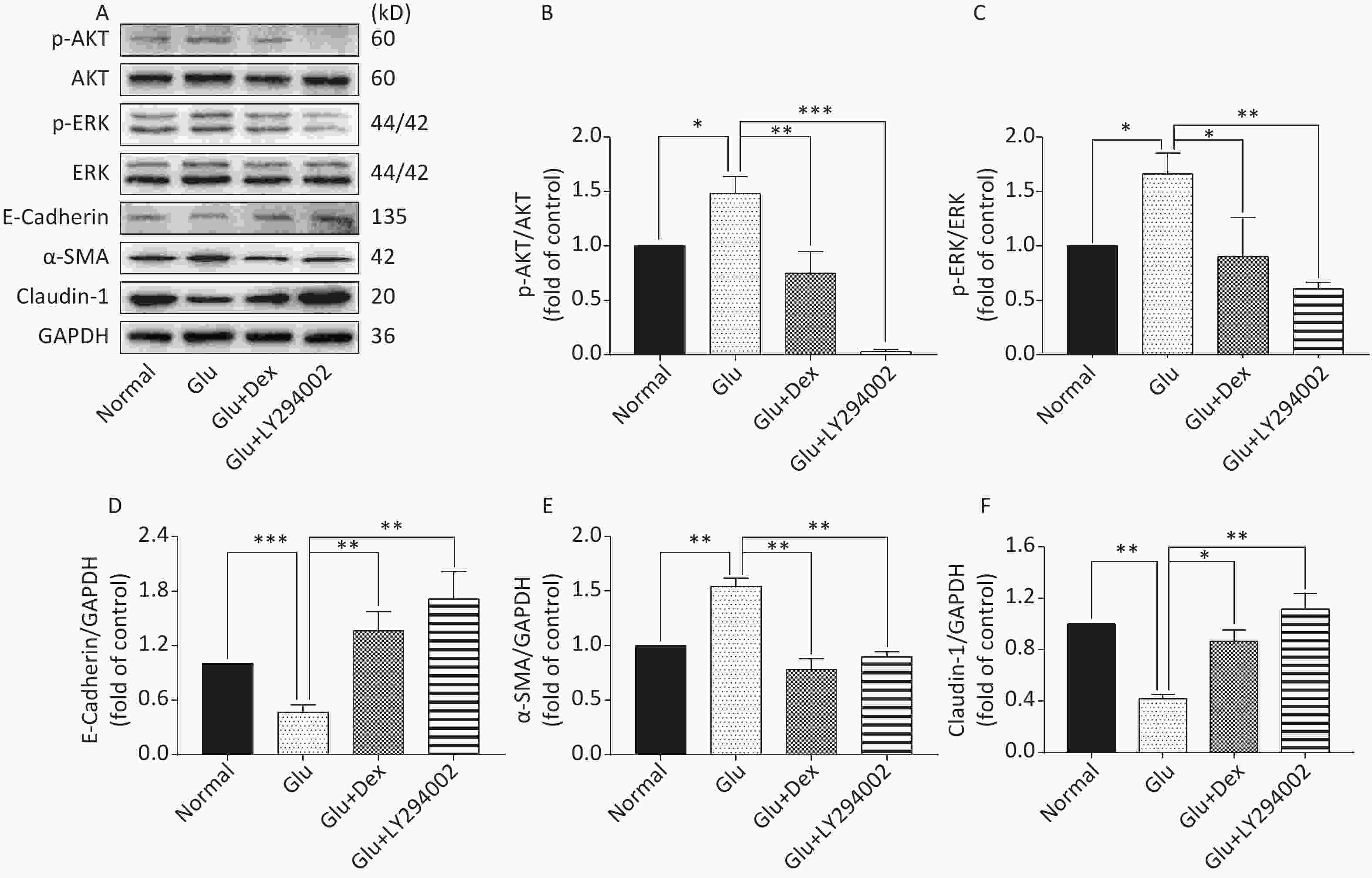
Figure 6. Expression of AKT, ERK, E-Cadherin, Claudin-1, and α-SMA in HK-2 cells exposed to glucose, Dex or PI3K/AKT inhibitor. (A) Western blot analysis of p-AKT, p-ERK, E-Cadherin, α-SMA, and Claudin-1. (B) The comparison of p-Akt/Akt relative expression level in HK-2 cells. (C) The comparison of p-ERK/ERK relative expression level in HK-2 cells. (D) The comparison of E-Cadherin relative expression level in HK-2 cells. (E) The comparison of α-SMA relative expression level in HK-2 cells. (F) The comparison of Claudin-1 relative expression level in HK-2 cells.
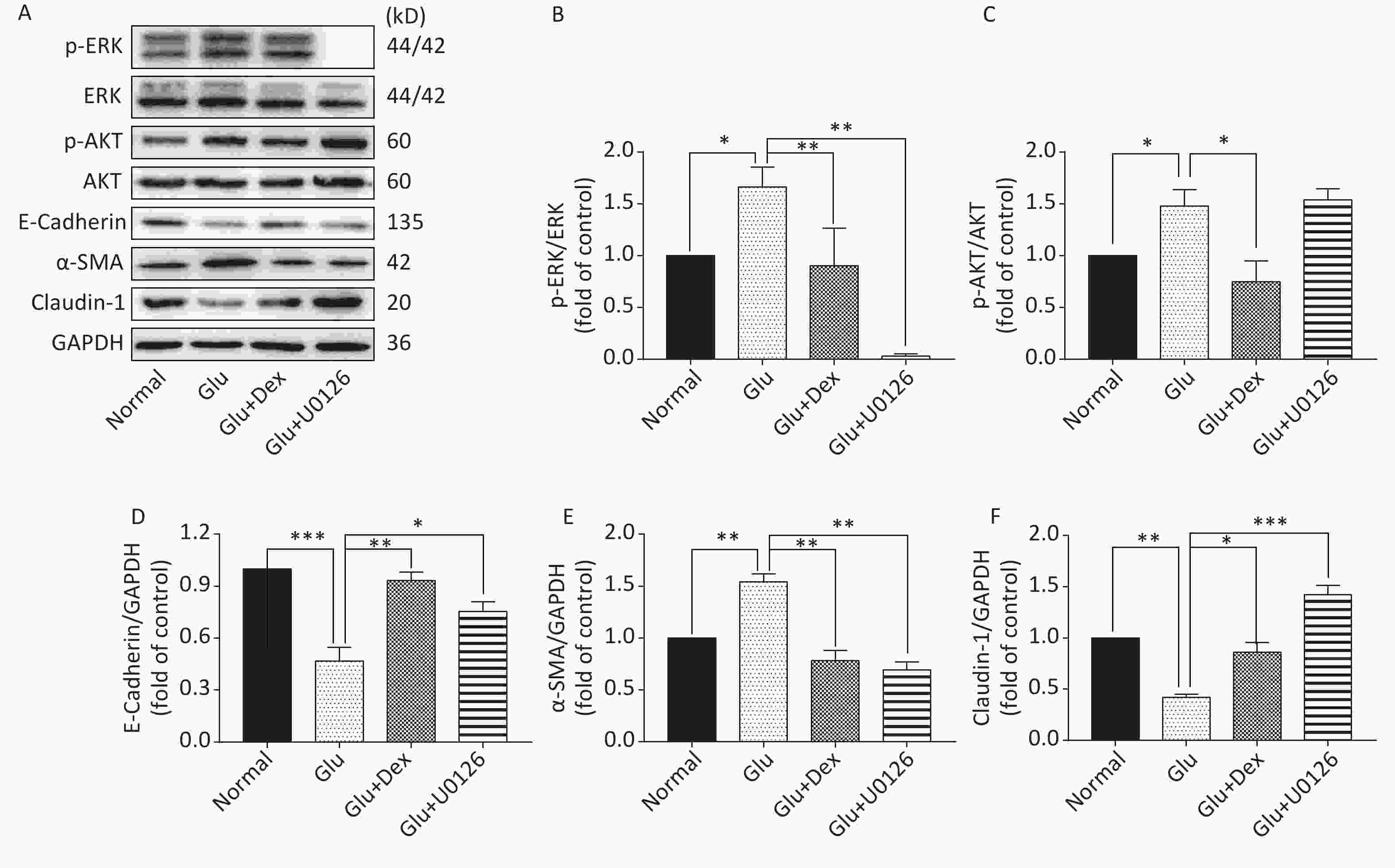
Figure 7. Expression of ERK, AKT, E-Cadherin, α-SMA, and Claudin-1 in HK-2 cells exposed to glucose, Dex or ERK inhibitor. (A) Western blot analysis of p-ERK, p-AKT, E-Cadherin, α-SMA, and Claudin-1. (B) The comparison of p-ERK/ERK relative expression level in HK-2 cells. (C) The comparison of p-AKT/AKT relative expression level in HK-2 cells. (D) The comparison of E-Cadherin relative expression level in HK-2 cells. (E) The comparison of α-SMA relative expression level in HK-2 cells. (F) The comparison of Claudin-1 relative expression level in HK-2 cells.
-
Dex as a selective alpha-2 receptor agonist is able to achieve appropriate level of sedation and pain relief. Moreover, it is also possible to reduce the dosage of other concomitant drug when it is combined with other anesthetic or sedative agents[6]. In addition, previous studies suggest that Dex would attenuate the damage caused by ischemia-reperfusion injury to the kidney through ameliorating oxidative stress[10]. It was also found out that high level of glucose was able to induce EMT in renal tubular epithelial cells by inducing oxidative stress[12]. Based on the previous research, we would like to investigate whether or not Dex would attenuate high glucose-induced HK-2 epithelial-mesenchymal transition and underlying mechanisms.
Our findings suggest that Dex could reduce the ROS production, attenuate the adverse effects of high level of glucose on HK-2 cells. Dex could also help more HK-2 cells enter G1 phase compared with HK-2 cells exposed to high level of glucose. Our results are in accordance with Li et al. findings[15].
It is known that the damage caused by high level of glucose to cells is related to the activation of AKT and ERK[10]. In our present study, our findings also suggest that the activation of AKT and ERK is significantly decreased by Dex, which indicates that Dex might protect HK-2 cells from high level of glucose by inhibiting AKT and ERK.
In our present study, we found out that high glucose could cause the activation of AKT and ERK in HK-2 cells, increasing the expression of p-AKT and p-ERK as well as the expressions of α-SMA which is an important marker of myofibroblast[12], decreasing the expression of E-Cadherin and Claudin-1 which are typical phenotypic markers of epithelial cell. Our findings are in agreement with those of Xue et al.[16] and He et al.[12], supporting the notion that high glucose-induced EMT is closely associated with AKT/ERK. It was reported that the decreased expression of E-cadherin was involved in the progression of HK-2 cell fibrosis and EMT is closely associated with diabetic nephropathy and prognosis[17].
The protective effects of Dex against acute kidney damage and kidney ischemia/reperfusion injury have been studied extensively. However, whether or not Dex will play a protective role in the diabetic nephropathy caused by high glucose is still unclear. Western blot results of our study demonstrate that the activation of AKT and ERK was significantly lower and the expression of E-Cadherin and Claudin-1 was significantly higher in HK-2 cells treated with both glucose and Dex than that treated with only glucose, moreover, the expression of α-SMA was also lowered by Dex, which suggest that the effects of Dex on alleviating EMT in HK-2 cells may be related to downregulating the activation of AKT and ERK. In order to verify the notion that Dexmedetomidine attenuates high glucose-induced HK-2 epithelial-mesenchymal transition by inhibiting AKT/ERK, we used PI3K/AKT inhibitor LY294002 and ERK inhibitor U0126 to test the notion. Western blot results reveal that the inhibitors are able to alleviate EMT, as shown by the decreased expression of α-SMA and increased expression of E-Cadherin and Claudin-1. Interestingly, PI3K/AKT inhibitor LY294002 can significantly inhibit the activation of ERK besides inhibiting of AKT activation, while ERK inhibitor U0126, in contrast, cannot significantly inhibit the activation of PI3K/AKT, which indicate that in the condition of high glucose-induced EMT in HK-2 cells, the regulation between AKT and ERK is not dual and Dex attenuates high glucose-induced HK-2 EMT by inhibiting AKT/ERK.
Our findings indicate that Dex might produce favorable, protective effects on diabetic kidney, might attenuate high glucose-induced epithelial-mesenchymal transition, therefore, our research is supportive of the notion that Dex may be preferable for patients with diabetes mellitus. Our findings are based on cellular experiments, not on animal experiment, which is also a limitation of our study. Further study needs to explore whether or not Dex will attenuate EMT in diabetic kidney using animal experiment.
-
Study concept and design: PAN Qi Zheng, RNE Shu Ping, and ZHAO Guo Qing. Analysis and interpretation of data: PAN Qi Zheng, REN Shu Ping, and SHI Jia Hong. Drafting and revision of the manuscript: REN Shu Ping. Obtained funding: ZHAO Guo Qing. Experiment conduction: PAN Qi Zheng, LI Kai, YANG Zhuo Dong, and GAO Ming. All authors have approved the final article.
-
No conflict of interest to declare.
Dexmedetomidine Attenuates High Glucose-induced HK-2 Epithelial-mesenchymal Transition by Inhibiting AKT and ERK
doi: 10.3967/bes2020.044
- Received Date: 2020-01-08
- Accepted Date: 2020-03-25
-
Key words:
- Dexmedetomidine /
- Epithelial-mesenchymal transition /
- High glucose /
- Oxidative stress /
- HK-2 cells
Abstract:
| Citation: | PAN Qi Zheng, LI Kai, YANG Zhuo Dong, GAO Ming, SHI Jia Hong, REN Shu Ping, ZHAO Guo Qing. Dexmedetomidine Attenuates High Glucose-induced HK-2 Epithelial-mesenchymal Transition by Inhibiting AKT and ERK[J]. Biomedical and Environmental Sciences, 2020, 33(5): 323-330. doi: 10.3967/bes2020.044 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: