-
In recent decades, the potential health hazards of microwave exposure have been attracting increasing attention. Our previous studies have demonstrated that microwave exposure impaired learning and memory in experimental animal models[1, 2]. The synapse is a particular structure for transmitting electrochemical signals between nerve cells in the central nervous system (CNS). Synaptic plasticity regulates learning and memory via releasing neurotransmitters, inducing expression of receptors at synapses, activating intracellular signaling cascades, and by inducing other changes[3, 4]. Studies from our group and others have reported that microwave exposure could impair synaptic plasticity in rat hippocampus[1, 5]. However, the underlying mechanisms by which this occurs are still largely unexplored.
MicroRNAs (miRNA) play pivotal roles in maintaining physiological functions of the CNS, such as synaptic activities, axonal transport, the release of neurotransmitters, and mitochondrial biogenesis[6, 7]. MiRNAs are also important regulators in the pathogenesis of neurodegenerative diseases, neuroblastoma, and schizophrenia [8]. Our previous study showed that miRNA-219-5p (miR219) was significantly down-regulated at seven days after 30 mW/cm2 microwave exposure. However, the effects of miR219 on synaptic plasticity have not been reported.
In this study, we confirmed that 30 mW/cm2 microwave exposure could dynamically regulate miR219 expression both in rat hippocampus and neuron-like cells. Importantly, synaptic structure- and activity-related molecules were negatively associated with miR219 expression and could be down-regulated by miR219 in neuron-like cells. We also showed that miR219 over-expression reduced expression of calcium/calmodulin-dependent protein kinase II (CaMKII) γ, N-methyl-D-aspartate receptor (NMDAR) 1, and phosphorylated cAMP response element-binding protein (p-CREB).
Forty-eight male Wistar rats (220 ± 20 g) were randomly divided into two groups: the microwave exposure (MW) group and the sham-exposure (SH) group. The exposure system was based on a klystron amplifier model JD 2000 (Vacuum Electronics Research Institute, Beijing, China) as described previously[1]. Animals in the MW group were exposed to microwaves at an average power density of 30 mW/cm2 for 10 min every other day for three days. Animals in the SH group were processed in parallel to the MW group but were not exposed to microwaves. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of the Beijing Institute of Radiation Medicine (IACUC-AMMS-2010-007). At 1, 7, and 14 d after the last exposure to microwave, rats were euthanized, and brains were removed and hippocampal tissues were isolated for analysis.
Rat pheochromocytoma (PC12) cell-derived neuron-like cells were induced in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Grand Island, NY) supplemented with 5 ng/mL nerve growth factor (NGF, Sigma, St. Louis, MO) and 1% horse serum for five days. The neuron-like cells were randomly divided into MW and SH groups. The MW group was exposed to 30 mW/cm2 microwaves for five minutes. Cells in the SH group were treated as the MW group but without microwave exposure. The cells were harvested at 1, 6, 12, and 24 h after exposure. pEGFP-C1-miR219 (GenePharma, Shanghai, China) and GV249-shmiR219 (GenePharma) were transfected into PC12 cells using Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA) and were induced to neuron-like cells at 48 h post-transfection. At 1, 6, 12, and 24 h after microwave exposure, cells were collected for analysis.
Total protein was extracted at indicated time points after microwave exposure, from both rat hippocampal tissues and neuron-like cells. The protein expression of VGLUT1, PSD-95, CaMKIIγ, NMDAR1, CREB and phosphorylated-CREB was detected by western blotting using rabbit anti-rat VGLUT1 antibody (Santa Cruz, Dallas, TX), rabbit anti-rat PSD-95 antibody, mouse anti-rat CaMKIIγ antibody (Abcam, Cambridge, MA), rabbit anti-rat NMDAR1 antibody, rabbit anti-rat CREB antibody, rabbit anti-rat p-CREB antibody (Cell Signaling Technology, Danvers, MA), mouse anti-glycolytic glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (KangChenBiology, Nanjing, China), and corresponding secondary antibodies. The relative expression was normalized to GAPDH.
Total RNA was also isolated from both rat hippocampus tissues and neuron-like cells. For analysis of synaptic activity-related factors, complementary DNA (cDNA) was synthesized using the AMV First-Strand cDNA synthesis kit (Sangon Biotech, Shanghai, China). The mRNA expression was measured by real-time RT-PCR using the Real Master Mix SYBR Green Kit (Tiangen, Beijing, China), and normalized to GAPDH. For miR219 expression analysis, 10 μg of template RNA was reverse transcribed using the TaqMan® microRNA reverse transcription kit (Applied Biosystems, ABI, Carlsbad, CA). Then, miR219 was detected using Gene Expression Master Mix with a specific miR219 probe (ABI) on an ABI 7300 real-time PCR system. The relative expression level of miRNA was normalized to U6 snRNA using the 2-ΔCt method.
Data were statistically analyzed using GraphPad Prism software version 5 (GraphPad Software, San Diego, CA, USA) and are presented as the mean ± se. Unpaired t-tests were used to analyze other data between two groups. One-way ANOVAs followed by Bonferroni post hoc tests were performed to analyze multiple groups. Differences were considered significant at a two-sided value of P < 0.05.
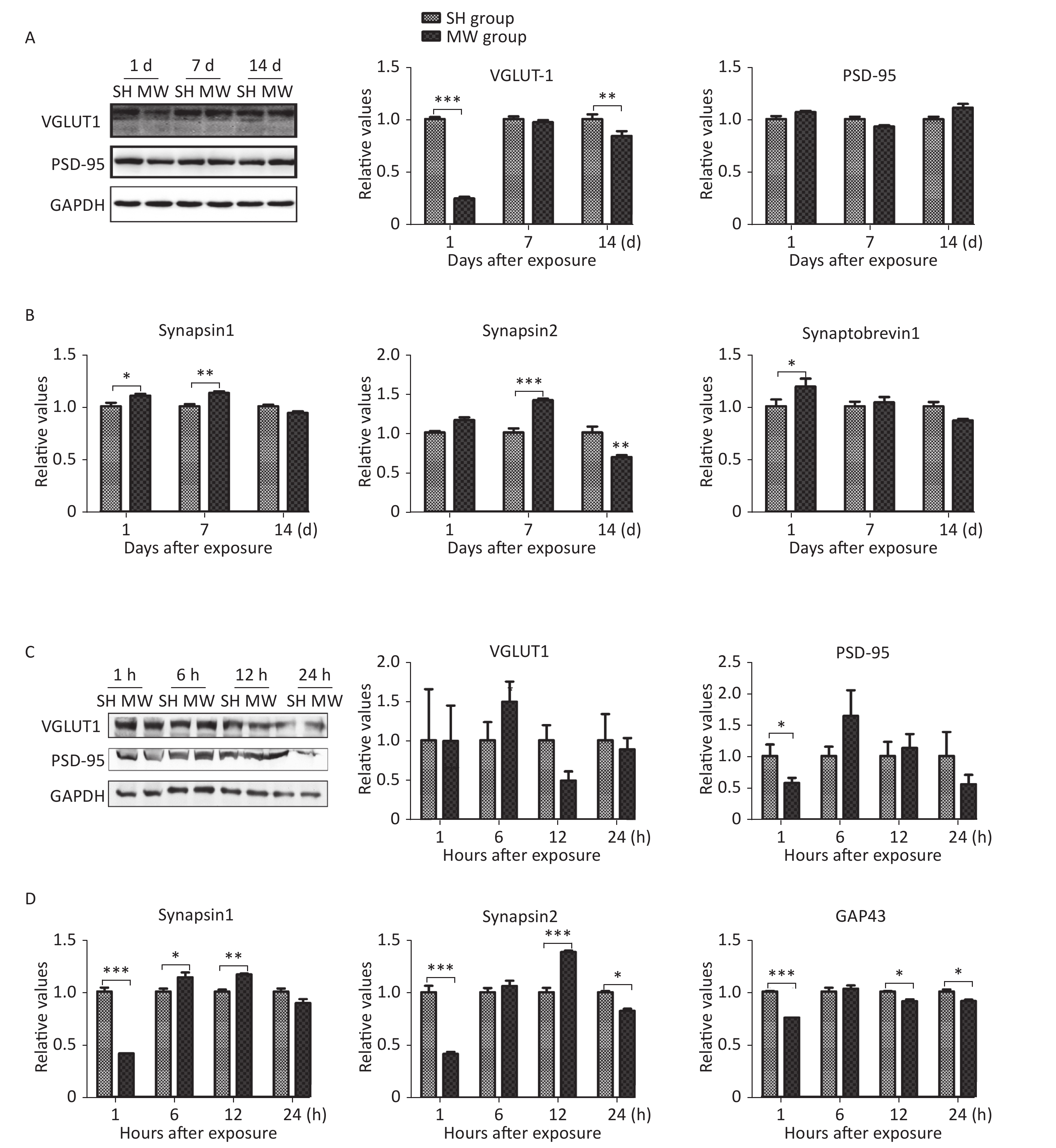
We previously reported that microwave exposure reduced the formation and the number of excitatory synapses in rat hippocampal neurons in vivo[5]. In this study, we evaluated several structural proteins, VGLUT1 and PSD-95, and synaptic activity-related molecules, such as synapsin 1, synapsin 2, synaptobrevin 1, and GAP43, in both rat hippocampus and PC12 cell-derived neuron-like cells. In rat hippocampus, the pre-synaptic marker VGLUT1 was down-regulated by microwave exposure at day 1 (P < 0.001) after exposure and then restored to a normal level at day 7. However, no obvious changes in the postsynaptic marker PSD-95 could be detected (Figure 1A). Interestingly, several synaptic activity-related molecules, such as synapsin 1 and synapsin 2, were up-regulated by microwave exposure on day 7 post-exposure (P < 0.01) (Figure 1B). In neuron-like cells, PSD-95, but not VGLUT1, was down-regulated at 1 h after exposure (P < 0.05) and then restored to normal levels (Figure 1C), suggesting that slight injuries were induced by microwave exposure. Moreover, the expression of synapsin 1, synapsin 2 and GAP43, indicators of synaptic plasticity activity, was down-regulated at 1 h (P < 0.001) and up-regulated at 6 and 12 h post-exposure (Figure 1D). These results suggested that microwave exposure induced injury of synaptic plasticity at an early stage and that some recovery mechanisms were subsequently activated.
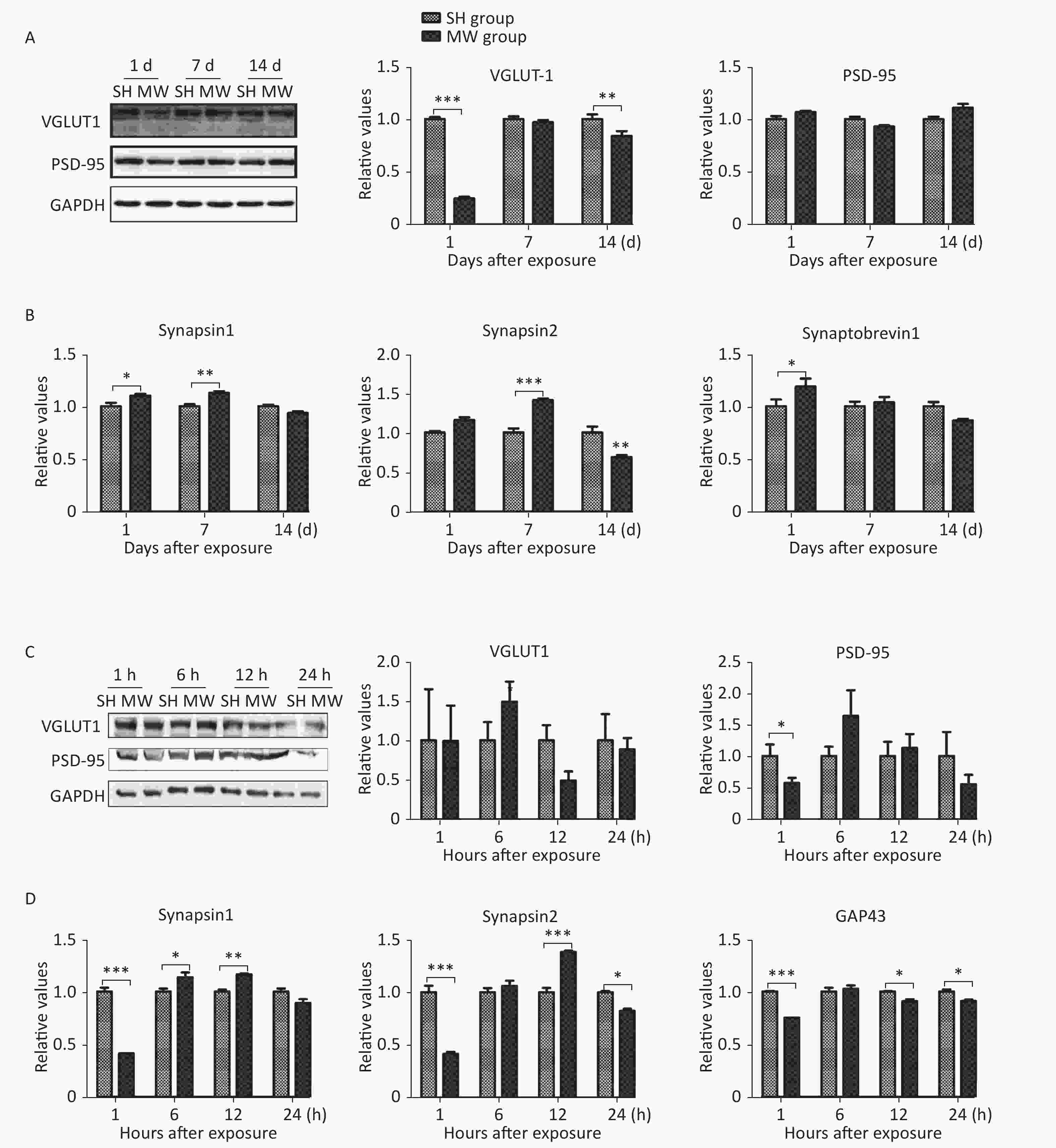
Figure 1. Microwave induced impairment of synaptic plasticity in both rat hippocampus and rat pheochromocytoma (PC12) cell-derived neuron-like cells.
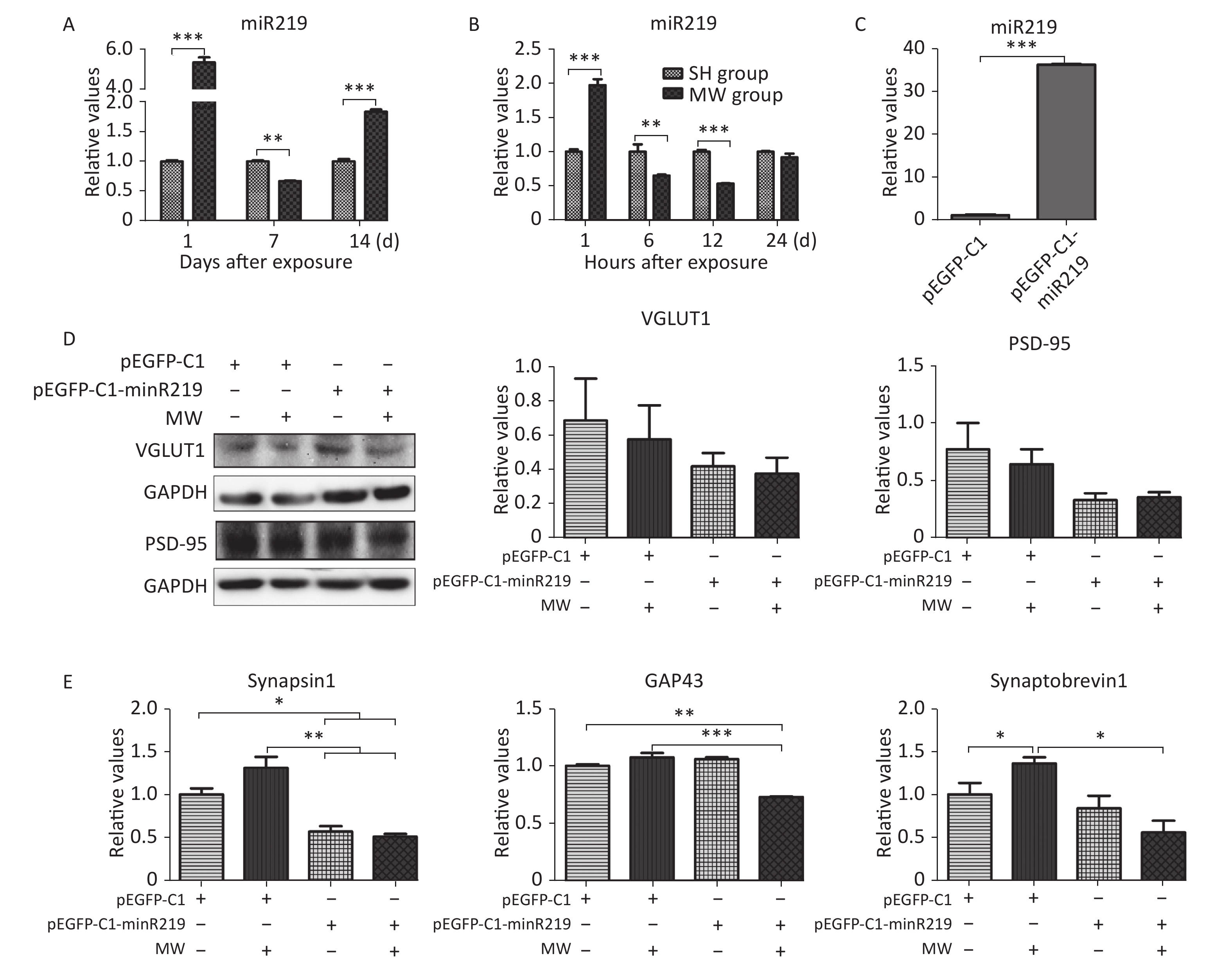
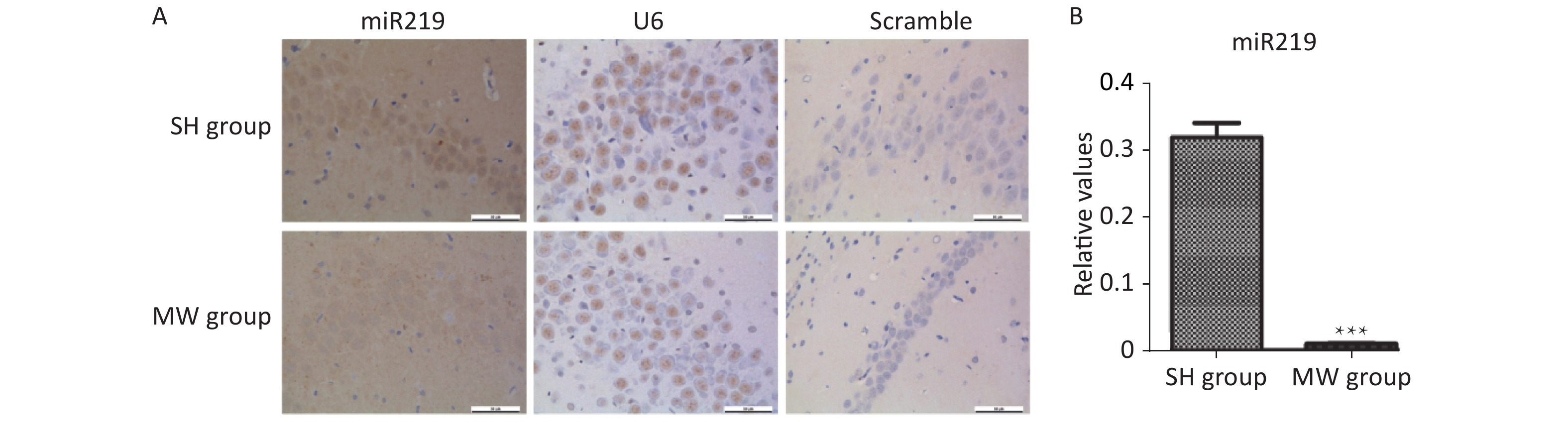
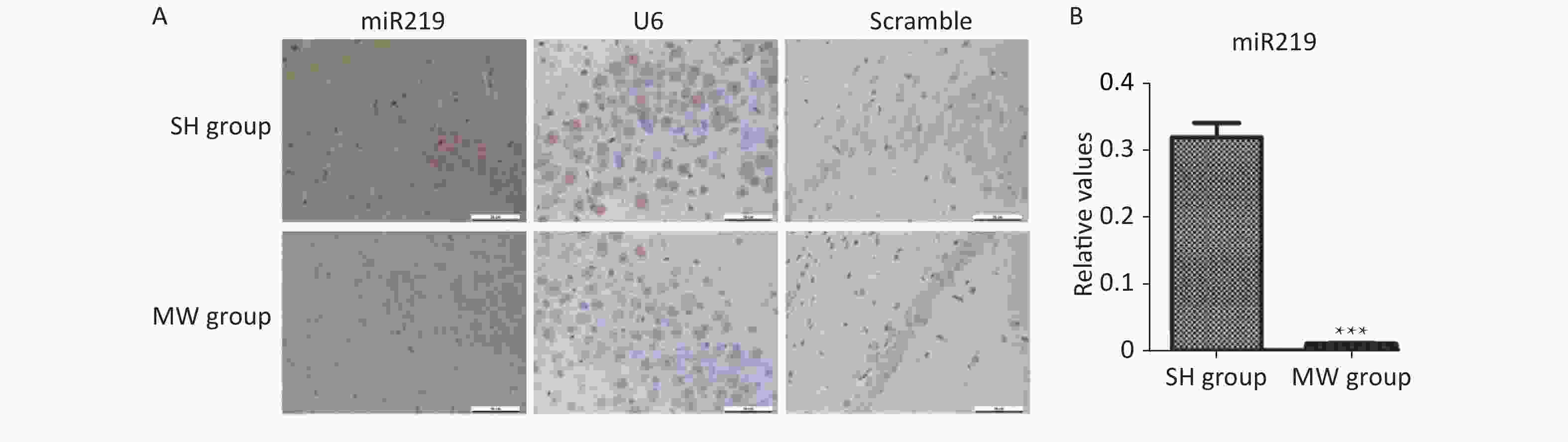
According to the results of microarray, miR219, an important molecule in the synaptic vesicle cycle, is a differentially expressed gene in rat hippocampus after 30 mW/cm2 microwave exposure. In this study, we confirmed that miR219 was markedly up-regulated in rat hippocampus at 1 d (P < 0.001), while it was down-regulated at 7 d (P < 0.01) after microwave exposure (Figure 2A, Supplementary Figure S1 available in www.besjournal.com). In neuron-like cells, miR219 expression increased at 1 h (P < 0.001), while a significant decrease could be detected at both 6 and 12 h (P < 0.01) after exposure (Figure 2B). To reveal the mechanisms underlying the protective effects of miR219 on synaptic plasticity, we prepared a miR219 over-expression vector, pEGFP-C1-miR219. pEGFP-C1-miR219 expressed miR219 in neuron-like cells effectively (Figure 2C). Compared to the pEGFP-C1 transfected neuron-like cells, pEGFP-C1-miR219-transfection slightly reduced VGLUT1 and PSD-95 protein expression at 6 h after exposure (Figure 2D). As shown in Figure 2E, mRNA expression of synaptic plasticity-related molecules, such as synapsin 1, GAP43, and synaptobrevin 1 was slightly up-regulated at 6 h after exposure, while miR219 over-expression significantly inhibited the mRNA expression of these genes (P < 0.05). These results indicated that miR219 down-regulation might initiate protective effects after microwave exposure via enhancing the activity of synaptic plasticity.
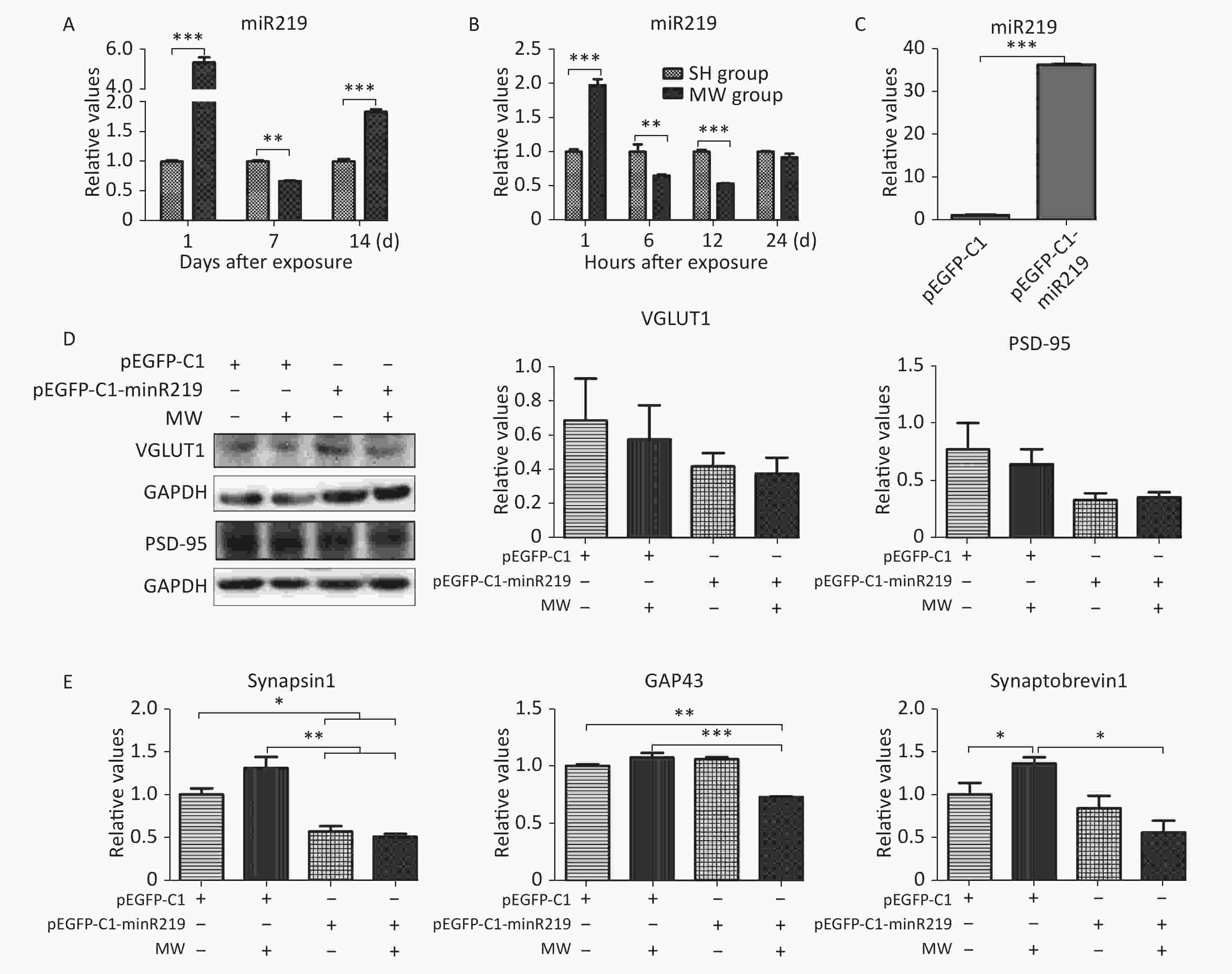
Figure 2. Microwave exposure (MW) dynamically regulates miR219 expression and protects synaptic plasticity from microwave induced injuries.
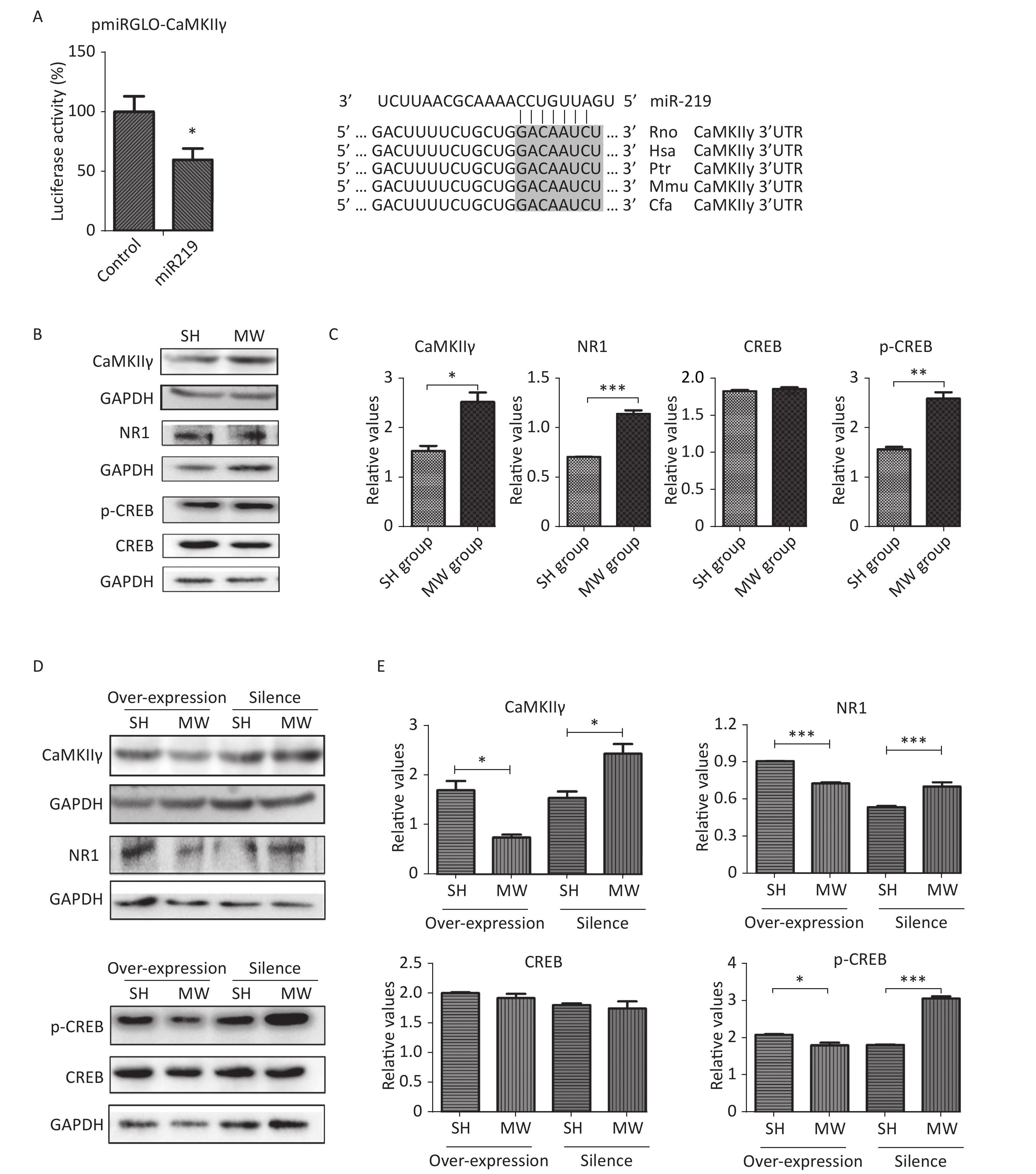
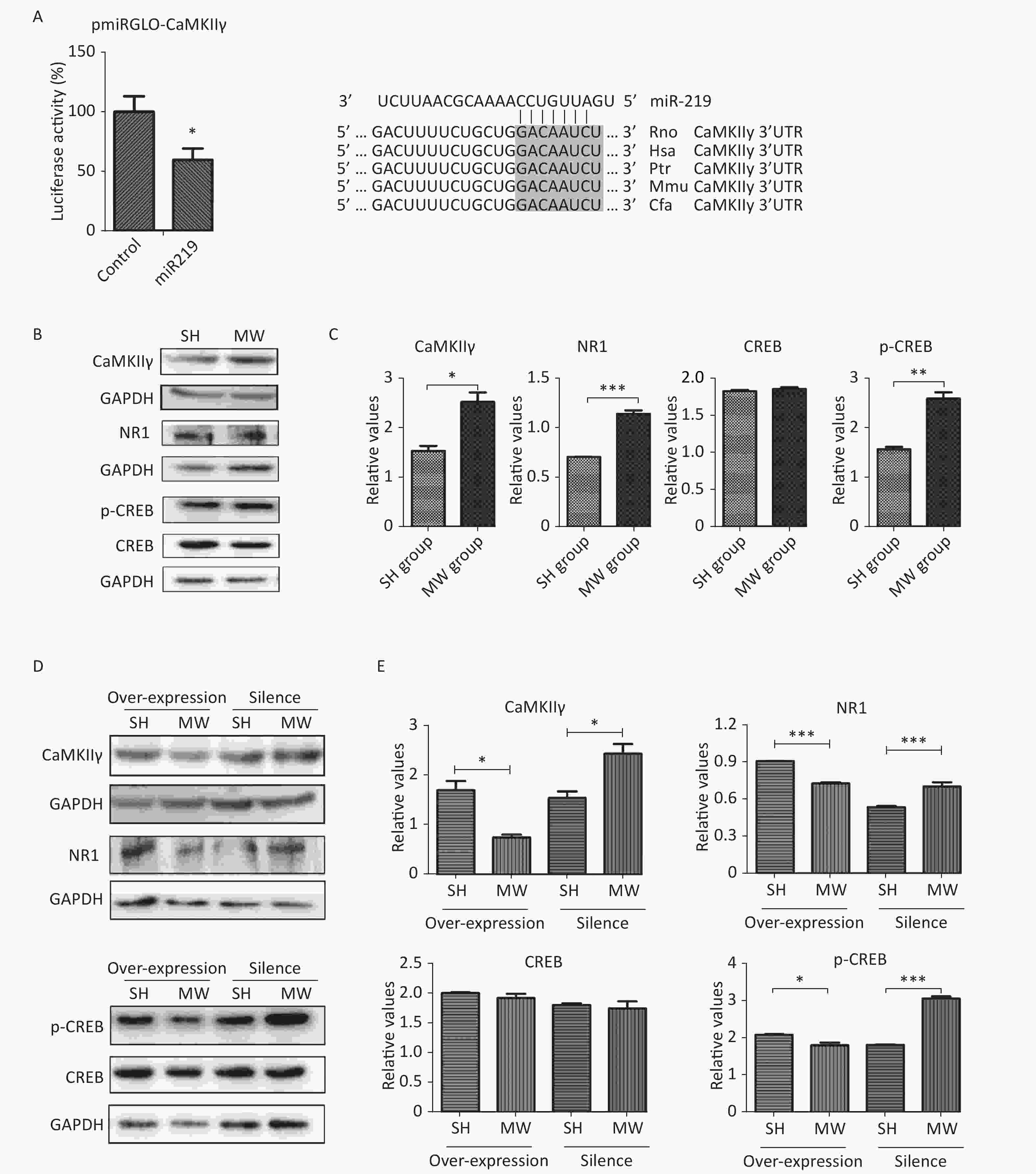
Numerous studies have demonstrated that NMDAR-related signaling plays an important role in regulating synaptic plasticity in the CNS[2, 9]. CaMKII, an unusual kinase accounting for 2%–6% of total protein in the PSD, can be activated by Ca2+ influx via NMDARs. CaMKII is composed of four different genes, CAMK2A, CAMK2B, CAMK2G, and CAMK2D, encoding CaMKIIα, CaMKIIβ, CaMKIIγ, CaMKIIδ, respectively[10]. CaMKIIγ is a pivotal molecule in the NMDAR signaling pathway, which is involved in excitatory synaptic transmission. According to software prediction, CaMKIIγ may be a target gene of miR219. Therefore, we constructed a vector with dual-luciferase reporter genes to verify the binding sequence of miR219 in CaMKIIγ. As expected, miR219 could bind to a corresponding site of CaMKIIγ, resulting in the observed down-regulation of luciferase (P < 0.05; Figure 3A) and indicating that CaMKIIγ is the target gene of miR219.
In the in vitro neuron-like cell model, we showed that microwave exposure increased CaMKIIγ protein expression (P < 0.05) at 6 h after microwave exposure. Moreover, the expression of NMDAR1, which lies upstream of CaMKIIγ in the canonical signaling pathway, and phosphorylated-CREB (p-CREB), which lies downstream of CaMKIIγ in the same pathway, were also enhanced (P < 0.01) by microwave exposure (Figure 3B–3C). To further investigate the roles of miR219 in regulating CaMKIIγ and NMDAR signaling, we exposed miR219-overexpressing and miR219-silenced (transfected with GV249-shmiR219) neuron-like cells to 30 mW/cm2 microwaves. As expected, miR219 over-expression significantly reduced CaMKIIγ, NMDAR1 and p-CREB expression (P < 0.05) after microwave exposure, while miR219 silencing enhanced the expression of these genes (P < 0.05; Figure 3D–3E). These results suggested that the down-regulation of miR219 after microwave exposure could promote the repair of synaptic structures and synaptic activity via increasing CaMKIIγ expression and activating NMDAR signaling.
In conclusion, microwave exposure reduced miR219 expression in the hippocampus, which, in turn, up-regulated CaMKIIγ expression and activated NMDAR signaling, which finally promoted synaptic plasticity to recover learning and memory.
The authors declare that they have no conflicts of interest.
Inhibition of MicroRNA 219 Expression Protects Synaptic Plasticity via Activating NMDAR1, CaMKIIγ, and p-CREB after Microwave Radiation
doi: 10.3967/bes2020.048
- Received Date: 2019-08-19
- Accepted Date: 2020-03-10
| Citation: | ZHAO Li, XIONG Lu, HAO Yan Hui, LI Wen Chao, DONG Ji, ZHANG Jing, YAO Bin Wei, XU Xin Ping, WANG Li Feng, ZHOU Hong Mei, PENG Rui Yun. Inhibition of MicroRNA 219 Expression Protects Synaptic Plasticity via Activating NMDAR1, CaMKIIγ, and p-CREB after Microwave Radiation[J]. Biomedical and Environmental Sciences, 2020, 33(5): 359-364. doi: 10.3967/bes2020.048 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: