-
Type 2 diabetes mellitus (T2DM) is typified by the increment of chronic blood glucose levels that is caused by an absolute and/or a relative deficiency of insulin, accounts for 90% of diabetes and causes a range of complications[1]. It has been suggested that inflammation contributed to the pathogenesis of T2DM, which may result in severe insulin resistance (IR)[2]. Liver plays a pivotal role in glucolipid metabolism as hepatic glycogen is synthesized and stored within the organ[3].
Pterostilbene (PTE), a natural polyphenolic compound, has been widely found in blueberries, blackberries and a series of other berries. Some previous studies have shown that PTE had the functions of anti-inflammatory, reducing fasting blood glucose (FBG) levels and oxidative stress in diabetic rats[4, 5]. However, the potential effect of PTE on liver injury and its associated metabolic complications induced by T2DM have yet to be further investigated. Therefore, we conducted this study to investigate the protective effect of PTE on liver injury in diabetic rats and its underlying mechanisms.
We purchased male SPF grade SD rats (4 weeks, weighed 170–220 g, n = 70) from Henan laboratory animal center. All animal experimental procedures were performed sternly compliance with the National Research Council Guide for the Care and Use of Laboratory Animals. The rats were housed under standard conditions with free access to food and water. After 1 week of adjustable feeding, control group (n = 10) were treated with ordinary feeding (carbohydrate 41.47%, protein 21.06% and fat 14.42%), and model group (n = 60) were fed with a high-fat and high-sugar diet (basic food 59.5%, sucrose 20%, lard oil 10%, yolk powder 10%, and sodium cholate 0.5%), all persisted to the end of the experiment. Diabetes induction was performed using streptozocin (STZ, 40 mg/kg BW)[6]. In our study, 50 rats were successfully established the diabetes model, and then were divided into four groups (n = 10): diabetes group, PTE 20 group [diabetes + PTE 20 mg/(kg·d)], PTE 40 group [diabetes + PTE 40 mg/(kg·d)], and PTE 80 group [diabetes + PTE 80 mg/(kg·d)], the rest of diabetes model rats and the unsuccessfully diabetes model rats were killed. And then PTE were suspended in 0.5% carboxymethylcellulose (CMC-Na) solution, PTE intervention groups received respective intervention dose, and the control and diabetes groups received 0.5% w/v sodium CMC-Na solution only, at a fixed time each day for another 6 weeks. After the experiment, blood and rat liver were collected, a part of each rat liver was stained with HE.
The levels of total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), FBG, and fasting serum insulin (FSI) levels were detected using automatic biochemical analyzer (KHB, Shanghai, China). The concentrations of interleukin-6 (IL-6), IL-1β and tumor necrosis factor-alpha (TNF-α) in the serum were analyzed by ELISA kits (IL-6, IL-1β: Bioss Biotech, Beijing, China; TNF-α: Neobioscience, Beijing, China), and serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were detected via colorimetric assay kits (Elabscience Biotech, Wuhan, China), according to the manufacturer’s instructions.
After that, rat livers were weighed and homogenized in tissue protein extraction kits. Quantified with BCA assay kit, proteins were denatured with 5 × SDS-PAGE loading buffer, ran on a SDS-PAGE and transferred onto PVDF membranes. The membranes were blocked and incubated at 4 °C with respective antibodies against β-actin (1:5,000, Dingguo Changsheng Biotech, Beijing, China), NF-κBp50 (1:1,000, Proteintech, China) and IL-6 (1:1,000, Proteintech, China). After incubation, the membranes were co-incubated with an HRP-conjugated antimouse or antirabbit antibodies (1:5,000, Dingguo Changsheng Biotech, Beijing, China) at room temperature for 2 h. The signals were detected and captured using chemiluminescence system and analysed by Image J software.
The results were performed using GraphPad Prism 8. Data were represented as mean ± standard deviation (SD). One way analysis of variance (ANOVA) following Tukey’s post hoc tests was used to find statistical relevance of the data. P < 0.05 was considered significant.
Hyperglycemia is one of the main metabolic disorders of T2DM. Table 1 showed the FBG and FSI levels in rats. We found that FBG of model group was significantly higher after 4 weeks high-fat and high-sugar fed than control group, which means a hyperglycemic state in model rats. After STZ injection, it may specifically destroy islet β cells, resulting in a large amount of insulin release and a sharp increase in model rats. However, we found that FBG did not decrease due to the large release of insulin in Table 1, indicating that the rats of model group have IR. After PTE treatment, PTE 20 group demonstrated significant reductions in FBG and FSI level (P < 0.05) as compared with diabetes group, which may means less IR; while other intervention groups did not find desired results. It is not known whether the target sites of different concentrations of PTE are consistent, and coupled with individual differences, the reasons for this phenomenon need to be further explored.
Groups FBG FSI −1 week 0 week 6 week −1 week 0 week 6 week Control 9.02 ± 1.16 5.68 ± 1.40 8.11 ± 1.39 3.23 ± 1.74 1.64 ± 1.17 0.82 ± 0.69 Diabetes 14.47 ± 3.92* 27.62 ± 5.22* 31.66 ± 2.04* 2.48 ± 2.06 48.35 ± 11.11* 95.11 ± 26.14* PTE 20 16.73 ± 3.56* 26.80 ± 5.11* 23.26 ± 6.53*# 5.85 ± 2.14 42.00 ± 17.73* 41.16 ± 9.79*# PTE 40 16.00 ± 1.65* 28.32 ± 7.19* 25.69 ± 2.61* 4.02 ± 3.40 52.09 ± 10.58* 54.04 ± 24.57* PTE 80 15.48 ± 1.03* 26.86 ± 3.59* 26.36 ± 3.72* 4.98 ± 6.10 59.61 ± 13.53* 53.89 ± 3.73*# Note. Data are presented as mean ± SD (n = 10); *P < 0.05 compared with control group; #P < 0.05 compared with diabetes group. PTE: pterostilbene; FBG: fasting blood glucose; FSI: fasting serum insulin. Table 1. Effects of PTE on FBG and FSI
Dyslipidemia is a common pathological change in patients with diabetes and may be caused by IR[7]. When IR occurs, free fatty acids in the body cannot be converted, which increases the synthesis of TC, TG, and LDL-C in the liver and induces dyslipidemia. Dyslipidemia can affect vascular endothelial function, destroy vascular endothelial cells, and cause atherosclerosis, which is the main cause of cardiovascular disease. Therefore, correcting T2DM-induced dyslipidemia can improve IR and reduce the risk of cardiovascular disease, which is important for the prevention and treatment of T2DM complications. Results of TC, TG, LDL-C, and HDL-C were presented in Table 2. We found that the levels of serum TC, TG, and LDL-C of diabetes rats were significantly increased compared with control group, while a marked decline in HDL-C levels was found in the serum of diabetes rats as compared to the control group (P < 0.05), which means that lipid disorders did exist in diabetic rats. After 6 weeks of PTE treatment, we found that rats in PTE 20 group showed significant decreases in serum TC, TG, and LDL-C levels compared with diabetes groups (P < 0.05) and produced an equivalent effect with controls; PTE 40 group rats experienced significant decreases in serum TC and LDL-C (P < 0.05); the serum TC, TG, and LDL-C levels of PTE 80 group didn’t decreased significantly. Additionally, we found no significantly difference between diabetes group and PTE intervention groups in HDL-C levels.
Groups TC
(mmol/L)TG
(mmol/L)LDL-C
(mmol/L)HDL-C
(mmol/L)IL-6
(ng/L)IL-1β
(ng/L)TNF-α
(pg/mL)AST
(IU/L)ALT
(IU/L)Control 1.48 ± 0.22 0.58 ± 0.17 0.29 ± 0.12 1.07 ± 0.11 15.59 ± 1.59 10.40 ± 2.27 114.61 ± 7.32 58.13 ± 25.74 31.15 ± 13.65 Diabetes 3.46 ± 0.80* 6.22 ± 2.06* 1.36 ± 0.48* 0.41 ± 0.22* 35.31 ± 6.88* 23.57 ± 4.47* 344.07 ± 41.86* 162.30 ± 63.77* 76.72 ± 11.96* PTE 20 1.85 ± 0.20# 3.04 ± 1.69# 0.46 ± 0.33# 0.57 ± 0.28* 25.70 ± 3.62*# 15.62 ± 3.36# 250.08 ± 56.65*# 74.85 ± 8.67# 38.02 ± 13.55# PTE 40 2.15 ± 0.58# 5.20 ± 1.18* 0.64 ± 0.42# 0.26 ± 0.29* 22.35 ± 3.63# 12.53 ± 1.48# 222.29 ± 31.70*# 70.13 ± 28.90# 33.92 ± 11.14# PTE 80 2.62 ± 0.65* 4.83 ± 1.38* 0.92 ± 0.34 0.32 ± 0.42* 27.36 ± 3.18* 17.70 ± 3.68* 267.97 ± 47.96* 99.07 ± 29.65 44.61 ± 21.70# Note. Data are presented as mean ± SD (n = 10); *P < 0.05 compared with control group; #P < 0.05 compared with diabetes group. PTE: pterostilbene; TC: total cholesterol; TG: triglycerides; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; IL-6: interleukin-6; IL-1β: interleukin-1β; TNF-α: tumor necrosis factor-alpha; AST: aspartate aminotransferase; ALT: alanine aminotransferase. Table 2. Effects of PTE on serum lipid profile, inflammatory factors and liver function
Inflammation plays an important role in the development of IR via multiple signal pathways, which has been implicated in the pathogenesis of diabetes[2]. A report by Yang et al. has demonstrated that the IR could be successfully improved by reducing inflammation, illustrating the unequivocal correlation between both[8]. Increases in pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 are thought to be related to the severity of diabetes[9]. As shown in Table 2, diabetes rats showed higher levels of serum IL-6, IL-1β, and TNF-α than control group (P < 0.05). PTE treatment of 20 and 40 mg/(kg·d) significantly decreased the serum levels of IL-6, IL-1β, and TNF-α in model rats (P < 0.05), especially PTE 40 group. However, PTE 80 group rats showed no significant difference in serum levels of IL-6, IL-1β, and TNF-α comparing with that of diabetes group.
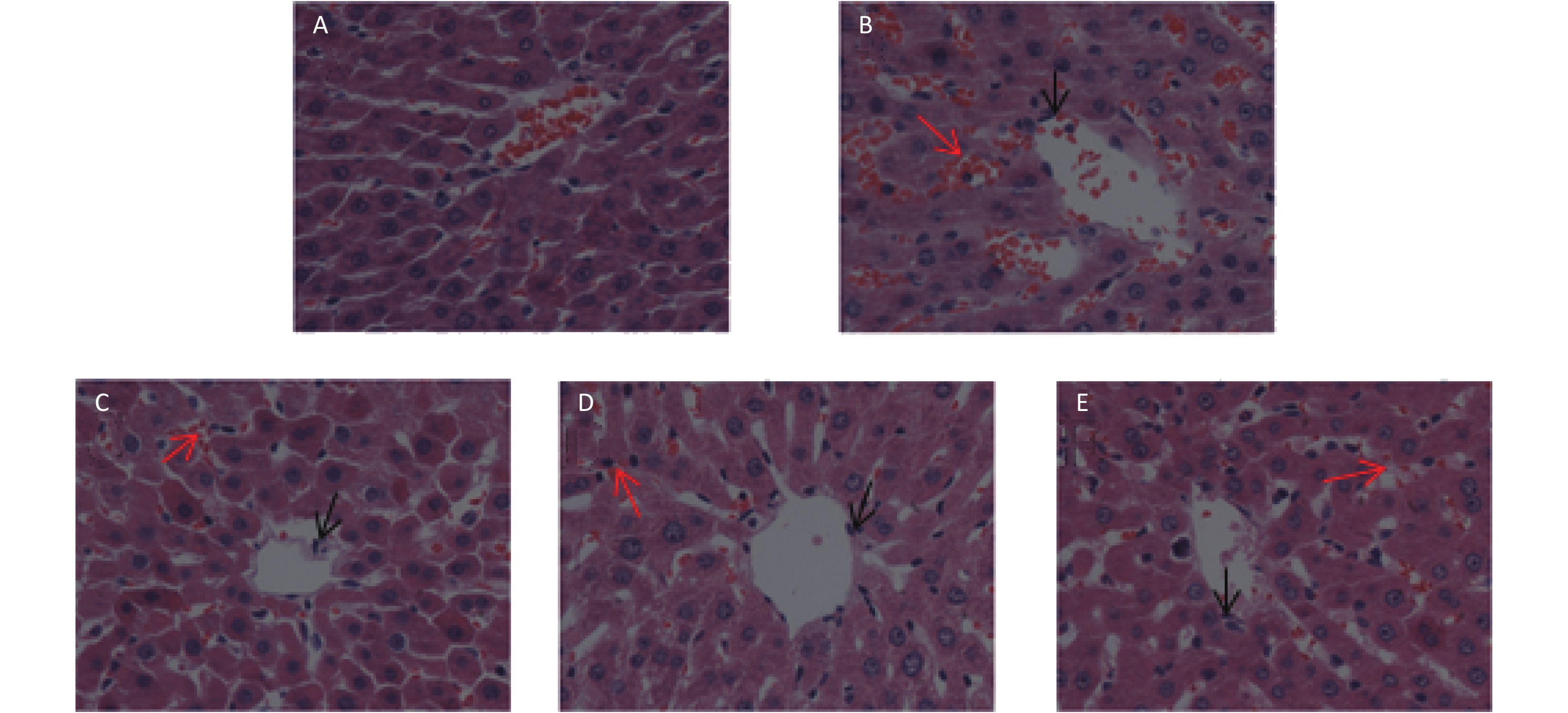
Liver tissue is a crucial primary metabolic tissue with high insulin sensitivity, and a main site for glycolipid metabolism. We measured serum AST and ALT levels to evaluate liver function (Table 2) and also performed HE staining of liver tissues (Supplementary Figure S1 available in www.besjournal.com). The serum levels of AST and ALT in the diabetes group were higher than control group (P < 0.05), suggesting that damages in liver might have occurred. Treatment of diabetic rats with PTE [20 and 40 mg/(kg·d)] induced a significant reduction in AST (P < 0.05); whereas no significant difference was observed between the diabetes and PTE 80 group. On the other hand, PTE treated diabetic rats experienced a decline in ALT levels (P < 0.05) as compared to the diabetic rats, which was 2.5-times the amount of control rats. We also found that the liver tissue of the control group rats was well-organized, the cytoplasm of hepatic cell was deeply stained and regularly arranged, and no obvious pathological change was observed in Supplementary Figure S1A. As for the diabetes group, the hepatic cord structure in the hepatic lobules was in disorder, the hepatocytes were swollen and enlarged, the cytoplasm became loosened, and local hepatic cell necrosis could be seen in the tissue, deep condensed or fragmented nucleus of necrotic cells (black arrow); accompanied by bleeding (red arrow) (Supplementary Figure S1B). Moreover, after 6 weeks PTE intervention, hepatic cord structure disorder reduced, and necrotic cells and bleeding decreased (Supplementary Figure S1C-E).
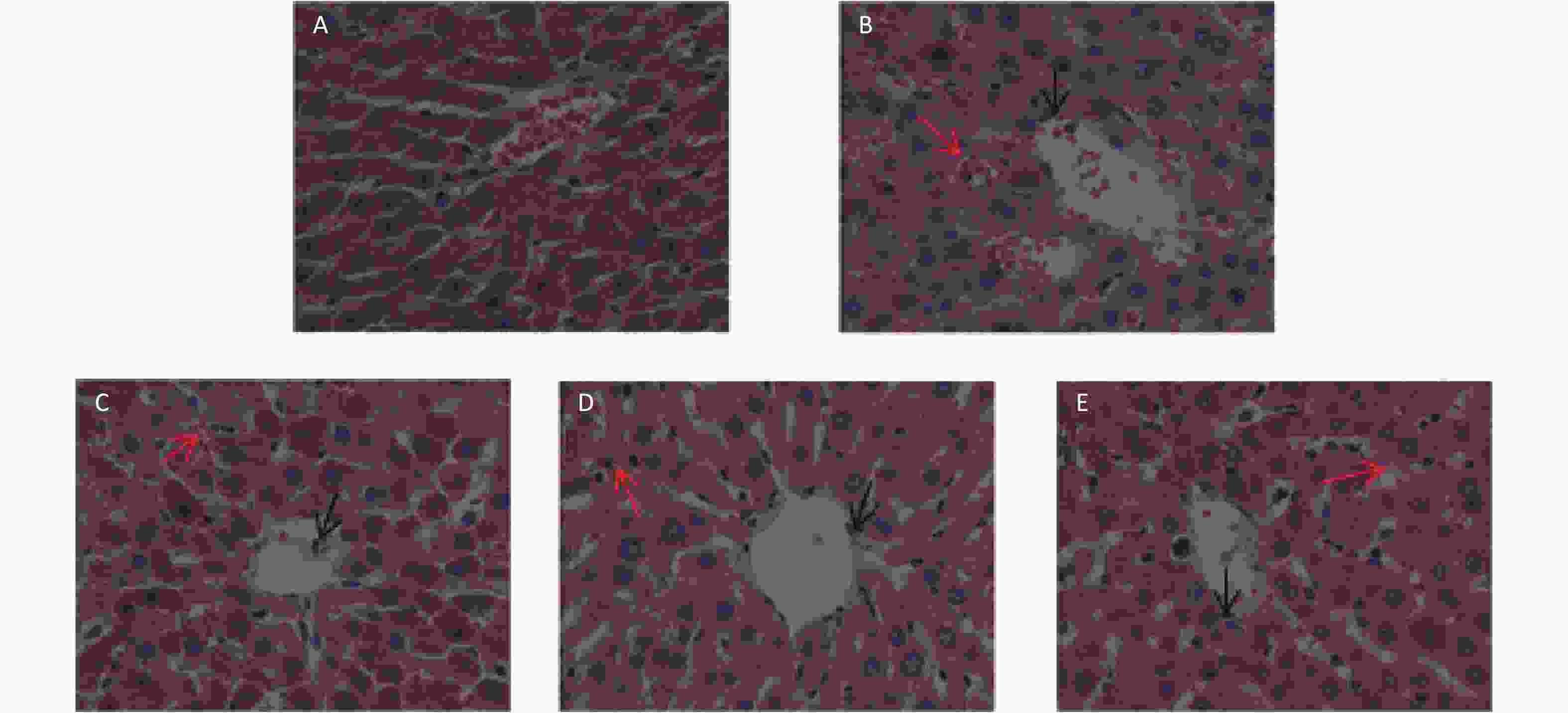
Figure S1. Effects of PTE on the morphology changes in the liver of control and experimental rats (40 × 10 magnification). (A) Control group; (B) Diabetes group; (C) PTE 20 group; (D) PTE 40 group; (E) PTE 80 group. Black arrow: hepatic cell necrosis; Red arrow: bleeding
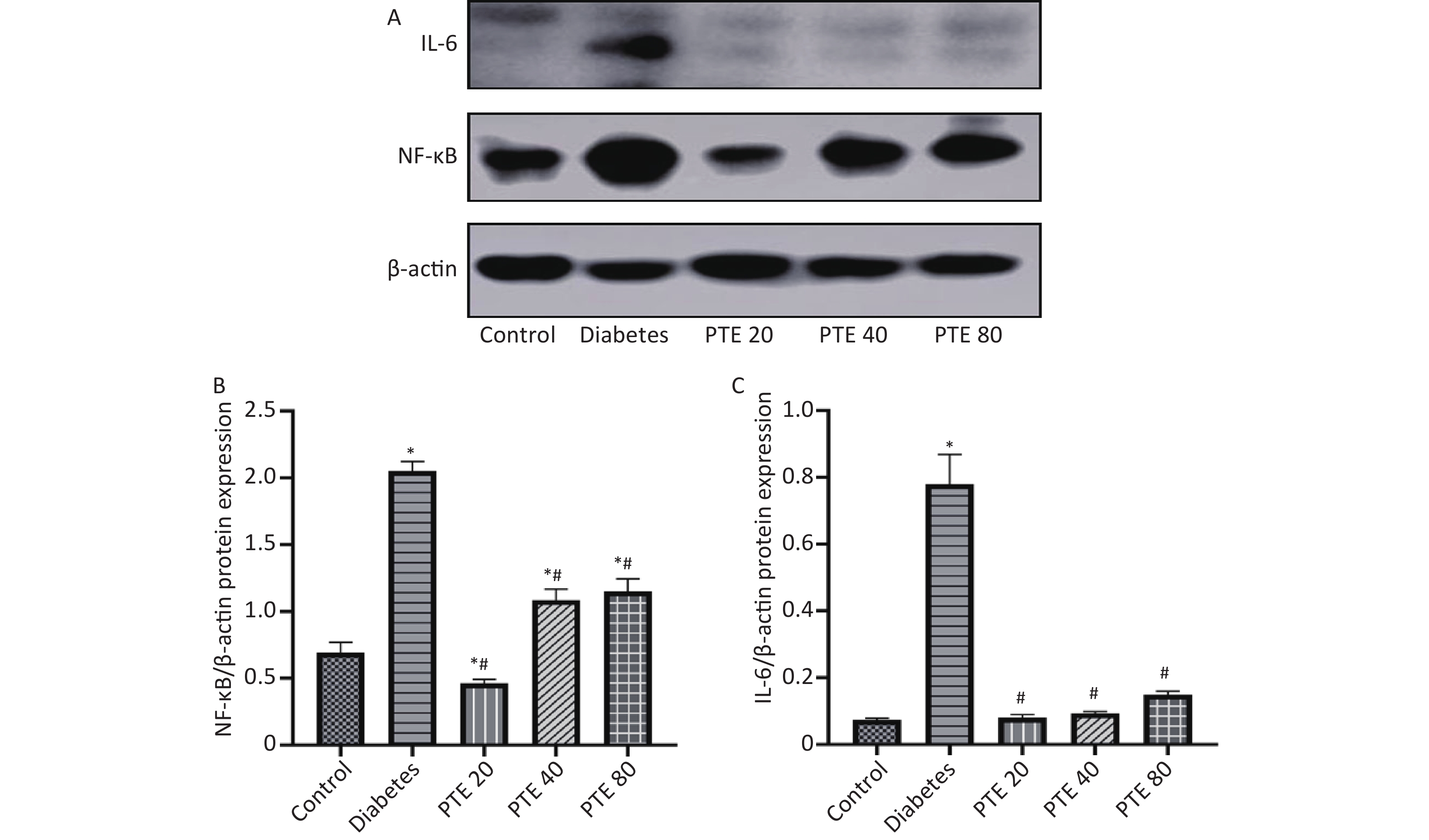
In addition, we also examined the expressions of NF-κBp50 and IL-6 protein in the liver of rats. NF-κB is a transcription factor and participates in the regulation of various gene expressions and plays an important role in the pathogenesis of diabetes[10]. Our results showed that the expressions of hepatic NF-κBp50 and IL-6 in diabetes rats were increased compared with control group (Figure 1; P < 0.05). After 6 weeks of treatment, PTE intervention groups showed significantly lower expressions of hepatic NF-κBp50 and IL-6 compared with diabetic rats (P < 0.05). Interestingly, PTE treatment of 20 mg/(kg·d) showed a stronger inhibitory effect on the expressions of NF-κBp50 and IL-6 protein in liver than the dosages of 40 and 80 mg/(kg·d). Moreover, there was no statistically significant difference in IL-6 protein levels between the PTE intervention groups and control group. Combined with the analysis of serum IL-6 levels, we found that the expression of IL-6 protein was not consistent with it, which may be related to individual differences, the sequence of PTE action sites, and the concentration of PTE at the target site may be different. This requires us to explore.
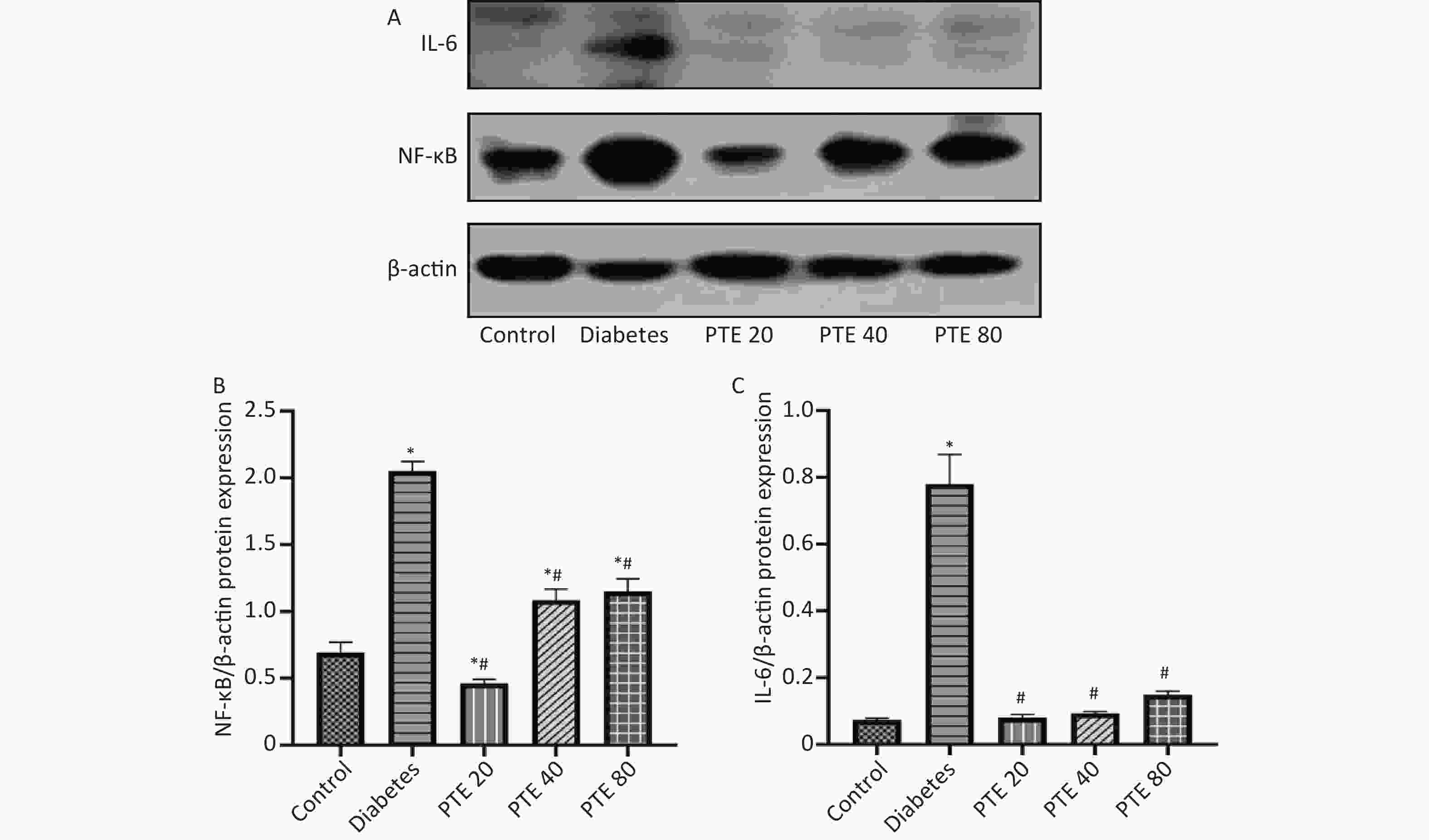
Figure 1. Effect of PTE treatment on the protein expressions of NF-κBp50 and IL-6 in the liver of experimental rats. (A) Protein levels of NF-κBp50 and IL-6 were detected by Western blot analysis. (B) NF-κBp50, and (C) IL-6 gray values were performed in control and experimental rats. Values were given as mean ± SD in each group (n = 10). Statistical analysis was performed by one-way ANOVA, followed by Tukey’s Post-hoc test. *P < 0.05, compared with control group; #P < 0.05, compared with diabetes group.
Our study shows that PTE can improve glycemic control, dyslipidemia, and liver injury in T2DM rats. This is the first one to explore the effect of PTE on various aspects of diabetes rats from the perspective of inflammation. Our study has significance for the prevention and treatment of diabetes. However, there are limitations: due to the complexity of the mechanism in rats, individual differences in rats, the uncertainty of the action of PTE on target organs in the body, and whether different concentrations of PTE will stimulate different mechanisms in the body, and so on. These uncertainties lead to effective doses between different indicators has a difference. We need further and more rigorous experiments to study the effectiveness and effective dose of PTE.
The authors confirm that they have no conflict of interest to report.
Pterostilbene Ameliorates Glycemic Control, Dyslipidemia and Liver Injury in Type 2 Diabetes Rats
doi: 10.3967/bes2020.049
- Received Date: 2019-08-26
- Accepted Date: 2020-03-22
| Citation: | ZHANG Yu Jing, SUN Hua Lei, WANG Teng, LIU Xin Xin, LIU Chang, SHEN Fang, WANG Bing Ya, DING Run Rong, LIU Yi Ming, HUANG Guo Yu, LI Wen Jie, LI Xing. Pterostilbene Ameliorates Glycemic Control, Dyslipidemia and Liver Injury in Type 2 Diabetes Rats[J]. Biomedical and Environmental Sciences, 2020, 33(5): 365-368. doi: 10.3967/bes2020.049 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: