-
Exosomes, a type of extracellular vesicles (EVs), were first discovered in sheep reticulocytes in 1983[1], and after four years, the name ‘exosome’ was officially given to them. A variety of cells secrete exosomes under both normal and pathological conditions. Exosomes are mainly products of the multivesicular body formed by intracellular lysosomal invagination, and released into the extracellular space by fusion of the outer membrane of the multivesicular body with the cell membrane. Exosomes are present in body fluids including blood, saliva, urine, cerebrospinal fluid, and breast milk[2]. Since their discovery, they have been found to play crucial roles in many physiological and pathological processes, and the last 10 years have witnessed the explosion of studies on exosomes and other EVs. They carry large amounts of materials, including proteins, lipids, and miRNA/mRNA/DNA, and are thus involved in intercellular communication[3-5]. For example, tumor-derived exosomes promote tumor growth by inhibiting anti-tumor immune responses and stimulating tumor proliferation and metastasis[6]. Disease-associated EVs play a role in disease pathogenesis and exert a wide range of effects on the target cell, therefore, EVs can be regarded as biomarkers for early diseases, and they may also be used as drug carriers for targeted therapy.
The male reproductive system consists of testes, ducts, accessory glands, and external genital organs: the penis and scrotum. The role of the testes is to produce sperm and hormones; the epididymal ducts transport, store, and assist sperm maturation. Accessory glands secrete most of the liquid part of the semen, and the penis containing the urethra serves for ejaculation. The glandular tissue of single testis contains 200–300 lobules, with up to three seminiferous tubules for each lobule. In the interstitial space, specialized Leydig cells secrete androgens[7]. Male sexual dysfunction is mainly divided into erectile dysfunction (ED), and hypogonadism[8]. In addition, infertility may also occur even when the sexual function is normal, sometimes due to sperm abnormalities such as azoospermia and oligozoospermia. The pathogenesis of many male infertility-related diseases has been uncovered recently[9].
The female reproductive system consists of the internal and external reproductive organs and related tissues. Female genitalia include the vagina, uterus, fallopian tubes, and ovaries. Reproduction requires a complex sequence of comprehensive events in the female body, including ovulation, sperm transport, sperm capacitation, fertilization, embryo implantation, fetal support, and childbirth[10, 11].
Recently, it was found that EVs are very important for tumor development, the cardiovascular system, and the immune system[1, 12]. In addition, there are many studies about the effects of EVs on reproductive processes such as gametogenesis, hormone secretion, and embryo development. This review summarizes the effects of EVs derived from various tissues or cells on male and female reproduction systems[13].
HTML
-
EVs include exosomes, microvesicles (also known as shedding vesicles, ectosomes, and nanoparticles), and apoptotic bodies. They are generally distinguished by their size and biogenesis. Exosomes are vesicles with a diameter of 20–100 nm[12, 14]. When the multivesicular bodies (MVBs) fuses with the membrane, it releases exosomes into the extracellular environment. By contrast, larger microvesicles (100 nm–1 μm) and apoptotic bodies (1–5 μm) directly bud outward from the plasma membrane[14]. Exosomes from different cell types contain different proteins, some of which are involved in MVBs biogenesis (for example, Alix and Tsg101)[15]. There are certain membrane proteins in EVs which accumulate in the plasma membrane or endosomes, such as four-molecule cross-linking family member 9 (TM4SF9, tetraspanins). TM4SF9 proteins such as CD63, CD81, CD82, CD53, and CD37 were first identified in B-cell exosomes in 1998, and enrichment of this protein suggests that it might be a potential marker for these exosomes as well[16]. Joanna Kowal and his team showed several classical exosomes by proteomic analysis, and proteins such as major histocompatibility complexes (MHC), flotillin, and 70 kD heat shock protein were found in almost all EVs. They finally identified proteins that are specifically enriched in small EVs and established that exosomes could be marked with CD63, CD81, or CD9[17].
The function of EVs during physiological and pathological processes depends on the load of EVs and their ability to interact with receptor cells to deliver proteins, lipids, and RNAs. EVs bind to target cells specifically, and these bindings depend on the EV contents (including proteins, miRNAs, etc.) and the specific receptors of the target cells or tissues. This principle was established based on several findings. For example, EVs derived from bone marrow mesenchymal stem cells (BMSCS) carry microRNA-31a-5p (mir-31a-5p), which specifically binds to osteoblasts and osteoclasts, inhibiting the formation and promoting the resorption of bone[18].
-
The embryo develops from the zygote formed by the fusion of gametes. In mammals, the gametes are sperm or eggs derived from either spermatogenesis or oogenesis in male or female reproductive systems, respectively. Spermatogenesis is closely related to reproduction, and there are many factors related to reproductive ability, such as hormone levels, sexual behavior, etc. Any problems in these processes may affect the final outcome of reproduction. The molecular mechanisms of sperm maturation have not been elucidated clearly, so it is still an emerging topic of reproductive medicine. Recent studies have shown that EVs have essential regulatory effects in the maturation and capacitation of sperm, acrosome reaction, and fertilization[19]. EVs derived from different tissues may be able to improve the condition of the penis by producing factors that inhibit apoptosis or by carrying non-coding RNA (Figure 1).
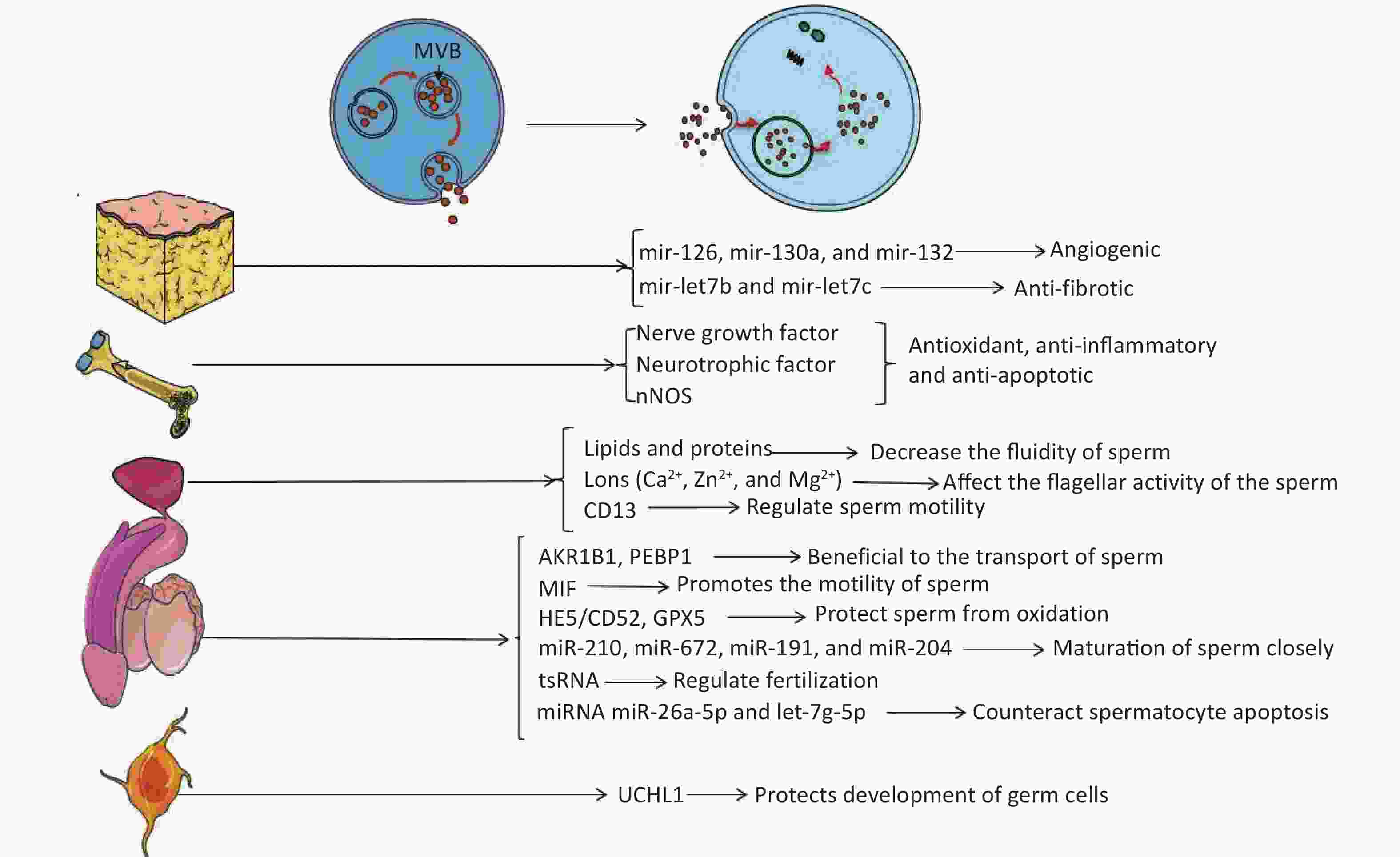
Figure 1. Extracellular vesicles affect the target through their contents. Endosomes form MVBs by inward budding. When MVBs fuse to the plasma membrane, exosomes are released into the extracellular environment. EVs carry active substances such as soluble proteins, lipids, metabolites, DNA, and RNAs (mRNA, miRNA, and other small regulatory RNA).
-
Studies have found that obesity can affect human reproductive activity, and EVs might provide a new mechanism contributing to this phenomenon. Recently, many groups have investigated therapies for ED through adipose tissue stem cells (ADSC)[20-26]. With the discovery of EVs in recent years, it was gradually recognized that stem cells might function through secretory pathways of EVs (mainly exosomes)[27]. ADSCs have been used to treat ED, and many studies have confirmed that ADSCs can improve the damage of cavernous nerves through paracrine pathways and restore penile erectile function[20-23]. Chen et al.[24] have successfully linked exosomes to ADSC-treated ED and demonstrated that ADSC-derived exosomes could improve type 2 diabetes-associated ED in rats via enhanced expression of CD31 and α-SMA and an increased ratio of smooth muscle to collagen in the corpus cavernosum of rats. Besides this, ADSC-derived exosomes exhibit proangiogenic properties in vitro, induce endothelial cell proliferation, restore erectile function, and reduce cavernous fibrosis; researchers suggest that exosomes derived from ADSCs may transport key functional miRNAs to target cells to improve functional recovery or to activate endogenous repair mechanisms, the major contributing components may be angiogenic miRNAs (mir-126, mir-130a, and mir-132) and anti-fibrotic miRNA families (mir-let7b and mir-let7c) contained in ADSC exosomes[25]. Li et al.[26] confirmed that ADSC exosome treatment could increase the mean intracavernous pressure/mean arterial blood pressure ratio significantly in rats with cavernous injury. In addition, melatonin can promote the secretion of exosomes by fat cells and increase the level of αKG in exosomes[28]. Melatonin is the most powerful antioxidant for the reduction of testicular ischemia/reperfusion injury by the metabolism of reactive oxygen species[29]; however, the functional role of adipose tissue-derived exosomes in this process requires further study (Figure 1).
-
Besides adipose tissue, one of the most abundant sources of stem cells in the human body is the bone marrow, which produces mesenchymal stem cells (MSCs). Experiments have shown that intravenous injection of bone marrow mesenchymal stem cells (BMSCs) can relieve ED caused by cavernous nerve injury in rats by increasing brain-derived nerve growth factor and glial cell-derived neurotrophic factor, or increasing nerve fiber nNOS[30]. Ouyang et al.[31] reported that MSC-derived exosomes can significantly enhance smooth muscle content and neuronal nitric oxide synthase in the corpus cavernosum. Using a rat model of cavernous nerve injury, they demonstrated that the ratio of smooth muscle to collagen in thevv corpus cavernosum was significantly improved with MSC-exosomes treatment. Additionally, cell viability of the culture corpus cavernosum smooth muscle cells was increased and caspase-3 expression was decreased in vivo and in vitro after treating with MSC-derived exosomes. Li et al.[26] demonstrated that in mice with bilateral cavernous nerve injury, treatment with exosomes derived from BMSCS can increase the mean intracavernous pressure/mean arterial blood pressure ratio and improve erectile function. BMSCS-derived exosomes might also prevent testicular ischemia-reperfusion injury because of their antioxidant, anti-inflammatory, and anti-apoptotic activities[32] (Figure 1).
-
The above-mentioned studies have shown that exosomes derived from BMSCs can increase brain-derived neurotrophic factor and glial cell-derived neurotrophic factor in penile tissue to improve erectile function. Cells in the nervous system also have secretory functions. For example, neurons can release soluble glial proteins into the medium. Studies have found that neurons and glia release UCHL1 into the extracellular matrix via the exosomes in mammals[33, 34]. Extracellular UCHL1 in exosomes is an important component of vesicles in fish and amphibians, and the vesicles separate these germ cells at different stages to ensure the simultaneous maturation of the sperm[35, 36]. It is postulated that extracellular UCHL1 can construct a barrier that protects the normal development of germ cells by reversing the pathway of protein ubiquitination[35] (Figure 1).
-
EVs from the prostate and epididymis are essential in the semen to maintain sperm motility, membrane integrity, protection from oxidization, and inhibition of premature spermatogenesis[37]. Such EVs from the prostate are called prostasomes[38]. The acinar epithelial cells of the prostate secrete a kind of microvesicle, so-called storage vesicle, which is equivalent to the MVBs of the late endosome origin[39]. Prostasomes are thought to play an important role in intercellular communication through direct interaction between fixed acinar cells and moving sperm. Experiments in pigs have confirmed that prostasomes mediate transfer of aminopeptidase to sperm cells. A significant increase of acrosome reaction extent in spermatozoa incubated with prostasome-like vesicles was observed. These findings indicate a prostasome-mediated transfer of molecules to the sperm membrane, inducing the acrosome reaction[40]. Carlini et al.[41] have shown that human sperm can be fused with prostasomes in an acidic environment and sperm rely on this phenomenon to transfer lipids and proteins to change the properties of sperm membranes. This results in a decrease in the fluidity of sperm membranes, benefiting the reception of signals of fertilization. Prostasomes can also resist sperm damage to increase sperm cell viability and help fertilization as a buffer in the acidic environment of the vagina[42, 43]. These mechanisms are contradictory, but a possible explanation is that the increase of sperm motility observed under different experimental conditions may be due to the combined effects of several factors such as proteins, ions, adenine, and/or other factors. As the role of EV contents during spermatogenesis has been studied since 1995, some prostasomes are rich in divalent cations such as Ca2+, Zn2+, and Mg2+ to regulate the concentration of divalent cations around the sperm and finally affect the flagellar activity of the sperm[44]; aminopeptidase N (CD13) can regulate sperm motility by affecting the level of enkephalin by transferring prostasomes into the sperm[45] (Figure 1).
-
In addition to the prostasomes in the semen, epididymosomes are a kind of exosomes produced by the apocrine secretion of epididymal epithelial cells[46-50].
The discovery of epididymosomes has enriched our knowledge of EVs in the semen. It is indicated that epididymosomes might contribute to the maturation of the sperm[51]. They are found to carry various substances related to the maturation of sperms, including AKR1B1, PEBP1, MIF, and HE5/CD52 GPX5. Epididymosomes also carry some proteins involved in the acquisition of the male gamete fertilizing ability[52-55]. Besides these, scientists have suggested that multiple miRNAs carried by epididymosomes are closely related to the maturation of sperm, including miR-210, miR-672, miR-191, and miR-204. These are secreted by EVs derived from the dendritic cell2 (DC2) epididymal principal cell line. These miRNAs are Dicer1-dependent factors and may have pronounced changes in epididymal functions, such as epithelial dedifferentiation, lipid homeostasis, and the profiles of epididymal gene expression in the downstream regions. Thus, these maybe the new molecular candidates that are important in the control of male fertility[56]. Epididymis-derived EVs in human and mouse semen load small non-coding RNAs [miRNA and tRNA-derived RNA fragments (tsRNA)], which have the potential to regulate functions that contribute to fertilization. tsRNA is rare in testicular sperm, but it is gained as sperm matures in the epididymis. Epididymosomes carry RNA payloads matching those of mature sperm and deliver RNA to immature sperm in vitro[57, 58]. Researchers have confirmed that miRNAs in seminal plasma exosomes have vital functions in male fertility. They have identified 16 miRNAs expressed differentially between the sperm of low testosterone pigs and control pigs, and these miRNAs are associated with the apoptotic pathway of P53, mitogen-activated protein kinase (MAPK) pathway, and mammalian target of rapamycin pathway. Researchers have also demonstrated that two of them — miR-26a-5p and let-7g-5p — are components of seminal plasma exosomes and have the ability to counteract spermatocyte apoptosis. Thus, testosterone deficiency may induce the sperm miRNA alterations by exosomes in seminal plasma, which are further responsible for the decline of sperm motility[59] (Figure 1).
-
In addition, other tissues or cells in the body can secrete EVs that affect reproductive processes. For example, the circulatory system also secretes EVs, and most of these originate from platelets, with a small amount from erythrocytes. There is a Met/miR-130b axis in EVs from peripheral blood, and the activation of c-Met can increase the level of miR-130b, which binds to the 3'UTR of the androgen receptor to inhibit its expression[60]. In addition, exosomes secreted by human adrenocortical cells carry CYP17A1, a member of the cytochrome P450 enzyme family, which is an important enzyme for de novo synthesis of androgens by acting on steroid hydroxylation and carbon-carbon bond cleavage reactions[61, 62]. The genital tracts may be related to the production and release of EVs. Defects of these EVs might affect the body fluids and/or mucous membranes of the genitourinary organs and may be associated with infertility[63]. In summary, EVs from various tissues or organs might be used to treat ED, and they can improve erectile function in rats by protecting the nerves, improving cavernous endothelial function, and reducing cavernous fibrosis and apoptosis[64]. Detailed mechanisms remain elusive and require further investigation before they are applied to clinical therapy.
EVs Derived from Adipose Tissue
EVs Derived from Bone Marrow
EVs Derived from the Nervous System
EVs Derived from the Prostate
EVs Derived from the Epididymis
Effects of Other Sources of EVs on Male Reproduction
-
After the summary of EVs in the male reproductive system, their significance in female genitalia also deserves to be mentioned. As described previously, the reproductive process that occurs in females is not limited to oogenesis but also includes transport and capacitation of sperm, fertilization, cleavage, embryonic development, and childbirth. In addition to these processes, many other factors are related to female reproduction, such as hormone levels (for example, estrogen and progestin), sexual behaviors, and libido. Any defects of these processes may influence the outcome of pregnancy. There are many studies regarding the role of EVs in female reproductive processes, and most focus on the influence of embryo-derived EVs on the mother and/or placenta. The relationship between reproduction and EVs from different maternal sources needs to be elucidated.
-
The main function of the ovary as the female gonad is to produce ova and secrete sex hormones, which promote and maintain the development of female sexual characteristics. The microenvironment of the ovary is maintained by follicular fluid, and miRNAs carried by EVs in the follicular fluid are associated with follicular growth and oocyte maturation, proliferation of granulosa cells, cumulus expansion, meiosis and mitosis of the early embryo[65-67]. Female patients who received intracytoplasmic sperm injection demonstrated increased CD63 and CD81-positive exosomes in their follicular fluid and upregulated miRNA such as miR-29a, miR-99a, miR-100, miR-132, miR-212, miR-214, miR-218, miR-508-3p, and miR-654-3p. Most of these upregulated exosomal miRNAs participate in WNT, MAPK, ERBB, and TGF-β signaling pathways, and it is speculated that these miRNAs play an important role in growth, maturation, and meiosis of oocytes and may be related to the development of fertilized eggs[68]. It is notable that the characteristics of miRNAs carried by EVs in the follicular fluid vary with the age of women, suggesting that miRNAs carried by EVs can be used as biomarkers of age-related decline of oocyte quality[69]. Subsequently, da Silveira et al.[67] found that EVs in the ovaries are associated with age-related fertility decline in horses. They found that the presence of mir-23a carried by exosomes in the ovaries is involved in apoptosis of granulosa cells. The mir-23a can cause changes to TGF-β signaling in granulosa cells, which in turn affects the development of the follicle and ovulation[70]. In addition, exosomes derived from ovaries/oocytes express Uroplakin (UP) protein, which can affect fertilization[71].
-
The fallopian tube connects the ovary and the uterus. Al-Dossary et al.[72] identified EVs derived from the fallopian tube in 2013 and named these oviductal bodies. CD9+ exosomes were also detected in the fluid of the oviduct, and plasma membrane Ca2+-ATPase 4a (PMCA4a) was identified in it. PMCA4a plays central role in the Ca2+ efflux pump in the sperm of mice, and this is essential for capacitation[73]. In addition, experiments in turtles suggest that EVs secreted by fallopian tube epithelial cells and glands can help the fusion of sperm with the egg[74]. Although there are only a few known results of mammalian experiments, the roles of EVs derived from mammalian fallopian tubes are mainly clear. It has been reported that EVs secreted by the oviduct can prolong the survival time of embryos and improve embryo quality[75]. Studies have proven that the EVs of the fallopian tube fluid can significantly increase the birth rate by inhibiting apoptosis and promoting differentiation, which may be considerably beneficial to the development of assisted reproductive technology[76]. By analyzing the contents of EVs secreted by the oviduct, it has been identified that miRNA miR-34c and miR-449a are associated with defects of cilia in fallopian tubes and infertility. Proteins contained in fallopian tube-derived EVs are related to maternal estrus. In addition, some RNAs in these EVs are associated with the expression of cilia, development of the embryo, and transcripts encoding ribosomal proteins[77].
-
There are EVs in the microenvironment of the uterus. Researchers have discovered EVs with strongly positive CD9 and CD63 signals from endometrial epithelial cell cultures (ECC-1). Specific miRNAs carried by these include hsa-miR-200c, hsa-miR-17, and hsa-miR-106a, which are closely related to the implantation of the embryo. Proteins or miRNAs transported by EVs may be very valuable biomarkers for endometrial diseases in humans[78]. EVs secreted by uterine epithelial cells into the uterine cavity are important for embryonic development, as confirmed by removal of uterine glands resulting in embryos often failing to develop or aborting[79]. Research from the group of Javier showed that EVs released from human endometrial MSCs can partially recover the youthful abilities of aged oocytes and improve the total blastomere count[80].
-
Besides the reproductive system, many studies have isolated EVs directly from the circulatory system of the mother. EVs secreted from leukocytes, erythrocytes, and platelets can affect the physiological status of women during pregnancy through different mechanisms. Pregnant women with eclampsia have more EVs in their peripheral blood. Platelet-derived EVs may cause eclampsia, and they might be associated with blood coagulation. The beneficial effects of aspirin on preeclampsia-related risk factors may be at least partially explained by the influence of aspirin on the activity of platelets. Other EVs secreted by endothelial cells are also increased in patients with eclampsia. In addition, EVs secreted by maternal intestinal flora may cause the rupture of embryolemma, thus leading to premature labor and other consequences (Table 1).
Sources of EVs Carrier/correlation factor of EVs Functions Literatures Leukocytes Inflammatory cytokines (IL-1, IL-8)
and nuclear factor (NF-Kβ) and tissue factorIn women with preeclampsia, the concentration of EVs derived from leukocyte is elevated, which can help remove and regulate syncytiotrophoblast-derived vesicles and placental fragments released into the maternal circulation, and initiate inflammatory coagulation through its cargo. [81-84] Platelets P selectin, tissue factor, etc. Platelet-derived EVs in women with preeclampsia may be involved in increased thrombin generation and fibrin clot formation [85,86] Erythrocytes Increased concentration of EVs from erythrocytes in preeclampsia may be due to erythrocytes rupture and hemolysis, which may be related to extensive thrombosis. [85] Endothelial cells Soluble fms-like tyrosine kinase 1 (sFlt1), soluble endoglin (sEnd) and placental growth factor (PlGF),
certain procoagulant molecules
(e.g. PAI-1)In patients with preeclampsia, circulating EVs from endothelium are increased, and sFlt1 competitively binds to PlGF and vascular endothelial growth factor, inhibiting endothelial protection. sEnd is an anti-angiogenic protein that prevents capillary formation and increases vascular permeability. PAI-1 in EVs helps elucidate the link between endothelial dysfunction and extensive coagulation [87,88] Plasma The signal to reduce prostaglandins Reducing the prostaglandin 2α (pgf2α) in the uterus, which is associated with uterine infection. [89] Plasma The miR-133, miR-30, miR-99,
miR-23, and so onIn diabetic pregnancy, these exosomes can be engulfed by embryonic cells through the placental barrier, affecting heart development, possibly via exosomal miRNAs. [90] Intestinal flora The EVs secreted by the intestinal flora of pregnant women are associated with premature rupture of membranes and neonatal infection. [91] Table 1. Effects of EVs from pregnant mothers
-
After the fusion of sperm and egg, the embryonic development process occurs in the uterus. This process includes the development of the fertilized egg to a blastocyst, trophoblast cells coming in contact with the endometrium, and blastocyst implantation in the endometrium. Then, the inner cell mass (ICM) proliferates and differentiates into the ectoderm and endoderm, forming the amniotic cavity and yolk sac. Meanwhile, the blastoderm appears and differentiates into inner, outer, and middle germ layers gradually, finally forming the embryo. During these processes, the embryo also produces some substances such as protein, tissue factors and immunosuppressive factors to affect development. The placenta amnion can secrete EVs with abundant content to regulate embryonic development. The placental trophoblast can secrete many EVs as well, thus influencing the reconstruction of intrauterine spiral arteries and invasion of cytotrophoblast cells. Additionally, these EVs may be associated with some diseases in pregnant women, such as eclampsia. Moreover, EVs derived from trophoblasts also affect the immune system, preventing fetal rejection, and protecting the placenta from viral infections (Table 2).
Sources Of EVs Carrier material Functions Literatures Amnion mir-21 EVs loading mir-21 are helpful for the growth of the embryo. [92] Amnion Molecules involved in blood coagulation EVs express phosphatidylserine and tissue factor, they also can significantly shorten the plasma coagulation time and increase the production of factor Xa and thrombin. [93,94] Amnion Histone (H) 3, heat shock protein (HSP) 70, activated form of pro-senescence
P-p38, MAPKProtein involved in stress response. The activated form of pro-senescence and term parturition associated marker p38 mitogen activated protein kinase (MAPK) (P-p38 MAPK) co-localized with exosome, so these exosomes are related with the outcome of pregnancy. [95] Trophoblast Synctin-1 Induce peripheral blood mononuclear cell (PBMC) activation by the production of cytokines and chemokines, so these EVs modulate immune cell activation and the responses of immune cells to subsequent lipopolysaccharide stimulation. [96] Trophoblast mir-141 and C19MC Promote invasion and proliferation of extravillous trophoblast cells (EVTs). Trophoblasts secrete EVs containing mir-141 and C19MC, which are associated with invasion. [97-99] Trophoblast EVs released from syncytiotrophoblast stimulated the production of inflammatory cytokines TNF-alpha, IL12p70, and IL-18, these EVs are potential contributors to altered systemic inflammatory responsiveness in pregnancy. [100] Trophoblast Tissue factor and soluble vascular endothelial growth factor receptor
1 (sFlt-1)Tissue factors activate the coagulation system; and sflt-1 have anti-angiogenic effect. [101] Trophoblast Immunosuppressive factors Prevent fetal rejection, inhibit maternal immune reactions, and activate T lymphocyte and natural killer cells to protect the fetus. [102] Trophoblast miRNA - C19MC Protect the placenta from viral infections and transfer antiviral infections to non-placental cells. [13,103,104] Embryo PIBF Inducing the increase of IL-10, and IL-10 contributes to the Th2 dominant immune responses during pregnancy. [105] Table 2. Effect of embryo-derived EVs on reproduction
EVs Derived from the Ovaries
EVs Derived from the Oviduct
EVs Derived from the Endometrium
Other Sources of Maternal EVs
EVs Derived from the Embryo
-
EVs contain abundant biological macromolecules including proteins, lipids, DNA, and RNA, which mediate cellular communication and participate in various physiological and pathological processes. Recent studies have revealed the important role of EVs in reproduction and have expanded our knowledge regarding reproductive physiology and pathology. However, many specific functions and mechanisms of EVs are still unclear, and further research is urgently needed. For men, EVs in the semen have been shown to be vital in reproduction. Other tissue-derived EVs may also participate in the process of spermatogenesis and the physiological and pathological processes of the penis and testes in a paracrine manner. During pregnancy, EVs are derived from various cells and tissues, including placental trophoblasts, embryos, endothelial cells, immune cells, and platelets, which mediate the necessary communication between maternal and fetal circulation during the pregnancy. Alterations in the EV content and functions might be used for diagnostic purposes in female fertility studies[106]. A few studies have paid attention to the EVs from other tissues or cells in the male reproductive system, and their effects on hormone production, sexual behavior, and gametogenesis in the female remain to be elucidated. Therefore, comprehensive research on the functional roles of EVs in both male and female reproductive systems are definitely needed to decipher the relationship between EVs from various tissues and the entire reproductive process. These studies will further widen our knowledge regarding the reproductive mechanisms, and contribute to the development of new strategies to treat various reproduction-related diseases.
-
All authors report no conflicts of interest.







 Quick Links
Quick Links
 DownLoad:
DownLoad: