-
Placenta accreta spectrum (PAS) is the general term used for abnormal adherence of the placental trophoblast to the uterine myometrium and is also referred to as morbidly adherent placenta[1]. Pathologists have classified three subtypes depending on the depth of trophoblast invasion into the myometrium: placenta accreta, placenta increta, and placenta percreta. Maternal morbidity and mortality can occur due to severe and life-threatening hemorrhage. As the number of cesarean sections of pregnant women has increased in recent years, the incidence of placenta previa and PAS disorders has also increased. In 2005, the incidence of PAS disorders rose to 1 in 533 births in the United States[2]. Similarly, the prevalence of PAS was 0.22% in a heterogeneous set of studies in China based on a meta-analysis[3]. Reported rates in the overall prevalence of placenta accreta range between 0.01% and 1% of livebirth[4,5]. Accurate prenatal prediction of PAS disorders severity and intraoperative blood loss (IBL) is important for preoperative preparation.
Ultrasonography is the most effective and primary screening tool with high accuracy for women suspected of PAS and facilitated differentiation between the three types of PAS[6]. In 2015, Peking University Third Hospital (PUTH) proposed an ultrasonic scoring system for PAS[7,8]. In suspected PAS patients, Ultrasonic Scoring System (USS) was performed to assess the severity of placental invasion based on objective ultrasonic features and evaluation of medical history. In the previous study, the sensitivity and specificity of USS were 81.5% and 95.7% for predicting placenta increta and percreta. Although USS can assess the types of PAS disorders, no definitive data can explain the efficacy of USS in predicting blood loss.
In 2017, the postpartum hemorrhage guideline from the American College of Obstetrics and Gynecology (ACOG)[9] stated that immediate preparation for transfusion should be performed in women with ongoing blood equivalent to a blood loss of 1,500 mL or more. Therefore, predicting a blood loss of more than 1,500 mL may provide valuable information for the preparation of blood product. In this study, we aimed to investigate the association between an ultrasonic scoring system with IBL in PAS disorders from a retrospective cohort study with 332 cases and provided evidence of a different amount of blood product preparation with different ultrasonic scores.
-
The present study was approved by the Medical Ethics Committee of PUTH, Beijing, China (IRB00006761-2016145). Informed consent was obtained from each participant for their clinical records.
-
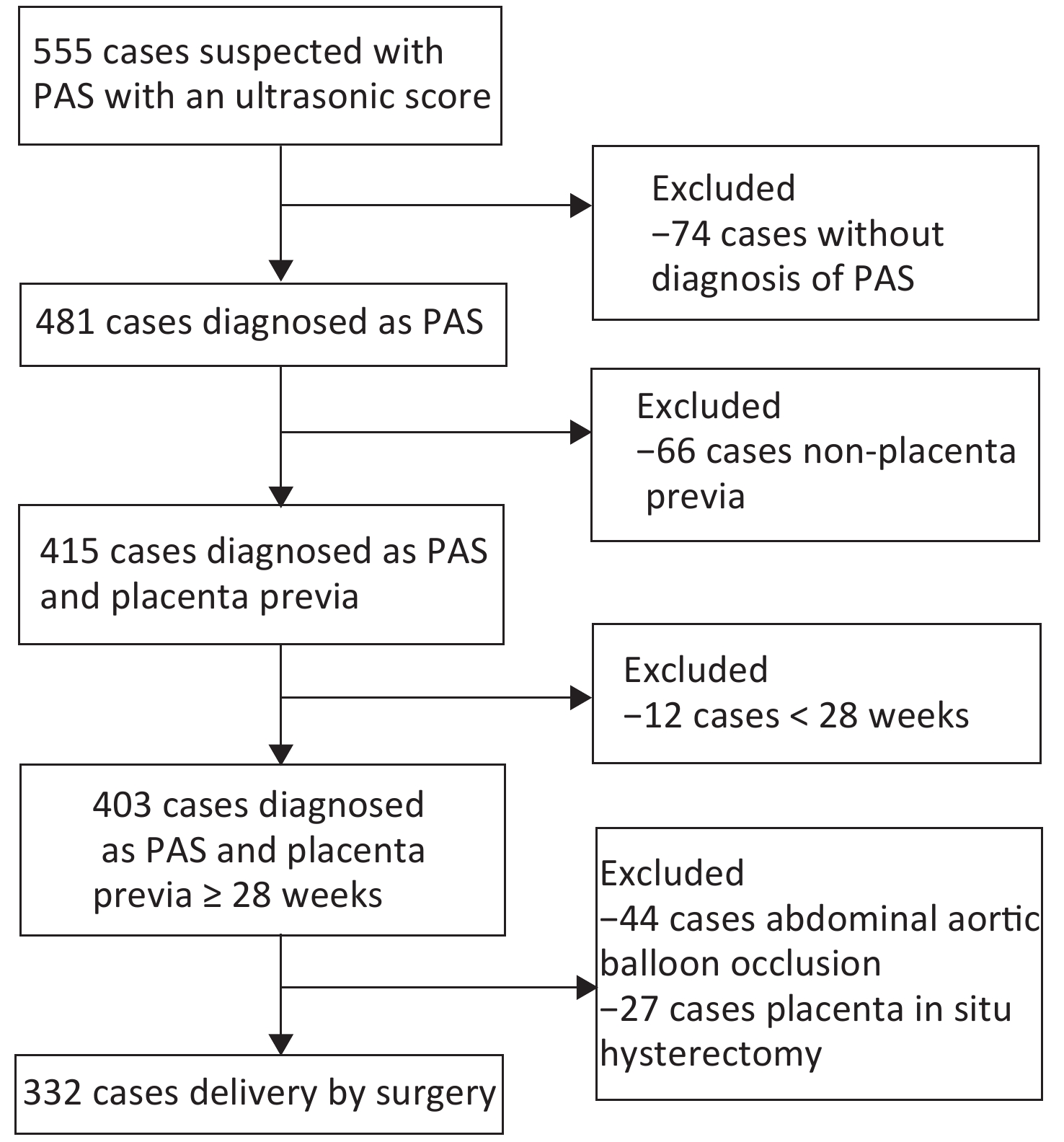
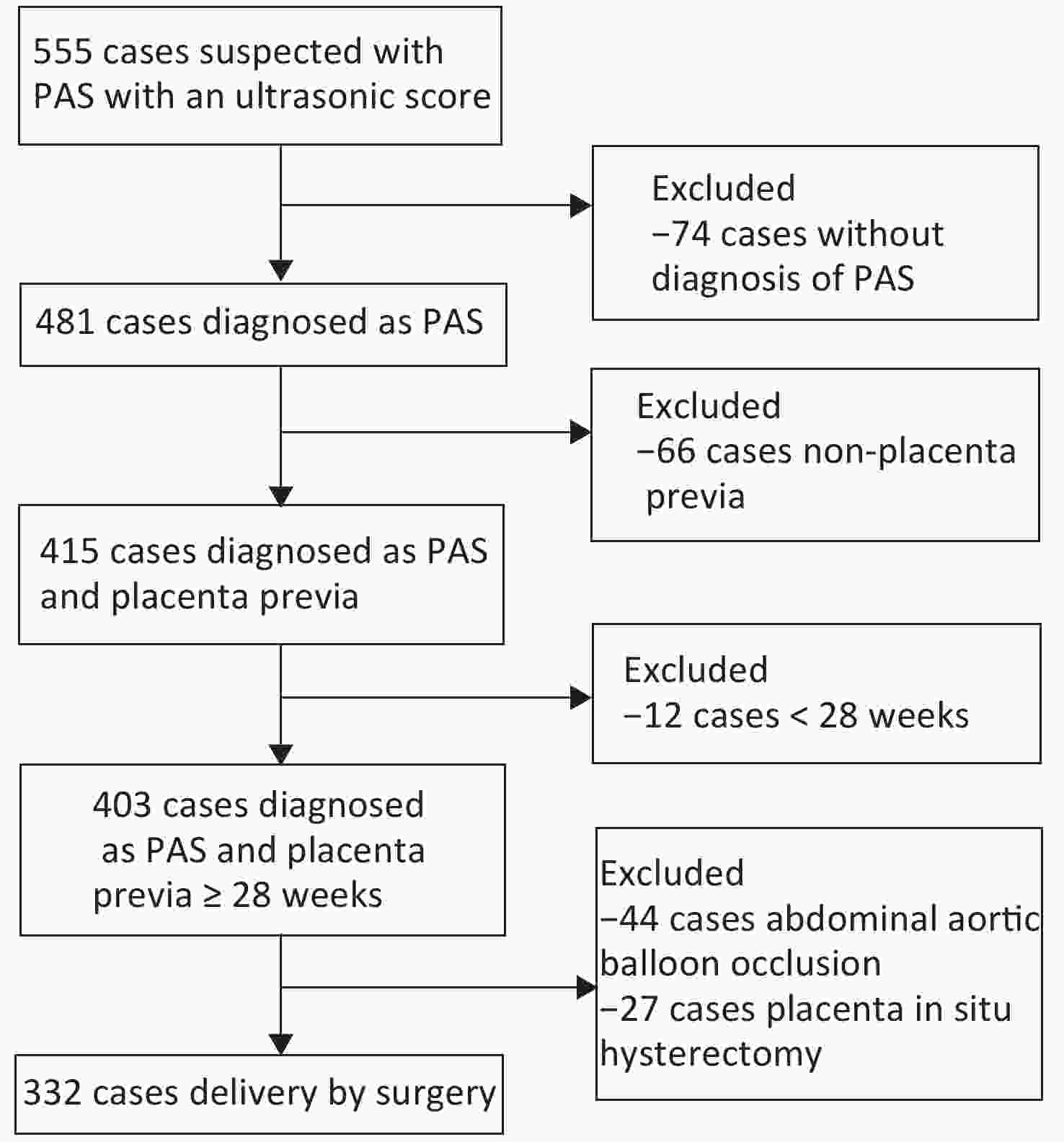
A retrospective cohort was established between January 2015 and November 2019. Patients with suspected PAS underwent ultrasonography and received an ultrasonic score through USS. The following exclusion criteria were applied: no diagnosis of PAS disorders through clinical observation or pathological examination, natural delivery, postpartum placenta accreta, gestational age less than 28 weeks, and non-placenta previa. Application of abdominal aortic balloon occlusion and placenta in situ hysterectomy may significantly affect the amount of blood loss, and these cases have been excluded. Cases with other hemorrhagic factors, including refractory contractions and coagulation disorders, have not been included in this study. A total of 332 cases were enrolled for the present analysis. The case inclusion process is shown in the flowchart in Figure 1. Clinical data (i.e., maternal medical history, gravity, parity, surgical approaches, and surgical teams) have been collected from medical records.
-
PAS disorders have been identified based on the clinical and/or histopathological diagnoses of the patients. From the perspective of clinical examination, PAS was defined as a failed attempt to remove the placenta during the cesarean section. On histological examination (either via curettement or the removed uterus, if applicable), PAS was characterized as the presence of placental villi, which were partially incorporated into the decidua basalis. Typical surgical approaches for PAS included bilateral uterine artery ligation, B-lynch sutures, focal sutures, and cervical pull sutures.
An ultrasonic scoring system is an effective diagnostic tool to inspect noninvasively. The color Doppler ultrasound—Philips iU22 and GE Voluson E8—were used for these studies in which the probe frequency used is 3.5 Hz. As a routine position, patients were initially placed in the supine position and lateral position whenever required. The scoring system included the following steps: Review signs including the location of the placenta, placental thickness, loss of clear zone, bladder line, placental lacunae, condition of subplacental vascularity, and cervical morphology in the cervical sinus, in addition to number of previous cesarean deliveries with the value of 0, 1, and 2 based on predefined subscale. After that, for each case, the values were added together to obtain the final score and classify the case according to the score. Blood loss was assessed objectively by counting the sponges and suction output. IBL that is equal to or greater than 1,500 mL was defined as an outcome indicator.
-
Means and standard deviations were calculated for maternal age and gestational age at birth. Counts and proportions were used to describe the year of birth, gravidity, parity, surgical approaches taken during delivery, and surgical teams.
Generalized additive models were used to explore the nonlinear relationships between ultrasonic score and blood loss. Several sensitivity analyses were conducted to assess the robustness of the findings by adjusting for different covariates in the models. In Model A, we adjusted for no covariate. In Model B, we adjusted for year of giving birth, maternal age, gravidity, parity, and gestational age at birth. In Model C, surgical approaches and surgical teams were adjusted for in addition to the covariates in Model B. Predicted blood loss had been estimated from the generalized additive models, and those from Models B and C were estimated with year set to 2019, maternal age and gestational age at birth set to means, gravidity set to 3 (the median), and other covariates set to the lowest values.
The medians and interquartile ranges for blood loss and proportions for IBL ≥ 1,500 mL were calculated by each ultrasonic score, according to which participants were divided into three groups of women with 1−6, 7−9, and 10−15 ultrasonic scores.
Finally, logistic regressions were used to determine the differences in the risk of IBL ≥ 1,500 mL between the three groups with different ultrasonic scores. Similarly, sensitivity analyses were also conducted in the generalized additive models, such as different covariates, which were included in Models A, B, and C were adapted for Models 1, 2, and 3, respectively. Adjusted odds ratios and 95% confidence intervals (CIs) of IBL ≥ 1,500 mL for women with 7−9 and 10−15 ultrasonic scores were calculated with 1−6 ultrasonic scores considering as the reference.
We did the posthoc power analysis in our study[10]. For detecting the difference in risk of blood loss ≥ 1,500 mL, the statistical power is more than 0.9 when both comparing the groups scored 1−6 and 7−9 and the groups scored 1−6 and 10−15, as Type I error rate of 0.05.
R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria) and package ggplot2 and mgcv were used for statistical analyses. A two-tailed P value of < 0.05 was used to reflect statistical significance.
-
A total of 332 pregnant women with PAS were involved in this study. The average age was 33.4 years old, and the average gestational age at delivery was 36.0 weeks. Among them, the proportions of placenta acrreta, increta, and percreta were 44.0% (146/332), 39.8% (132/332), and 16.3% (54/332), respectively. Table 1 depicts the proportions of different surgical approaches. The median value of blood loss was 1,000 (500, 2,000), whereas the average ultrasonic score was 7 (5, 9) points.
Characteristics Value Maternal age, mean (s) 33.4 (4.4) Gestational age, mean (s) 36.0 (1.7) Year, n (%) 2015 39 (11.7) 2016 56 (16.9) 2017 94 (28.3) 2018 67 (20.2) 2019 76 (22.9) Gravidity (not include this pregnancy), n (%) 0−1 47 (14.1) 2−3 159 (47.9) 4 126 (37.9) Para, n (%) 0 78 (23.5) 1 209 (63.0) 2 43 (13.0) 3 2 (0.6) Placenta accreta spectrum, n (%) Placenta accreta 146 (44.0) Placenta increta 132 (39.8) Placenta precreta 54 (16.3) Surgical approaches, n (%) Cervical pull sutures 119 (35.8) Bilateral uterine artery ligation 246 (74.1) Focal sutures 201 (60.5) B-lynch sutures 182 (54.8) Hysterectomy 12 (3.6) Surgical teams, n (%)* 1 227 (68.4) 2 41 (12.3) 3 35 (10.5) 4 29 (8.7) Note: *Divided into four surgical teams according to the experience of surgical participants. Table 1. Baseline characteristics of cases with PAS
-
The median value of blood loss was 1,000 (500, 2,000) mL, whereas the median value of ultrasonic score was 7 (5, 9) points. Table 2 presents the correlation between every single score value and blood loss. The median blood loss increased significantly as the score value increased. Similarly, the proportion of blood loss equal to or greater than 1,500 mL shows an increasing trend as the score increases. When the ultrasonic score was no more than 6 points, the median blood loss was no more than 1,000 mL, and the proportion of IBL ≥ 1,500 mL was below 30%. When the ultrasonic score was no more than 10 points, the proportion of IBL ≥ 1,500 mL was less than 50%.
Score Median (IQRs) of
blood loss (mL)Blood loss ≥ 1,500 mL,
n (%)1 700 (400, 1,000) 0 (0.0) 2 585 (400, 1,000) 2 (20.0) 3 500 (400, 600) 0 (0.0) 4 500 (400, 700) 4 (12.9) 5 500 (400, 1,000) 7 (14.6) 6 800 (500, 1,000) 4 (11.4) 7 1,000 (800, 1,500) 16 (35.6) 8 1,200 (800, 2,300) 19 (40.4) 9 1,350 (800, 2,400) 16 (47.1) 10 2,150 (1,350, 3,400) 18 (75.0) 11 2,550 (2,000, 3,950) 14 (87.5) 12 2,475 (1,500, 3,100) 10 (83.3) 13 2,650 (1,750, 4,000) 3 (75.0) 14 2,600 (1,200, 4,000) 1 (50.0) 15 4,000 (4,000, 4,000) 1 (100.0) Note. IQRs, interquartile ranges. Table 2. Status of intraoperative blood loss of every single ultrasonic score
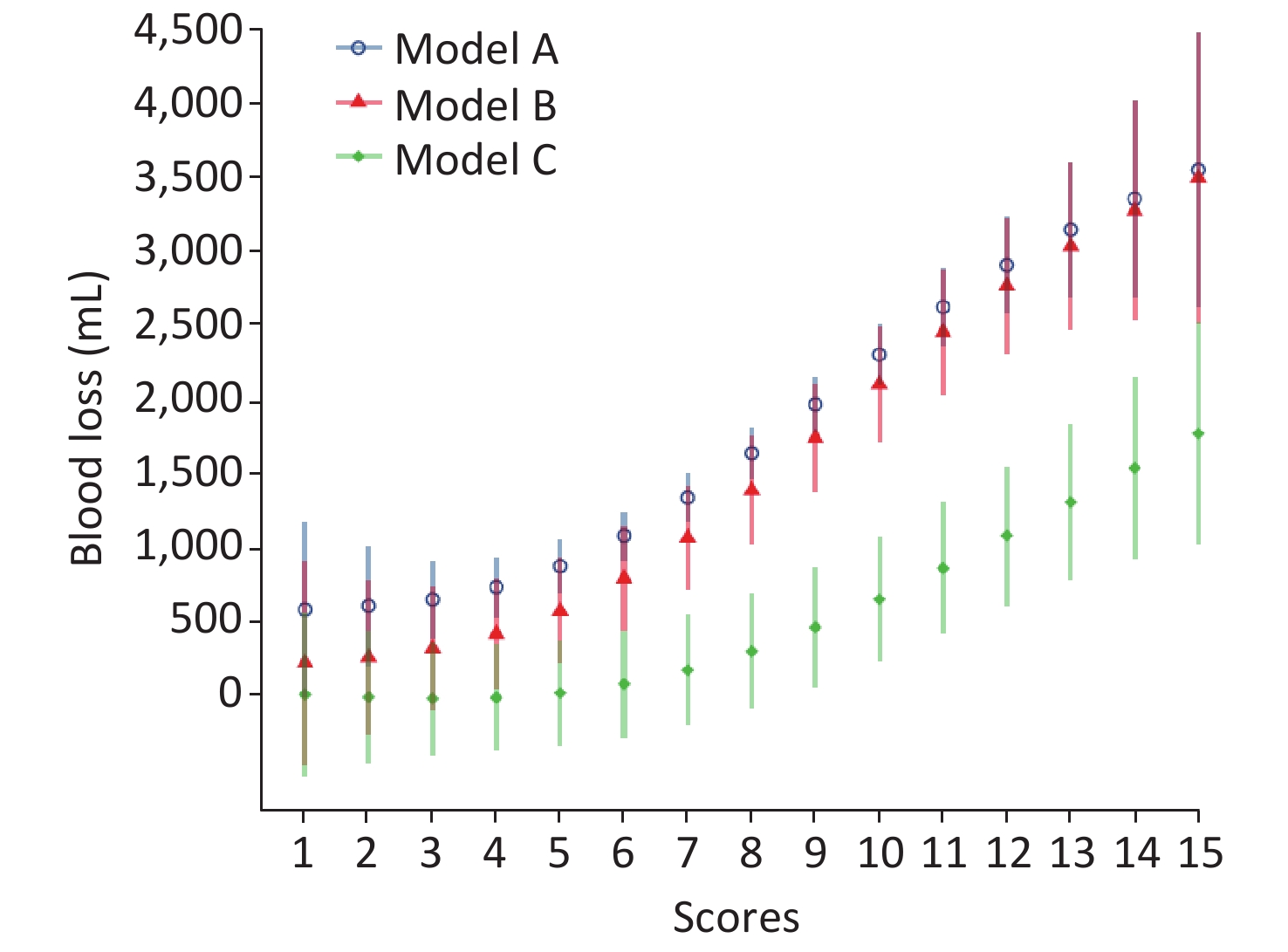
Generalized additive models have been drawn to explore the correlation of score and blood loss after adjustment of multiple covariates mentioned before, as shown in Figure 2. All models show a significant positive correlation between score and blood loss (P < 0.05).
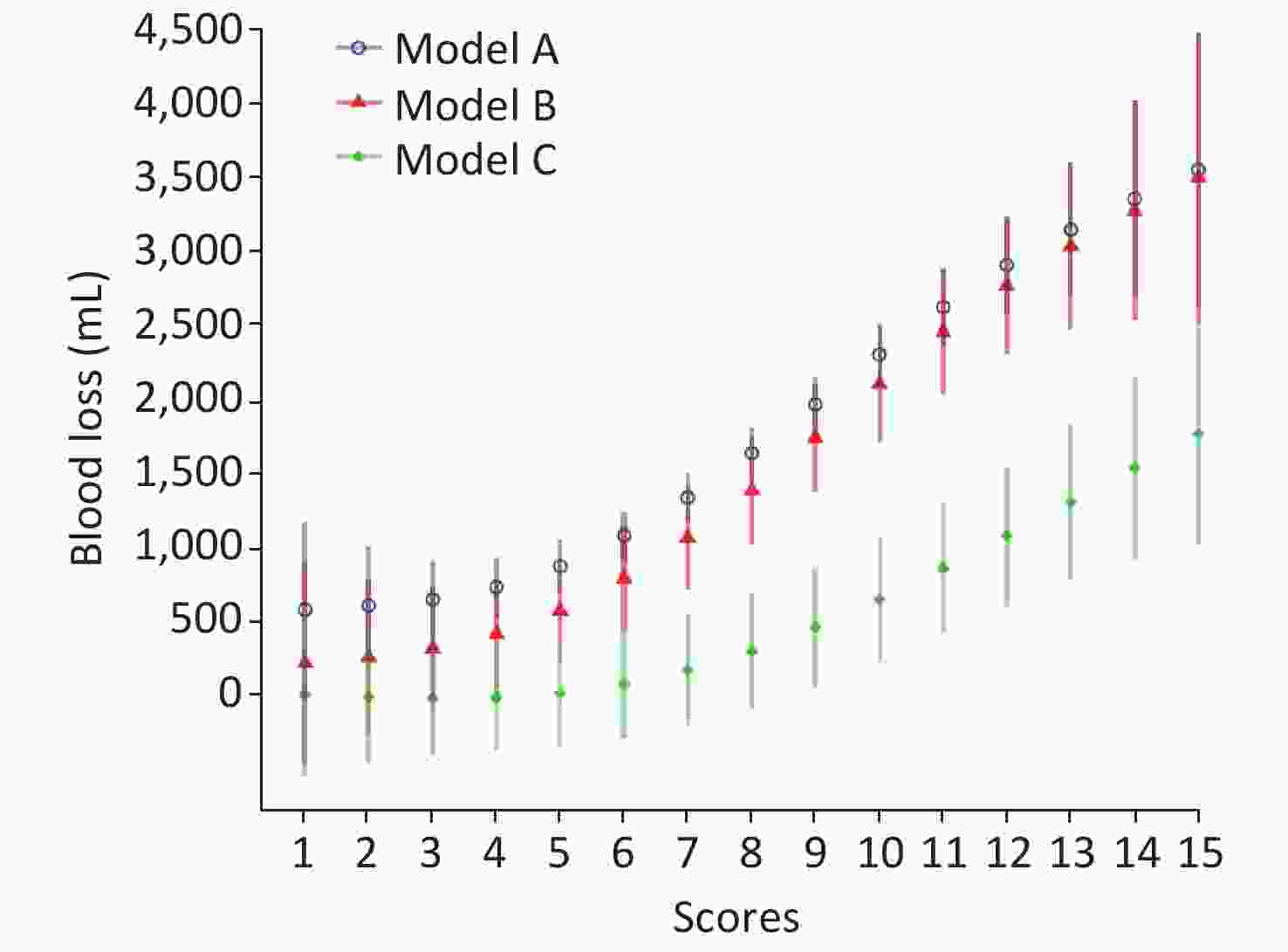
Figure 2. The correlation of ultrasonic score and intraoperative blood loss. Model A: adjusted for no covariate; Model B: adjusted for year of giving birth, maternal age, gravidity, parity, and gestational age at birth. Model C: surgical approaches and surgical teams were adjusted for in addition to the covariates in Model B.
-
According to the ultrasonic score value, all cases were divided into three score groups: low, median, and high. Low score group refers to less than 6 points, whereas scores between 7 and 9 points refer to the median score group, and 10 points or more are considered as high score group. Table 3 shows the comparison of the results obtained from maternal age, gestational age, IBL, transfusion blood product, and hysterectomy rate in the three groups. It was found that there were significant differences in gestational age, IBL, blood transfusions, and hysterectomy rate except for maternal age. The higher score signifies earlier termination of gestational age. It was likely that delivery time would be 34 weeks of gestation in advance in a high score group, whereas cases in low score group often terminated the pregnancy at nearly 37 weeks. Similarly, IBL, transfusion proportion, packed red cell transfused, and hysterectomy rate increased as the ultrasonic score increased. The median of IBL was 600, 1,200, and 2,500 mL in three groups, respectively. The proportion of IBL ≥ 1,500 mL in three groups was 11.6%, 40.5%, 79.7%, respectively. Table 4 presents the lists of the risk of IBL ≥ 1,500 mL in three groups. The risk of median and high score groups was 5.20 and 29.95 times compared with the low score group prior to covariate adjustments. After the adjustment of all covariates, the high score group remained at a high risk, which was 15.09 times than the low score group.
Group Low score Median score High score P value* n 147 126 59 Age (years), mean (SD) 34.0 (4.0) 33.0 (5.0) 34.0 (5.0) 0.376 Gestational age (weeks), mean (SD) 36.7 (1.5) 35.9 (1.6) 34.5 (1.4) 0.000 IBL (mL), median (IQR) 600 (400, 1,000) 1,200 (800, 2,000) 2,500 (1,500, 3,800) 0.000 Transfused, n (%) 37 (25.2%) 82 (65.1%) 57 (96.6%) 0.000 Packed red cell (mL), median (IQR) 0 (0, 400) 400 (0, 1,200) 1,200 (800, 2,400) 0.000 Hysterectomy, n (%) 1 (0.7) 3 (2.4) 8 (13.6) 0.000 Note. *Data were analyzed by chi-square test. IBL, intraoperative blood loss. Table 3. Comparison of different groups based on the ultrasonic score
Score Model A Model B Model C OR (95% CI) P OR (95% CI) P OR (95% CI) P 1−6 1.00 1.00 1.00 7−9 5.20 (2.80, 9.65) 0.000 6.94 (3.46, 13.94) 0.000 2.03 (0.82, 5.02) 0.126 10−15 29.95 (13.31, 67.38) 0.000 69.49 (22.84, 211.38) 0.000 15.09 (3.85, 59.19) < 0.001 Note. OR, odds ratio; CI; confidence interval; IBL, intraoperative blood loss. Table 4. Risk of IBL ≥ 1,500 mL in three groups
-
The findings of this study indicate that the ultrasonic scoring system correlated with hemorrhagic morbidity defined by blood loss during the operation equal to or greater than 1,500 mL and the requirements of blood transfusions. Stratification of pregnant women with PAS at risk of hemorrhage could potentially optimize the allocation of blood product resources and antenatal preparation. Our findings suggested that the amount of blood loss during operation increased with a higher ultrasonic score. The result provided some evidences for hemorrhage preparedness in the case of suspected PAS, irrespective of the extent of invasion.
As we all know, the prenatal diagnosis of PAS has mainly based on ultrasound. Pagani et al.[11] reported overall good diagnostic accuracy of ultrasound while determining the depth of placental invasion with a sensitivity of 90.6%, 93.0%, 89.5%, and 81.2% for placenta accreta, increta, accreta/increta, and percreta, respectively, in a systemic review. A number of studies have investigated the diagnostic accuracy of various ultrasonic signs. However, only a few studies have tried to explore the diagnostic performance of an ultrasound-based scoring system[12-15]. In this context, Rac et al.[12] made a combination of five ultrasonic imaging indicators and generated a predictive equation, known as the ‘Placenta Accreta Index,’ to explore the predictive value of the PAS scoring system. Tovbin et al.[13] reported that a scoring system, including the number of placental lacunae and the presence of bladder wall interruption, had a high diagnostic performance for PAS, with an area under the receiver operating characteristic (ROC) curve of 0.94 (95% CI, 0.86–1.0). Marsoosi et al.[14] developed a scoring system on five indicators with an area under the ROC curve of 0.98. However, none of the above studies investigated the performance of the scoring system in predicting clinical outcomes. Only two studies by Gali[16] and Dall’Asta[17] have shown increased blood loss and surgical complications in women with a higher PAS category based on a new ultrasonic staging system. Chong of PUTH set up the ‘placenta accreta scoring system,’ which has been shown to be relatively accurate in predicting pathological PAS types from 83.9% to 92%. To our knowledge, this was the first ultrasonic scoring system for PAS proposed in China and has now been used in several hospitals of different regions.
In the case of PAS disorders, hemorrhage occurs on a spectrum, and both over-preparation and under-preparation for transfusion carry risks and benefits. Risk stratification of women affected by a PAS is, of course, challenging. Previous studies had focused on classifying PAS disorders based on prenatal ultrasonic findings and have been shown to be effective in predicting the severity of PAS when associated with intraoperative or pathology findings[18]. Nevertheless, there is still a gap between prenatal ultrasound imaging and the risk of severe surgical morbidity, such as massive hemorrhage and the need for transfusion. The depth of the placental invasion is not the only determinant of the perioperative outcome of women at risk of PAS. Therefore, we have attempted to explore the correlation between USS and IBL as the application maturity of USS has been achieved.
In the present study, when we encounter a case with suspected PAS disorders, if the score is equal to or below 6 points, transfusion therapy is less likely to be needed. If the score is between 7 and 9 points, the risk of blood loss over 1,500 mL does not show the statistical difference after adjusting the other variables, such as surgical approaches. But the proportion of transfusions occurs in more than half of all cases. This suggests that cases with median scores should be well prepared, including optimal surgical approaches, experienced surgeons, estimated blood loss, and blood product preparation. And if the score is equal to or greater than 10 points, the risk of blood loss over 1,500 mL increased significantly irrespective of all other variables. Almost all the cases required transfusions. If the medical institutions do not have adequate blood product resources, it is always better to transfer these cases in advance to a referral center or a competent institution. We confirm that the PAS scoring system performs well when applied to risk stratification management for PAS, in particular, at the referral center, where the application can be extended.
As far as delivery time is concerned, ACOG recommended that a window of 34 0/7−35 6/7 weeks of gestation be suggested as the preferred gestational age for scheduled cesarean delivery or hysterectomy in a stable patient[19]. Similarly, the guidelines of the Society of Obstetricians and Gynecologists of Canada suggest that elective surgery is optimal for approximately 34−36 weeks. In our study, the median gestational age was 36 weeks, as stated in the two guidelines. There was a statistically significant difference in gestational age between the three groups. High scores cases were more likely to have a cesarean delivery in advanced gestational age. It appears that the severity of PAS disorders will affect the timing of delivery, but the optimal timing remains unclear.
The limitations of the study relate to a single-center retrospective analysis, which inevitably leads to bias. The cases included in the analysis were strictly limited to certain conditions: Over 28 weeks, placenta previa, preforming cesarean section, and clinically and pathologically proved to be PAS disorders to avoid the confounding factors. The predictive value of the ultrasonic scoring system for IBL can, therefore, be applied restrictively in these cases. Some unpredictable factors affecting blood loss, such as refractory contractions, have been excluded from this study. Further study was needed to provide a comprehensive insight into the prediction of IBL in PAS disorders.
Despite these limitations, the strengths of our study are the size of the cohort. As a result, we report a correlation between the ultrasonic scoring system and IBL in PAS disorders, which is a more clinically relevant risk gradation method. In conclusion, our study shows that a standardized risk assessment of blood loss based on the ultrasonic score can identify those women at highest risk for PAS disorders. The risk of blood loss equal to or greater than 1,500 mL refers to ultrasonic score ≥ 10 points, considering transfusion and subsequent referral mechanism. If verified prospectively, the assignment of the PAS may be helpful for patient counseling and delivery planning, all of which may ultimately improve pregnancy outcomes.
Correlation of An Ultrasonic Scoring System and Intraoperative Blood Loss in Placenta Accreta Spectrum Disorders: A Retrospective Cohort Study
doi: 10.3967/bes2021.022
- Received Date: 2020-08-04
- Accepted Date: 2020-12-04
-
Key words:
- Ultrasonic scoring system /
- Intraoperative blood loss /
- Placenta accreta spectrum disorders
Abstract:
| Citation: | CHEN Lian, SHI Hui Feng, JIANG Hai, SHI Xiao Ming, WANG Yuan Yuan, ZHANG Ai Qing, CHONG Yi Wen, ZHAO Yang Yu. Correlation of An Ultrasonic Scoring System and Intraoperative Blood Loss in Placenta Accreta Spectrum Disorders: A Retrospective Cohort Study[J]. Biomedical and Environmental Sciences, 2021, 34(2): 163-169. doi: 10.3967/bes2021.022 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: