-
Brucellosis is a zoonosis global importance. Brucella abortus and Brucella melitensis are the main species responsible for this disease in humans and production losses in domestic ruminants. Unpasteurized milk, home-made cheese and ice cream, and meat present a risk for human infection[1]. Brucellosis is an occupational hazard for professionals that are in regular contact with animals and animal products, such as veterinarians, butchers, and livestock farmers[2]. This disease is often overlooked by public health authorities in developing countries[3] due to insufficient human resources and laboratory capacity for diagnosis or surveillance[4] as well as the presence of other acute febrile illnesses, such as malaria and typhoid, that confound clinical diagnosis of the disease[5]. As a result, brucellosis is re-emerging worldwide[6], with global estimates of over 500,000[7] cases per year.
Reports on human brucellosis in Namibia are limited. As a result, the epidemiology of the disease and its burden on the human population remain poorly described. However, serological evidence of the disease in cattle, sheep, and goats abounds. The aim of this study was to estimate the serological prevalence of human brucellosis and identify plausible risk factors for the disease. This should provide evidence empirical to guide public health and veterinary authorities in formulating and refining strategies for preventing and controlling the disease in the country.
Namibia is located between −22°58'1.42"S and 18°29'34.80"E in the South-West of Africa and covers a total land area of 824,292 km2. It is divided into 14 administrative regions and has an estimated total population of 2.5 million inhabitants. This study was a cross-sectional seroepidemiological study on suspected human brucellosis cases that were screened for brucellosis between 2012 and 2017 at the Namibia Institute of Pathology (NIP). For the purposes of this study, a presumptive or suspected case of brucellosis was defined as a patient who visited a certified private or state physician and, after examination, was suspected to have brucellosis-like symptoms and whose blood sample was submitted to the laboratory for brucellosis screening. A positive case of brucellosis was considered as a presumptive or suspected case that showed a positive titer on the agglutination test.
Human brucellosis testing results from around the country from 2012 to 2017 inclusive were retrieved from the NIP database with prior authorization of the Ministry of Health and Social Services. The data were filtered to retain only the cases that met the case definition for presumptive and confirmed cases of brucellosis. For each case, the date of sampling, patient identification code, region in which the health facility was located, age, sex, and outcome of the serological test were recorded. Patient exposure history and clinical symptoms were missing from the data, thus, were not part of this study.
Serological screening for brucellosis was carried out at the NIP using a commercial rapid slide agglutination test kit (Fortress Diagnostics Limited, UK) as the principal assay, following the manufacturer’s test procedure. The test used standardized and stained B. abortus (FEBBAB05) and B. melitensis (FEBBME05) somatic ‘O’ antigens (febrile antigens) to detect antibodies against Brucella spp. in human serum. The results were read under a bright artificial light by checking for the presence or absence of agglutination (clumping). Results were validated by comparing the outcome to the positive and negative controls that were supplied with the kit and run for each test batch. As part of the quality assurance program of the laboratory, the quality of test antigens was routinely verified using known positive and negative sera.
Samples that tested positive on the rapid slide agglutination test were confirmed and titers determined using the semi-quantitative agglutination test by the same test kit manufacturer (Fortress Diagnostics Limited, UK). Positive results were identified as the highest dilution showing agglutination, while negative results were those that did not show any agglutination. Positive and negative controls were prepared for each run of tests and used to validate the performance of the test.
Brucellosis testing results were stored in Microsoft Excel® spreadsheet version 2007 (Microsoft Corporation, USA) and exported to STATA version 15 for data analysis. Demographic data were summarized using descriptive statistics. The z test was used to test the significance of differences between the proportion of reactor males and females. Incidence was estimated per region and for the country as the proportion of seropositive cases in the population over the six-year study period and determined for the 100,000th person. P values < 0.05 were taken as statistically significant. A log binomial regression model was used to determine the effect of region on relative risk (RR) of infection. RR for age and gender were entered into a multivariable log binomial model.
A total of 971 presumptive cases of brucellosis were tested for anti-Brucella antibodies over the six-year study period. The number of patients presenting with suspected brucellosis declined from 199 cases in 2012 to 107 cases in 2017. The median age of the patients was 36 years (interquartile range [IQR] 25–49 years). Most of the participants were aged between 30 and 40 years (26.78%, n = 260) followed by those aged between 41 and 50 years (18.02%, n = 175), 20–29 years (16.58%, n = 161), 51–60 years (15.65%, n = 152), 13–19 years (8.55%, n = 83), 61–84 years (6.18%, n = 60), 6–12 years (5.56%, n = 54), and 0–5 years (2.68%, n = 26) in descending order. The majority of the patients were female (59.32%, n = 576, z = 5.7, P < 0.001).
Overall brucellosis seroprevalence was 17.92% (174/971, 95% CI: 15.64–20.46). The positive titer recorded in the agglutination test was from 1:20 to 1:320. The estimated prevalence was adjusted to 11.64% (113/971) based on the recommended diagnostic cut-off point of ≥ 1:80 for rapid agglutination tests. The prevalence of active infections among the tested individuals was 6.90% (67/971) based on the widely recommended diagnostic titer of ≥ 1:160 for brucellosis. The majority of positive cases (56.32%, 98/174) detected in this study were examined by physicians at private medical facilities, while the remainder were from public hospitals and clinics.
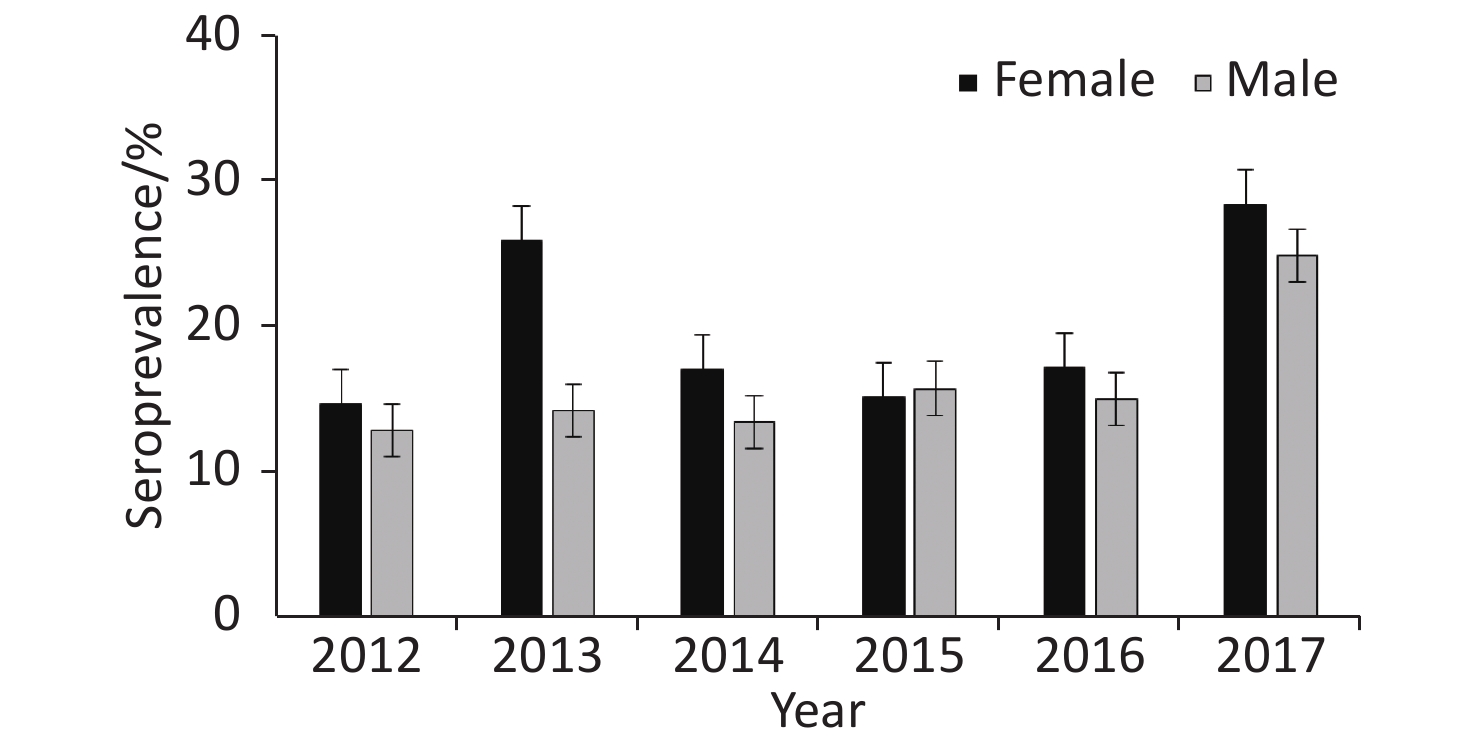
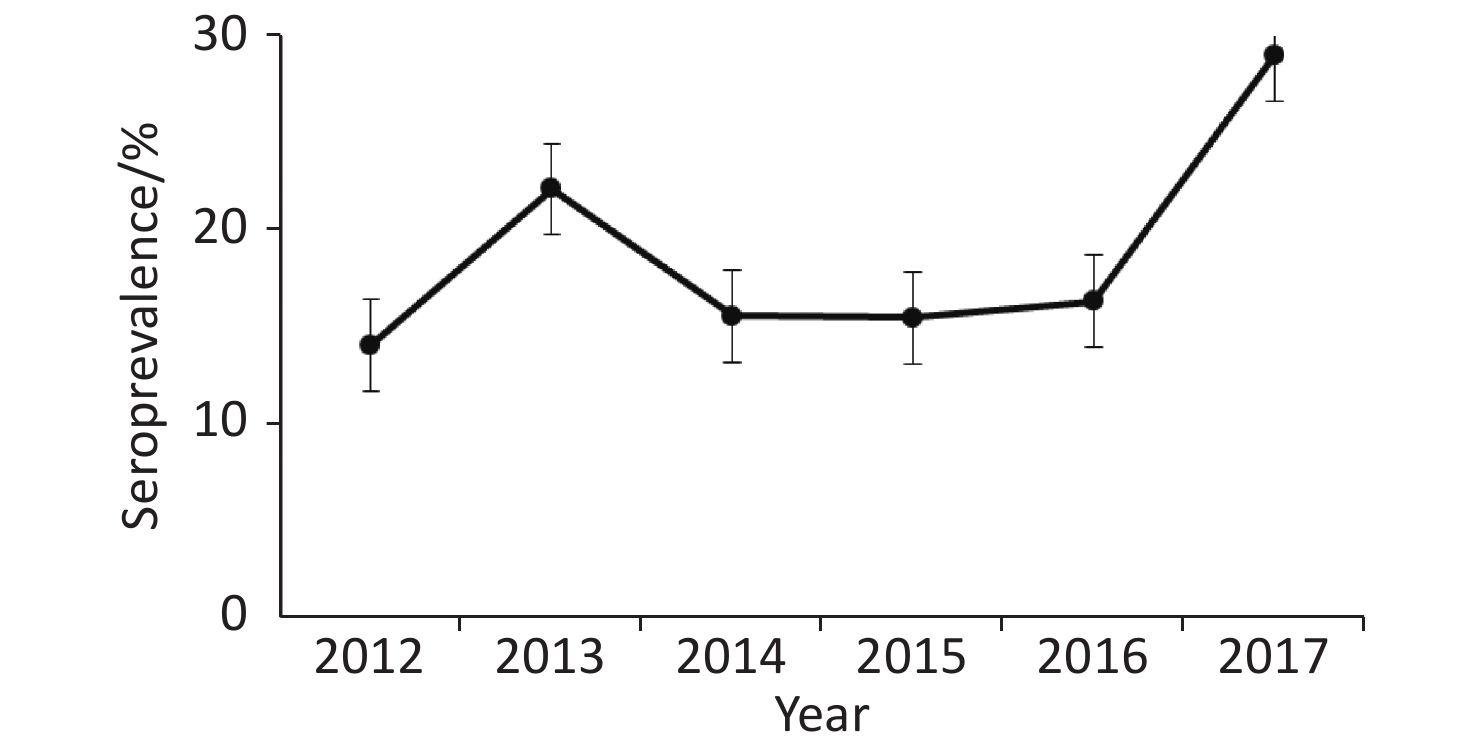
Annual prevalence of seropositive patients (Figure 1) differed between 2012 and 2013 (P = 0.04) as well as 2016 and 2017 (P = 0.015), but not between 2013 and 2014 (P = 0.11), 2014 and 2015 (P = 0.98), or 2015 and 2016 (P = 0.84).
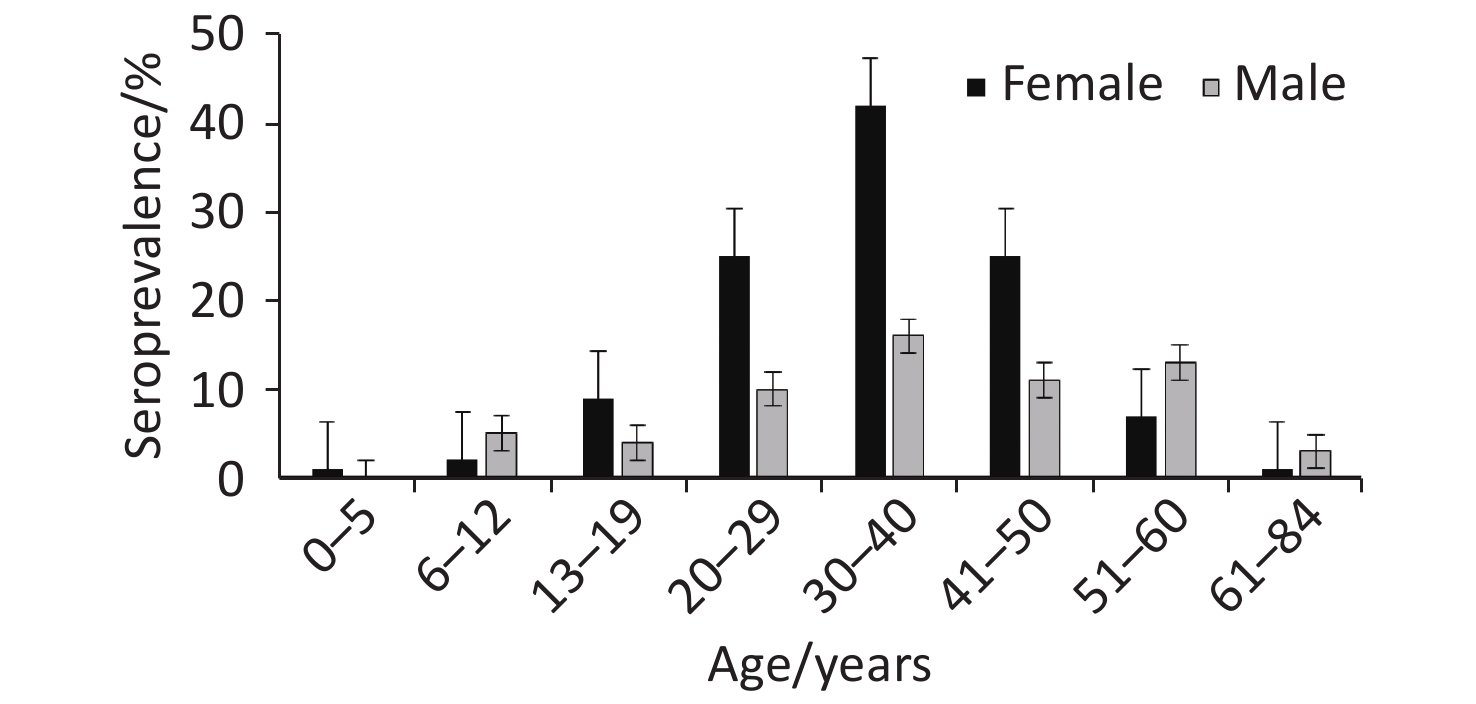
For each age category (Figure 2), there were proportionally more female seropositive cases (64.37%, 112/174) than male (35.63%, 62/174), except in the age categories 6–12, 51–60, and 61–84 years. Of the 113 patients confirmed as positive based on the recommended cut-off titer of ≥ 1:80, 68.14% (77/113) were female, while 31.86% (36/113) were male. Overall, positive cases were clustered in the 20 to 50 year age range, with the highest proportion of positive cases in the 30–40 year age group (33.33%, 58/174) and the lowest in the 0–5 year age group (0.57%, 1/174). The prevalence of seropositive patients in both males and females increased with age from 0 to 5 years of age to reach peak prevalence at 30–40 years and declined thereafter (Figure 2). Annual and overall prevalence of Brucella antibodies was higher in females than in males (Figure 3), but the differences were not significant (χ2 = 2.24, P = 0.14).
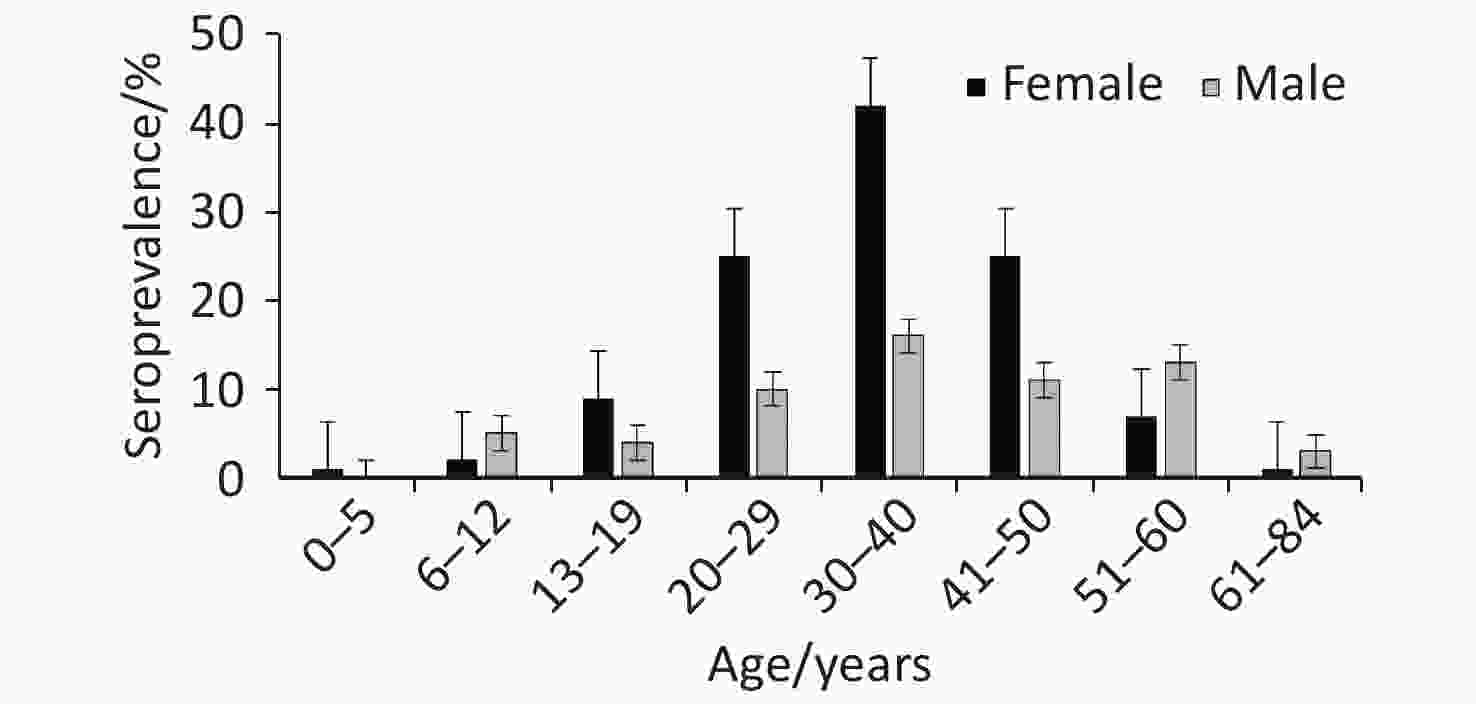
Figure 2. Seroprevalence of human brucellosis among suspected cases in Namibia by age and gender from 2012–2017.
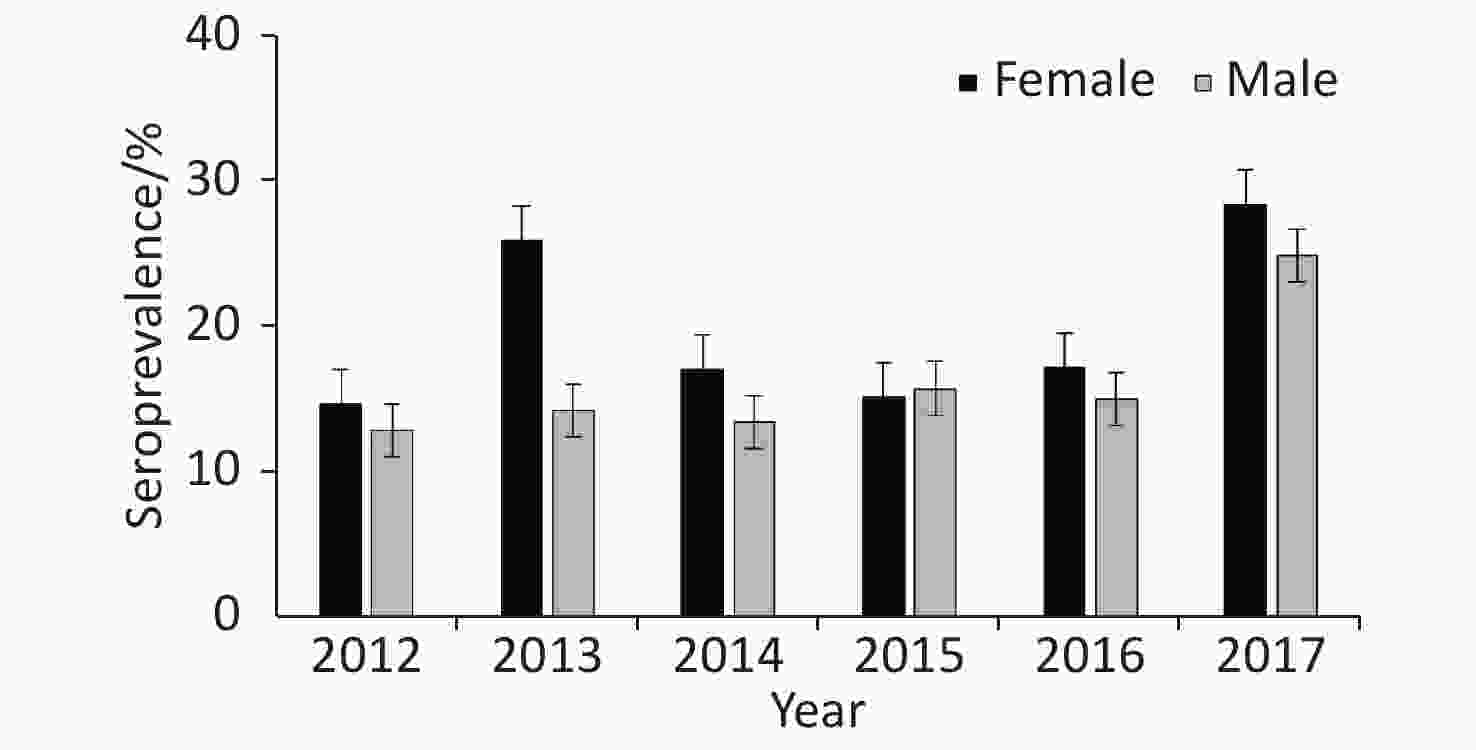
Figure 3. Seroprevalence of human brucellosis among suspected cases by gender in Namibia from 2012–2017
A higher proportion of positive cases (55.17%, 96/174) were confirmed during the rainy season (October—March) than the dry season (April-Sept; 44.83%, 78/174), with November (n = 27) recording the highest number of positive cases. However, the observed seasonal differences in prevalence were not statistically significant (z = 1.9, P > 0.05).
All 14 regions of the country recorded at least one incident case of brucellosis. The highest number of incident cases were in Khomas (n = 66), followed by Hardap (n = 41), Kunene (n = 17), Karas (n = 15), Oshana (n = 11), and the rest of the regions (1–10 cases). Most regions where the human population lives in communal areas, such as Kavango, Zambezi, Omusati, Ohangwena, and Oshikoto regions, had lower incidence of the disease (1–4 cases) than regions with urban areas.
A log binomial regression model was used to determine the RR of infection for age and gender, using Oshikoto as the reference region. The risk of infection was significant in the Erongo region (RR = 0.398, 95% CI: 0.164–0.962) compared to Oshikoto, while no statistical differences in the risk of infection was observed between Oshikoto and other regions. Neither age category (RR = 0.980 95% CI: 0.907–1.05) nor gender (RR = 0.800, 95% CI: 0.598–1.069) were significant predictors of risk of Brucella infection.
Overall the incidence of brucellosis over the six-year study period was 6.96 per 100,000 persons. This was calculated based on an average population of 2,500,000 people. Among administrative regions, relatively high incidence was recorded for the Hardap (44.27) followed by Karas (17.35), Kunene (15.49), and Khomas (14.11), while the other regions had an incidence of 0.49–4.58 per 100,000 persons.
Human brucellosis cases are a good indicator of the presence of infection in animal populations. Control of the disease in animals often results in reduced incidence in humans. In Namibia, brucellosis is a notifiable disease that is endemic in cattle, sheep, and goat populations.
In this study, we documented an apparent serological prevalence of 11.64% for brucellosis among patients of varied ages that presented with symptoms suggestive of human brucellosis in Namibia from 2012 to 2017. Since sero-positive patients were 5 years and older, congenital infection is unlikely to have played a role. In contrast to the high seroprevalence determined in humans in this study, studies in cattle and sheep in Namibia (the most likely sources for human infection) have consistently yielded prevalence of less than 2% over the years[8]. The seroprevalence recorded in this study is comparable to a prevalence of 12.80%[9] reported by other studies, but is higher than the 2.19% reported by previous studies in Namibia[8]. Seropositive cases were placed on a six-week treatment course of a combination of doxycycline and rifampicin as per the World Health Organisation (WHO) recommended treatment protocol. Presumptive patients with a seronegative test result probably had other infections that are prevalent in the country, such as malaria and typhoid, that manifest clinical symptoms that are similar to brucellosis[5]. Since patients tested in this study had brucellosis-like symptoms, the positive titers detected can be considered with confidence as confirming disease in the tested individuals, rather than as evidence of exposure to the pathogen. The assay used in this study has a lower sensitivity (36.60%) and specificity (69.30%) than other tests, which often leads overestimation of brucellosis prevalence, especially in endemic areas and regions with low prevalence[10].
The highest infection rate was in the 30–40 year age group, which comprises the most active and working part of the country’s population, thus, is most likely to be exposed to infection through activities, such as assisting with cow delivery, milking, or slaughtering. The predominance of female cases may be a reflection of the roles played by females, which increase exposure to Brucella infection, such as milk processing, home cheese preparation, and working in the meat industry.
The majority of positive cases (79.89%) were in the major sheep farming regions (Khomas, Hardap, and Karas) and in a region (Kunene region) where pastoralism is practiced. Therefore, brucellosis control strategies should be focused on these regions to decrease national disease incidence as sheep and goats are sources of Brucella infection for humans worldwide. Surprisingly, fewer cases of the disease were reported in regions where livestock rearing is communal, which may be due to confusion with endemic diseases, such as malaria, that are prevalent in these regions.
It was encouraging that suspected cases of brucellosis decreased from 2012 to 2017, concurrent with a decrease in brucellosis cases in cattle and sheep in Namibia[8]. A strategy with measures to prevent and control the disease is recommended. The measures, preferably involving a one health approach, should include health education and awareness campaigns to disseminate knowledge on risk factors for the disease to the public, including medical and occupationally exposed professions, such as butchers and farmers.
The absence of a full clinical history for each patient precluded the complete assessment of clinical symptoms, exposure, and risk factors.
This study determined a relatively high prevalence of Brucella antibodies in the tested patients, with cases reported in all regions of the country. Age, gender, and geographical location were not risk factors for Brucella seropositivity.
The authors wish to thank the Ministry of Health and Social Services for authorizing the study and providing access to the data. The research protocol was approved by the University of Pretoria (V55-18). The University of Namibia granted the first author time to carry out this study.
MADZINGIRA Oscar and VAN HEERDEN Henriette conceived and designed the study; MADZINGIRA Oscar performed the study and drafted the manuscript; VAN HEERDEN Henriette and FASINA Oludayo Folorunso supervised the study, and KALINDA Chester carried out data analyses. All authors reviewed and approved the final manuscript.
Seroprevalence of Brucellosis among Clinically Suspected Human Cases Presenting at Health Facilities in Namibia from 2012 to 2017
doi: 10.3967/bes2021.029
- Received Date: 2020-06-12
- Accepted Date: 2020-10-13
| Citation: | MADZINGIRA Oscar, FASINA Oludayo Folorunso, KALINDA Chester, VAN HEERDEN Henriette. Seroprevalence of Brucellosis among Clinically Suspected Human Cases Presenting at Health Facilities in Namibia from 2012 to 2017[J]. Biomedical and Environmental Sciences, 2021, 34(3): 232-235. doi: 10.3967/bes2021.029 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: