-
Adverse environmental factors or prenatal insults may cause developmental and pathological changes in the brain [1]. Previous studies showed that a number of unfavorable prenatal factors might trigger the pathological changes of adult diseases such as hypertension and diabetes mellitus [2]. It is rational that inner-utero hypoxia could be a common problem of prenatal insults-induced development. Hence, this study focused on prenatal hypoxia (PH) during pregnancy.
Cerebral artery is sensitive to low oxygen and critical in the cerebral blood flow. As all known, potassium channels and protein kinase C (PKC)/extracellular signal-regulated kinase (ERK)/Rho kinase (ROCK) pathways play the vital role in the regulation of cerebral vasculature. However, there are very limited information about the regulations of potassium channels and PKC/ERK/ROCK signaling pathways on middle cerebral artery (MCA) form PH offspring. Therefore, the present study focused on the effect of PH on the MCA from the offspring, and its correlation with potassium channels and PKC/ERK/ROCK signaling pathways [3].
Notably, vascular functions as well as several molecules in vessel tissue were affected by prenatal insults, which is related to RAS. Ang II can stimulate vasoconstrictions via three major pathways: 1) release of Ca2+ from intracellular stores; 2) induce of trans-membrane influx of Ca2+ predominantly through L-type Ca2+ channels (Cav1.2); 3) activation of one or multiple subtypes of protein kinase C (PKC)[4]. The present study aimed to investigate the regulatory role of PKC/ERK/ROCK signaling pathway in Ang II--mediated vasoconstrictions in the MCA of the PH offspring.
Rat offspring: Pregnant Sprague-Dawley rats from the Animal Center of Soochow University were housed at 22 ℃ with a 12-hour light/dark cycle. They were randomly divided into 2 groups: 1) Control group: treated as the same as the PH group except without hypoxia; 2) PH group: kept in chambers with 10.5% O2 from gestational day 5 to 21. All rats were given tap water and standard rat food during and after pregnancy. Five-month old male offspring were tested. All experimental procedures were approved by the Institutional Animal Care Committee and in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, 1996).
Small segments of MCA (fourth-order branch) were isolated in Krebs-Henseleit solution, mounted in a multi-myograph system, and maintained in oxygenated KHS (95% O2–5% CO2) at 37 ℃. Vessel tone was normalized by the maximum contraction elicited by 120.0 mmol/L KCl. After equilibration for 60 min, vessel rings were contracted with cumulative Ang II (10−11–10−6 mol/L). Losartan (AT1R antagonist, 10 μmol/L), PD123319 (AT2R antagonist, 10 μmol/L), or GF109203X (PKC antagonist, 1 μmol/L)[5], was used for pre-treating segments for 30 min, respectively, before the adding of Ang II. Concentration-response curves to the PKC activator, phorbol 12, 13-dibutyrate (PDBu, 10−9–10−5 mol/L) were performed in the presence of the ERK antagonist U0126 (10 μmol/L) or the ROCK antagonist Y-27632 (1 μmol/L). Signals were recorded by Power-Lab system.
RNA was isolated by Trizol. Reverse transcriptase was employed for oligo dT primed first-strand cDNA synthesis (Supplementary Table S1, available in www.besjournal.com). Real-time PCR was carried out on Mx3000P detection system (Stratagene) by EvaGreen dye (Biotium). ΔΔCt method was used to quantify the amount of mRNA. 18S was used as an internal control. The amount of expressed target gene was normalized to 18S to obtain the relative threshold cycle. The relative gene expression (RGE) was normalized to 18S to obtain the relative threshold cycle and calculated as RGE = 2−(TΔCtNΔCt), where T represents the level of experimental groups, N represents the control group whose expression level was calculated, and ΔCt is the difference of threshold cycle (Ct) between the gene of interest and 18S.
Gene Name Primer (5’-3’) AT1α F: CTTCTCAATCTCGCCTTGGC R: AAGGAACACACTGGCGTAGA AT1β F: GGTTCAAAGCCTGCAAGTGA R: CGGTTAACAGTGGCTTTGCT AT2 F: TGGCTTCCCTTCCATGTTCT R: TCTCTCTCTTGCCTTGGAGC PKCα F: TTCAC AAGAG GTGCC ATGAG R: ATTCA TGTCG CAGGT GTCGC AT PKCδ F: CCGTT CCTGC GCATC TCCTTC R: GTCGA ATGTT GACTT CCACTC ERK1 F: CAG AGTGG CTATC AAGAA GATCA R: CGTCT CCATG AGGTCC TGAACA ERK2 F: GACAC AGCAC CTCAG CAATG AT R: GCAGAT CCAGA CCATG ATC ATACA ROKα F: GTGA AGTTC AGTTG GTTCG TCA R: AGAGC TGAAC CACCC AC GGACTGTT ROKβ F: GATAG GAAGA AAGCT AATAC TCAAG R: ACGCA AC TGCTC AATAT CACTCTCT Table S1. Primer sequences
The MCA was cut into pieces in Ca2+-free ice-cold (4 ℃) physiological salt solution (PSS) containing 137 NaCl, 5.6 KCl, 1 MgCl2, 10 HEPES, 10 glucose, 0.42 Na2HPO4, 0.44 NaH2PO4, and 4.2 NaHCO3 (pH 7.4). Segments were incubated for 30 min at 37 °C in PSS supplemented with papain (4 mg/mL; Solarbio), dithiothreitol (1 mg/mL; Biosharp), and bovine serum albumin (2 mg/mL; Biosharp). After washed by PSS, tissues were triturated for isolation of single SMCs.
Potassium currents were measured with conventional whole-cell configuration by an Axopatch 700B amplifier (Axon Instrument, Foster City, CA). Outward K+ currents were elicited by progressive 10 mV voltage steps (500 ms duration) from a holding potential of −70 mV to +60 mV using 3–5 MΩ patch electrodes. Currents were low-pass filtered at 2 KHz, digitized at 10 KHz, and stored in a PC compatible computer by pCLAMP 10.0 software.
Data are expressed as mean ± SEM Significance (P < 0.05) was determined by t-test or two-way ANOVA followed by bonferroni test. Curve fitting was performed with SigmaPlot 11 and GraphPad Prism 5.
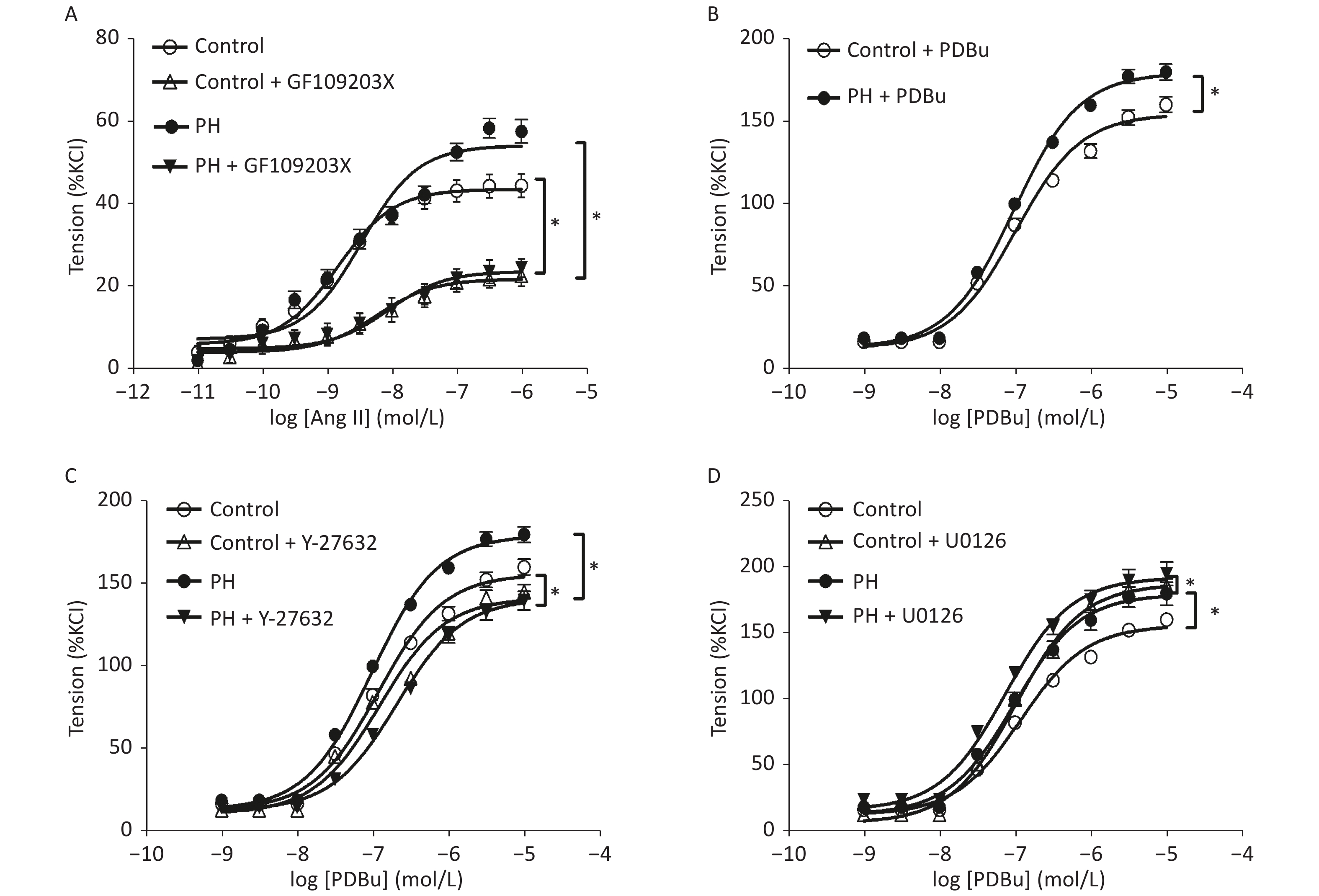
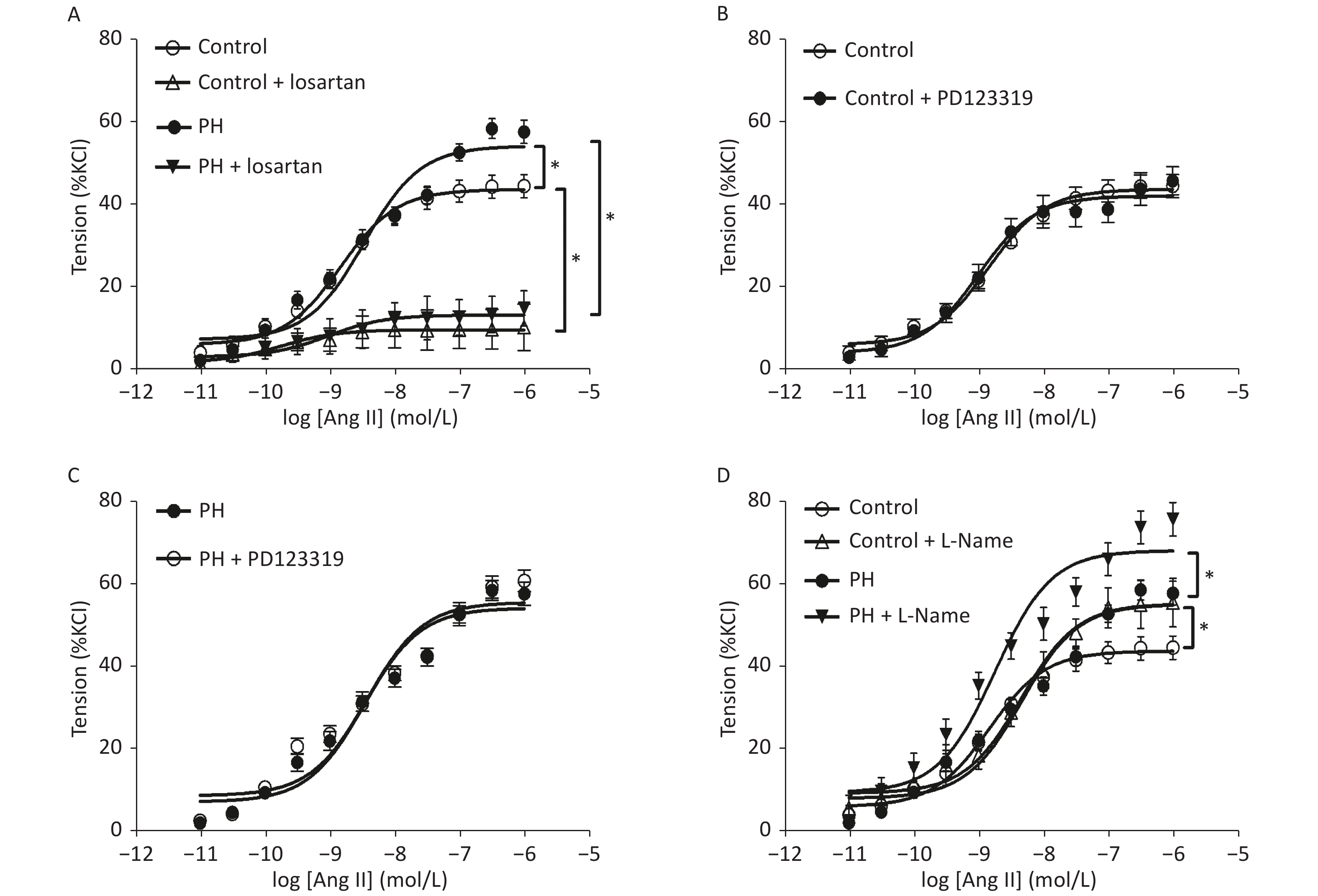
Angiotensin II mediated-vasoconstrictions were stronger in the PH offspring than that in the control. Pre-treating vessel rings with losartan almost completely blocked Ang II-mediated vascular contractions in both groups (Figure 1). Ang II-induced vasoconstrictions were obviously reduced in the presence of GF109203X, and the inhibition of contractile response to Ang II was greater in the PH offspring than that in the control. In addition, PDBu produced greater contractions in the PH offspring. Furthermore, PDBu-induced contraction was lower in the presence of the ROK inhibitor Y-27632 in both groups, while the gap was greater in the PH group. In the presence of ERK inhibitor U0126, PDBu-induced contraction was higher in the PH offspring than that in the control, while the gap was greater in the control group (Figure 2).
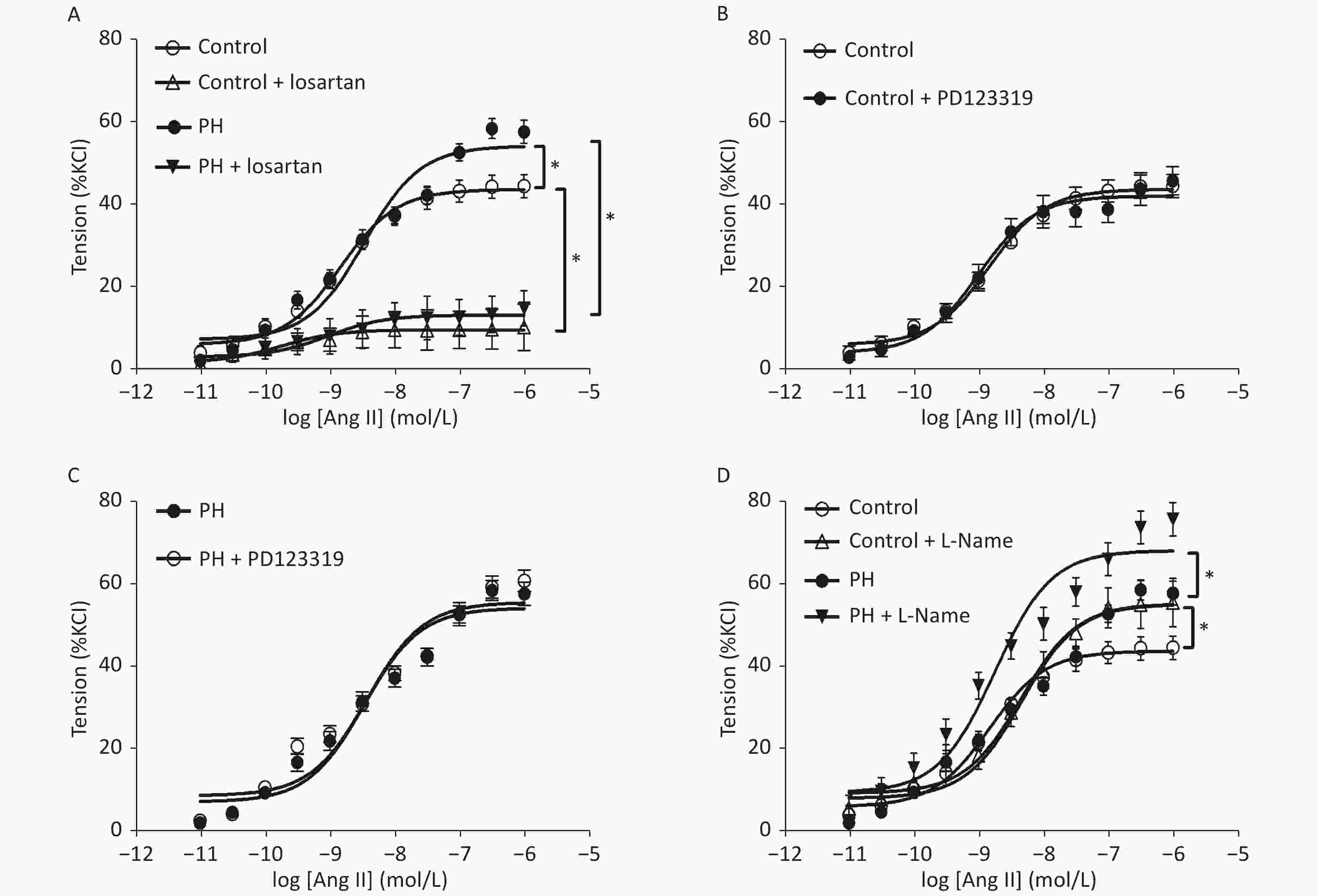
Figure 1. Contractile responses to Ang II (A) and the effect of losartan (A), PD123319 (B, C), or L-Name (D) on Ang II-induced vasoconstriction in middle cerebral arteries of the offspring (n = 8 each group). *P < 0.05. PH: prenatal hypoxia; Control: control.
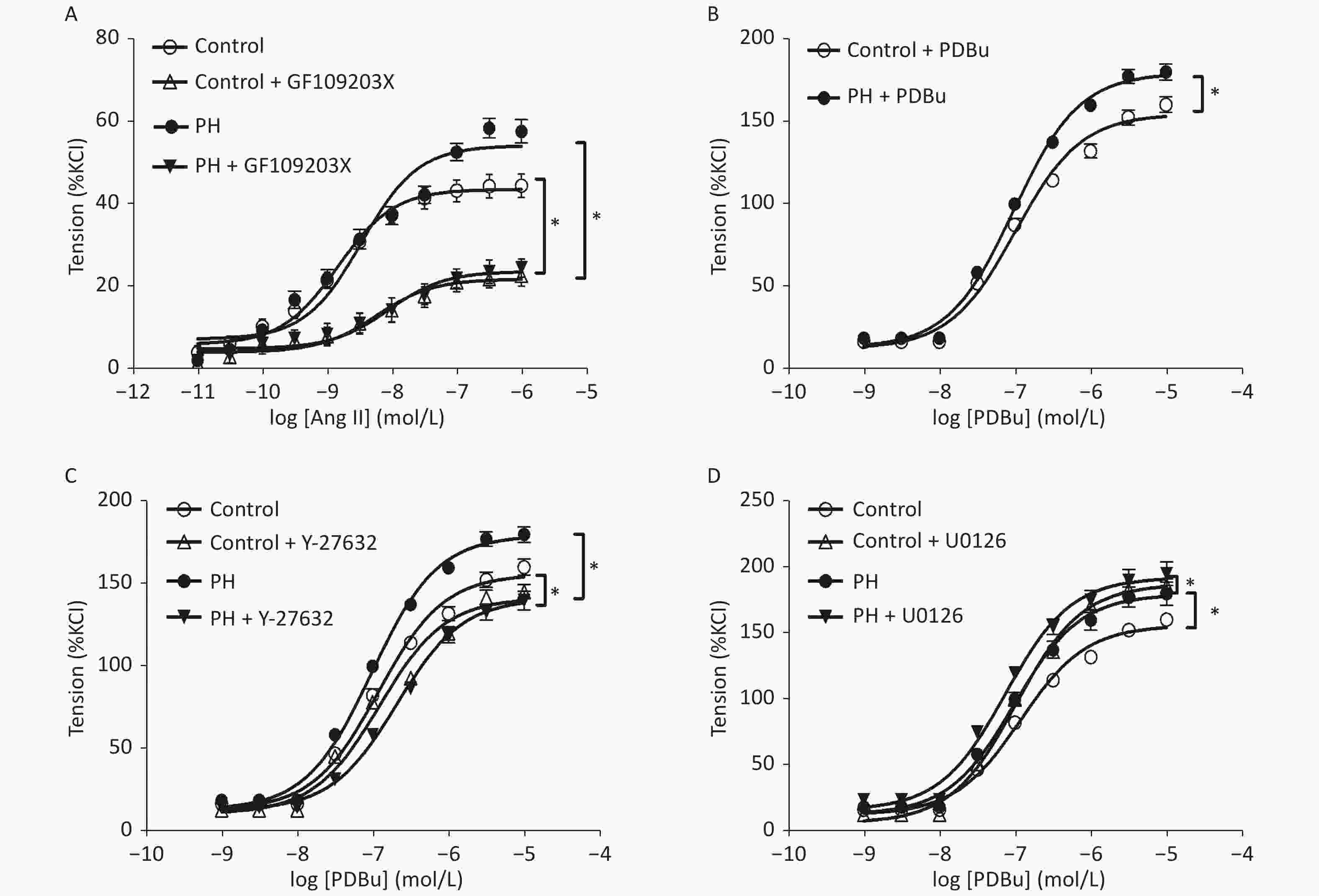
Figure 2. The effect of GF109203X (A) on Ang II-mediated vasoconstrictions, and PDBu (B) -mediated vasoconstrictions (B) with or without Y-27632 (C), and U0126 (D) in the MCA of the offspring (n = 5, each group). *P < 0.05. MCA: middle cerebral artery; PH: prenatal hypoxia; Control: control.
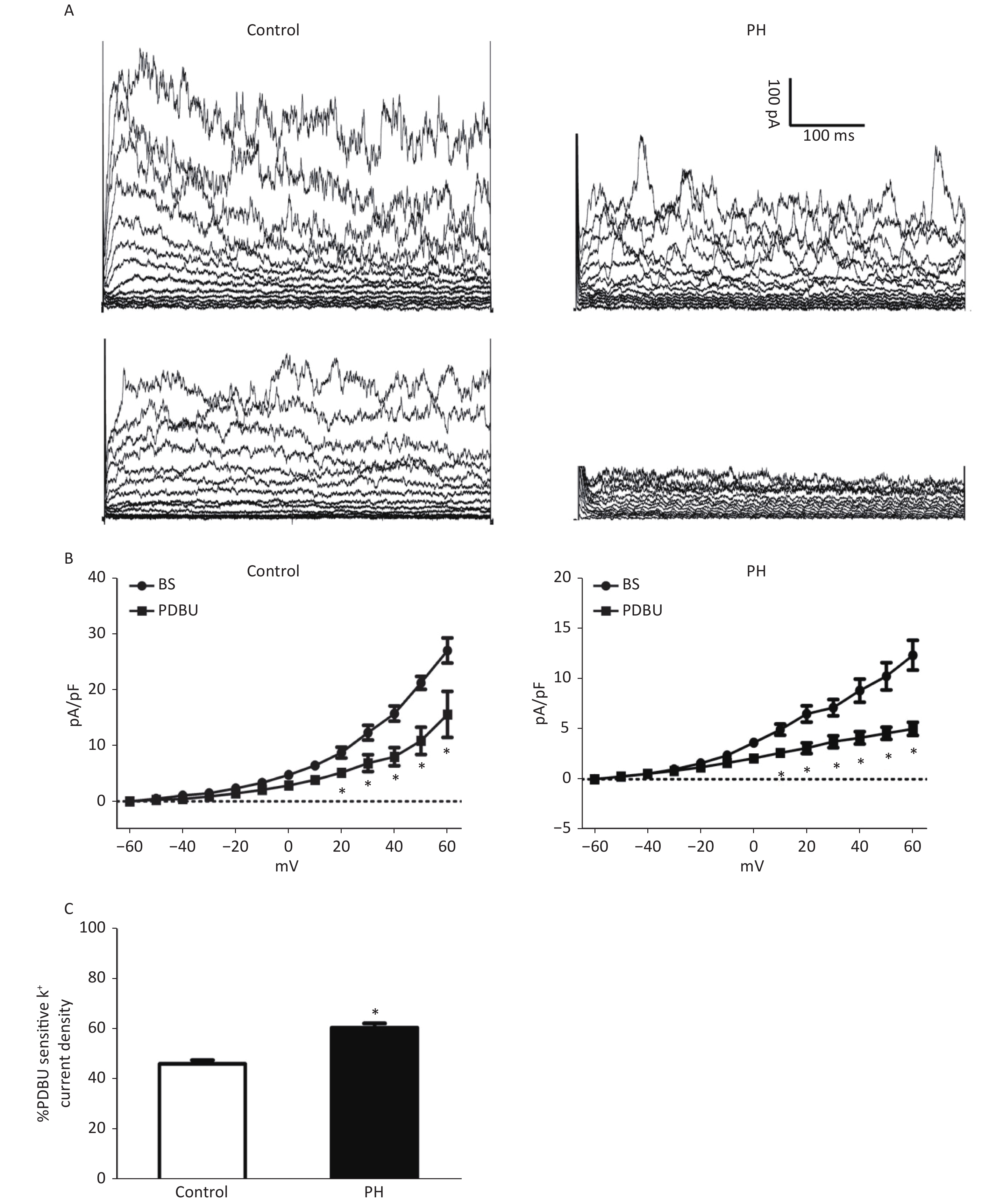
Whole-cell K+ channel currents were decreased by PDBu (100 nmol/L) in SMCs isolated from the MCA of PH offspring. PDBu (100 nmol/L) could decrease whole-cell K+ channel currents in both groups. The proportion of PDBu sensitive currents was obviously larger in the PH group, (PH vs. Control: 46.33% ± 1.04 vs. 60.67% ± 2.01. TP = +60 mV, P < 0.05) (Figure 3), indicating that the effect of PKC pathway on K+ channels was potentiated in SMCs of the PH offspring.
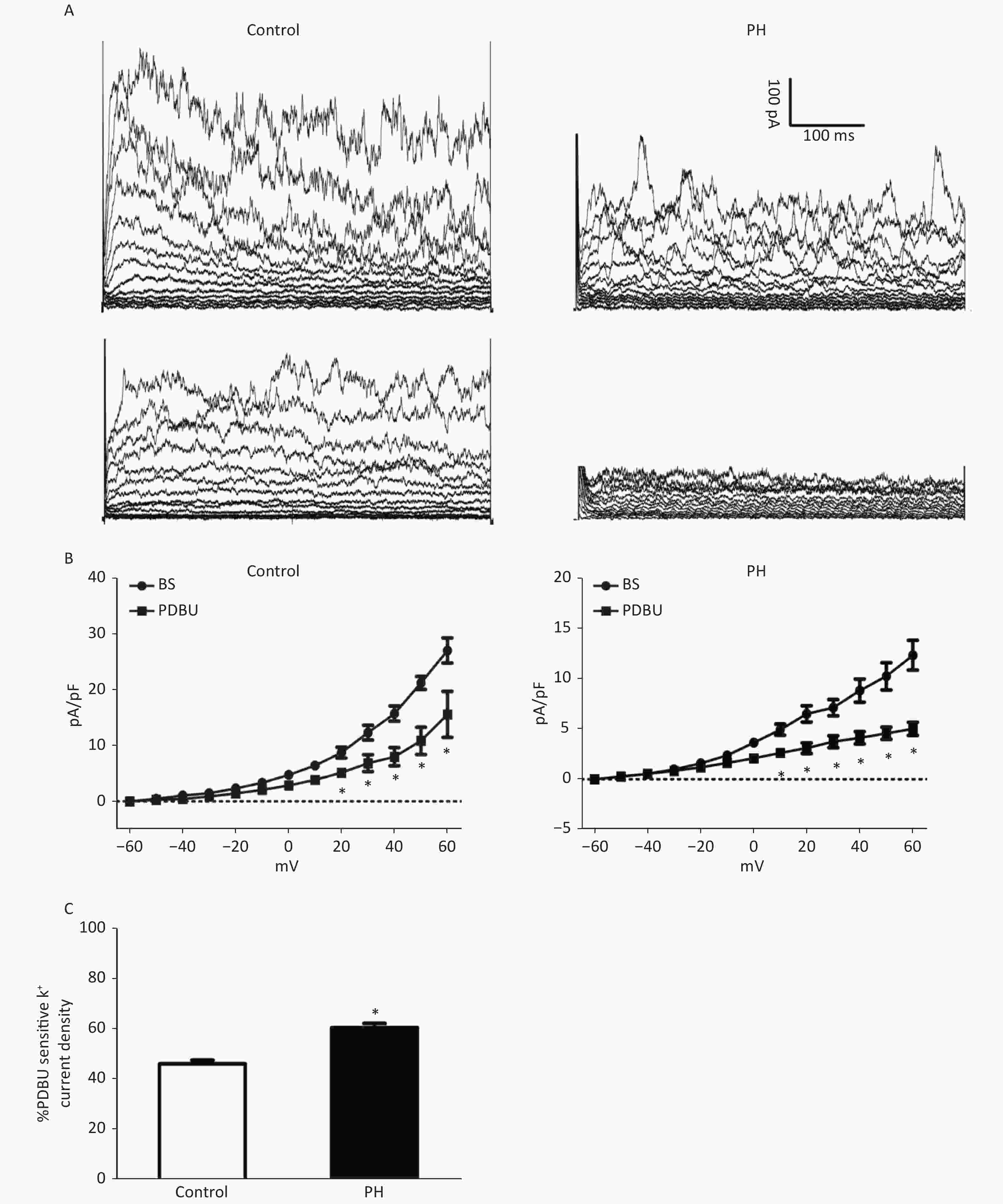
Figure 3. (A), Representative K+ current traces recorded on SMCs from the MCA of the Control (left) and PH group (right). Outward K+ currents elicited in the absence (upper) and presence of PDBu. (B), Current–voltage relationships of pooled data from the Control and PH group, demonstrating the effect of PDBu in reducing total currents. The difference between basal (BS) and PDBu-treated traces indicates PDBu sensitive current densities (C), The PDBu sensitive current as a percentage of total currents. A larger proportion of the current can be observed in the PH group (HP = +60 mV, animal = 7, cell = 15). Bars represent the mean ± SEM *P < 0.05. MCA: middle cerebral artery; PH: prenatal hypoxia; Control: control.
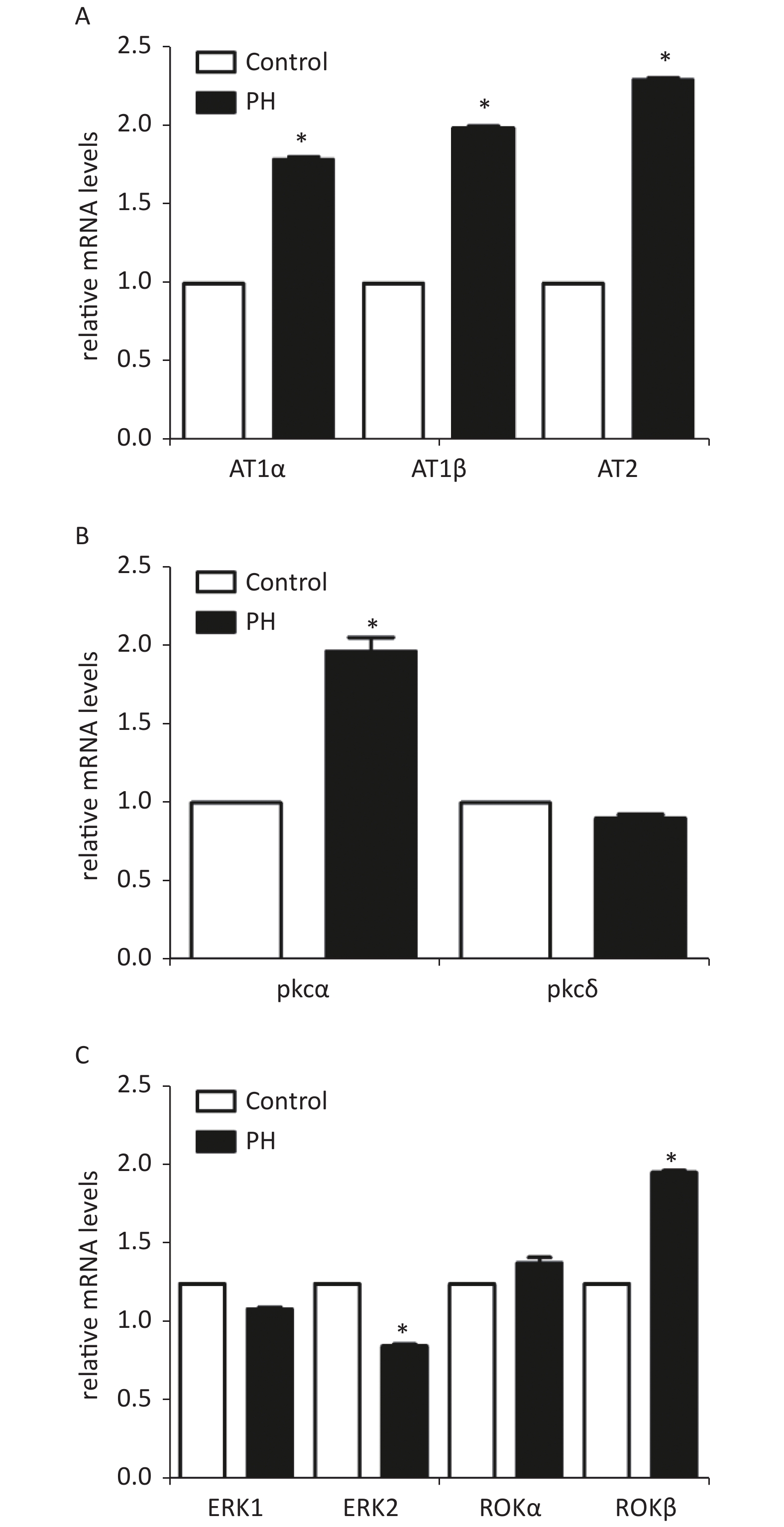
RT-PCR analysis showed that mRNA levels of AT1α, AT1β, and PKCα in the MCA were significantly elevated in the PH group. In addition, the results showed that PKC downstream protein ERK2 mRNA was obviously lower in the MCA of the PH offspring; ROKβ mRNAwas obviously higher in the PH offspring (Supplementary Figure S1, available in www.besjournal.com).
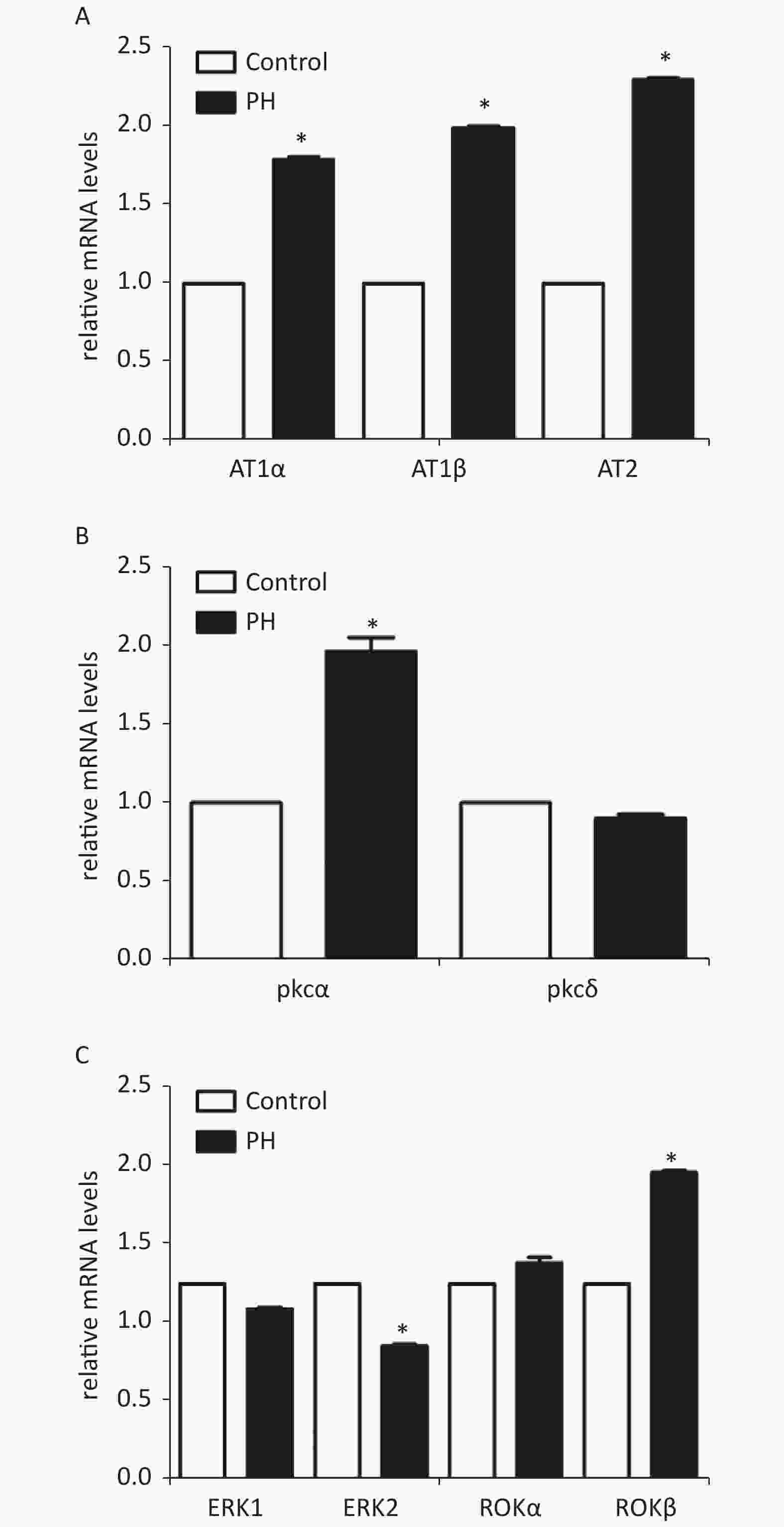
Figure S1. Expression of mRNA of AT1α, AT1β, and AT2 (A), PKC α, PKCδ (B), and ERK1, ERK2, ROKα, and ROKβ(C) in MCA of the offspring (n = 5, each group). *P < 0.05. PH: prenatal hypoxia; Control: control.
The present study demonstrated that prenatal hypoxia could affect central arterial functions in rat offspring. It demonstrating that the MCA could react more sensitively to vascular stimuli Ang II in the offspring exposed to prenatal hypoxia.
RAS plays a critical role in regulations of vessel functions in the central vascular system, including the MCA [6]. Angiotensin II, which is the predominate bioactive peptide in the renin-angiotensin system, promotes the maintenance of systemic pressure through various mechanisms in the cardiovascular and renal systems[7]. The present study demonstrated that hypoxia during perinatal period caused a higher Ang II-mediated vascular re-activity in the offspring MCA. Although angiotensin II is crucial to salt and water Canonical angiotensin II signaling occurs through a membrane bound heterotrimeric G-protein coupled receptor (GPCR). To date, there are two GPCRs known to mediate angiotensin II function, the AT1 and AT2 receptors[8,9]. The angiotensin II signaling cascade via the AT1 receptor is a consequence of the particular G-protein and/or signaling cascade which is activated: Gq/11, Gi, G12, and G13[8,9]. The AT1 receptor signals through three main pathways: classical phospholipase C (PLC) signaling leading to inositol trisphosphate (IP3) and diacylglycerol (DAG) cleavage, phospholipase D (PLD), also culminating in DAG generation; and phosphatidylcholine, phosphatidic acid (PA) and phospholipase A2 (PLA2), which is responsible for arachidonic acid (AA) and prostaglandin (PG) production. To investigate the possible mechanisms underlying the increased Ang II-stimulated MCA tension, Ang II receptors were tested, and it was found that hypoxia during pregnancy could lead to an over-expression of AT1R in the rat offspring. In the present study, losartan, a specific antagonist of AT1R, almost inhibited the enhanced vascular responses to Ang II in the MCA, whereas the AT2R blocker PD123319 had no effects, suggesting that AT1R pathway, not AT2R, mediated Ang II-increased MCA vasoconstrictions in the PH offspring. The present study provided novel information that AT1α and AT1β, as well as AT2R, were increased in the MCA of the PH offspring. The increased AT1R may contribute to the enhanced vascular tension-generated by Ang II in the offspring MCA.
DAG mediates vascular smooth muscle contraction via activation of protein kinase C (PKC). PKC is a serine/threonine kinase known to exist in several isoforms, all which contribute to various physiological activities and are activated differentially. Of the three known vascular smooth muscle isoforms, PKCα and PKCβ are dependent upon DAG activation and intracellular Ca2+ concentrations, while PKCε is DAG dependent only. PKC modulates vascular smooth muscle contraction by directly phosphorylating MLCK, which subsequently phosphorylates MLC. Furthermore, PKC activation leads to the activation of several other target proteins promoting smooth muscle contraction. PKC phosphorylates, and activates, ERK1/2, Rho-kinase (p160ROCK), and calmodulin-dependent protein kinase II. In addition, intracellular Ca2+ elevation was suggested to regulate angiotensin II-induced epidermal growth factor receptor (EGFR) and ERK activation. Various ion channels and ion transporters have been demonstrated as downstream targets of PKC.
To further study of AT1R signal pathway mediated vascular changes, PKC and other key elements may be considered. In general, Ang II is binds with AT1R to activate phospholipase C, and then hydrolyzes membrane phosphoinositides into diacylglycerol to activate PKC. Subsequently, PKC can stimulate vascular contractions through ERK and ROK pathways, while ERK has a negative feedback on PKC. By using PKC antagonist GF109203X, the present study showed that PKC may be involved in Ang II-stimulated vasoconstrictions in the MCA of the PH offspring. However, such in vitro vessel responses to Ang II was inhibited by the pre-treatment of the PKC blocker may indicate that the AT1R over-reaction could be blocked at the down-stream level at PKC, while the properties of the MCA to PKC activation could remain normal. However, in this study, the data of PKC activator PDBu demonstrated that enhanced PKC activation was involved in Ang II-stimulated vasoconstrictions in the MCA of the PH offspring.
PKC activation can increase ERK1/2 levels, while ERK1/2 has a negative feedback on PKC, and then inhibits the ROK pathway. The present study examined the down-stream pathways by ERK and ROK inhibitor. The results showed that PDBu-stimulated vasoconstrictions were altered by either U0126 or Y27632, and the MCA from the PH offspring appeared to have stronger inhibitory responses to both the inhibitors, indicating that a suppressed ERK and enhanced ROK signaling pathways could be involved in Ang II and PDBu-stimulated vasoconstrictions.
Prenatal hypoxia may result in increased activity and tissue distribution of several PKC isoforms during early organogenesis. PKC isotypes, including PKCα, PKCγ, PKCδ, PKCε, and PKCζ were detected in the rat MCA. Only α and δ isoforms appeared to be involved in PDBu-induced vasoconstriction [10]. This study measured mRNA expression of PKC isotypes. RT-PCR showed that the mRNA level of PKCα was significantly increased in the MCA of the PH offspring. In addition, mRNA levels of ERK2 were lower and those of ROKβ were higher in the MCA of the PH offspring, compared with those in the control group. Combined with the functional experiments, the results showed that the feedback of ERK to PKC was weakened while the ROK signaling was enhanced in the PH group, which might lead to constriction response increasing in PH MCA.
In analysis of above functional and molecular data in MCA study, AT1R, not AT2R, PKCα, not PKCδ, ERK2, not ERK1, and ROKβ, not ROKα, were involved in Ang II- and PDBu-stimulated vessel tension in the MCA of the PH offspring. It seems that PKC is a key in AT1R-PKC-ERK/ROK signal pathways. Hence, we performed patch clamp experiments in smooth muscle cells isolated from the offspring MCA. The data showed that whole-cell K+ currents were decreased by PDBu. The proportion of PDBu sensitive currents was significantly greater in the cells of the PH group compared with those of the control group, indicating that the effect of PKC signaling on K+ channels was significantly potentiated in the PH offspring cells. To the best of our knowledge, this is the first study to show PKC stimulation affecting K+ currents on SMCs from central blood vessels in the offspring exposed to prenatal insults.
In summary, this study demonstrated that Ang II-mediated increased constriction was closely related to PKC pathways in the MCA exposed to prenatal hypoxia. Micro-vessel and molecular experiments showed that either functional or molecular alterations, or both, in AT1R, PKCα, ERK2, and ROKβ, were involved in the AT1R-PKC pathways. Cellular experiments confirmed that PKC activity was changed in the smooth muscle cells of MCA from the PH offspring. The novel information gained may provide an interesting insight into the increased risk of stroke in developmental origins, which also may contribute to further understanding of early prevention or treatments of cerebral vascular diseases programmed in utero.
No competing financial interests.
Prenatal Hypoxia Altered Angiotensin II-mediated Vasoconstrictions via PKC/ERK/ROCK Pathways and Potassium Channels in Rat Offsrping Middle Cerebral Artery
doi: 10.3967/bes2021.033
- Received Date: 2020-07-02
- Accepted Date: 2021-01-14
| Citation: | LI Da Wei, BO Le, TU Qing, ZHOU An Wen, SHI Lin Ling, YANG Xiao Jun, MAO Cai Ping. Prenatal Hypoxia Altered Angiotensin II-mediated Vasoconstrictions via PKC/ERK/ROCK Pathways and Potassium Channels in Rat Offsrping Middle Cerebral Artery[J]. Biomedical and Environmental Sciences, 2021, 34(3): 250-255. doi: 10.3967/bes2021.033 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: