-
Exosomes are nano-sized (30–200 nm) phospholipid bilayer vesicles that are widely distributed in eukaryotic fluids, including plasma, saliva, and urine[1, 2]. They were long regarded as byproducts of membrane biosynthesis and abscission[3, 4], and then, exosome involvement in antigen presentation during immune cell activation in vivo was determined during the 1990s[5]. Thereafter, accumulated evidence has revealed that exosomes carry various biological molecules, including mRNAs, miRNAs, lncRNAs, DNA, lipids, and proteins, and are cell type-specific and have various biological functions[6, 7]. For example, the quantity and quality of exosomes derived from normal and malignant cells considerably differ, and they play important roles in various processes via intercellular communication[8, 9]. Exosomes not only influence the biological characteristics of adjacent cells but can also alter the fate of the recipient cells over a long distance[3, 10]. Chronic exposure to environmental chemicals results in alterations in exosomal content, secretion, and consequent pathological processes of environment-related diseases[11]. However, associations among chemical exposure, alteration of exosomal content, secretion, and disease progression remain largely unclear [11].
MicroRNAs (miRNAs), small short-chain (19-25 nucleotides) and non-coding RNAs, are important epigenetic regulators that promote the degradation of their target mRNA or inhibit their translation via complementary binding to 3’-untranslated region (3’-UTR) [12, 13]. The reported biological effects of miRNA include cell proliferation, differentiation, apoptosis, migration, and carcinogenesis[14, 15]. Abnormal miRNA expression is associated with the pathological processes of various diseases, and miRNA can function as typical oncogenes[16-18]. The most widely studied miRNA is miR-221, which is often upregulated in tumors and related to poor outcomes[19-21]. Nonetheless, the function of exosomal miR-221 in benzene-induced toxicity remains elusive.
Benzene is a common chemical that is also an established environmental carcinogen[22]. However, the mechanism of benzene-induced carcinogenesis remains unclear. We previously showed that exposure to benzene is associated with elevated miR-221 expression in peripheral blood lymphocytes from petrol station attendants[23]. However, the adverse health effects of elevated miR-221 content and whether it can be transferred to other cells and influence their biological characteristics via intercellular communication through exosomes is essentially unknown.
Here, we constructed hydroquinone (HQ, a representative metabolite of benzene with biological activity) -transformed malignant cell line, and investigated whether exosomal miR-221 derived from the transformed cells would influence apoptosis induced by HQ in normal recipient cells.
-
The Human Bronchial Epithelial Cells (16HBE) (American Type Culture Collection, Manassas, VA, USA) and HQ-transformed 16HBE (16HBE-t) cells were cultured in 1640 medium (Gibco, Laboratories, Gaithersburg, MD, USA) containing 10% fetal bovine serum (FBS), 10 U/mL penicillin, and 10 U/mL streptomycin (Hyclone Laboratories Inc., South Logan, UT, USA) in 5% CO2 at 37 ℃. The cells were digested with trypsin (Solarbio Co., Ltd., Beijing, China) when they reached 80% confluence and then seeded and passaged.
-
Soft agar assay was conducted to identify the biological characteristics of malignant 16HBE-t. Briefly, 1 mL per well of normal melting point agar (NMA; Solarbio) (0.6 w/v) in 1640 medium containing 10% FBS was added to 6-well plates and then solidified at 4 ℃ for 30 min. Thereafter, 3 × 103 cells/well in 1 mL of low melting agar (LMA, Solarbio) (0.3 w/v) in 1640 medium was added to the plates and incubated at 37 ℃ with 5% CO2 for 3 wk. Thereafter, we assessed 16HBE-t colony formation.
-
The viability of 16HBE and 16HBE-t cells was analyzed using CCK-8 assays (Dojindo Laboratories Co., Ltd., Kumamoto, Japan) as described by the manufacturer. Briefly, 1 × 103 16HBE or 16HBE-t cells/well in 100 μL complete medium were seeded into 96-well plates and incubated at 37 ℃ with 5% CO2 for 1–6 d. Thereafter, CCK-8 (10 μL/well) was added, and the cells were incubated for another 4 h at 37 ℃. Optical density (OD) was then determined using a spectrophotometer (BioTek Instruments, Winooski, VT, USA) at 450 nm. All groups of cells were assessed in triplicate.
-
A miR-221 inhibitor sequence was designed to inhibit miR-221 signals in 16HBE-t cells and synthesized at a commercial laboratory (RiboBio Co., Ltd., Guangzhou, China). The inhibitor was transfected into 16HBE-t cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), and then exosomes were isolated.
-
A total of 1 × 106 cells were seeded into 10 cm2 cell culture plates and cultured with 100 mL serum- free conditioned culture medium for 3 d, washed three times with PBS, incubated with exosomes-free serum medium for 24 h, then the supernatant of which was collected. In order to remove dead cells and cell debris, the supernatant was centrifuged separately at 300 ×g for 30 min and at 16,500 ×g for 30 min in 4 ℃. Exosomes were then isolated via 120,000 ×g centrifugation for 1 h at 4 ℃ by using polypropylene centrifuge tubes (Beckman Coulter Instruments, Inc. Brea, CA, USA) on ultracentrifuge (HITACHI, Japan). Exosomal pellets were then resuspended in 100 μL PBS and stored in 4 ℃ for following research.
-
The morphological characteristics of exosomes were determined using a JEM-1200EX transmission electron microscope (TEM, Jeol Ltd., Tokyo, Japan). Briefly, 20 μL of the exosomal pellet supernatant was added to copper grids, dried at room temperature for 10 min, and then negatively stained with phosphotungstic acid (pH 7.0) for 5 min before analysis by transmission electron microscopy (TEM).
-
Exosome uptake was determined by confocal laser scanning microscopy. We agitated a mixture of 1 μL of the lipophilic stain PKH 67 (BestBio Co., Shanghai, China) that stably binds to the lipid region of the exosomal membrane, 25 μL of exosome suspension, and 250 μL of diluent C (BestBio) and then incubated it at 37 ℃ for 5 min. The reaction was terminated by adding 1 mL of PBS, and then, exosomes were incubated with recipient cells for 3 h at 37 ℃. The nuclei of recipient cells were stained with 4,6-diamidino-2-phenylindole (DAPI; BestBio Co.) at 37 ℃ for 15 min, and then the cells were fixed in 4% paraformaldehyde in PBS for 30 min. Exosome uptake was then assayed using an LSM700B laser scanning confocal microscope (Carl Zeiss Microscopy GmbH., Jena, Germany) at room temperature.
-
Total RNA was extracted from 16HBE and 16HBE-t using TRIzol (Invitrogen), and then cDNA was synthesized using Beastar qPCR RT Kits (DBI Bioscience, Ludwigshafen, Germany). Relative levels of miR-221 were also analyzed by qRT-PCR using the following internal control U6 and primer sequences.
miR-221 stem-loop (RT primer): 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGAAACCCA-3′.
miR-221: Forward: 5′-ACACTCCAGCTGGGAGCTACATTGTC-3′.
Universal Reverse (Reverse primer): 5′-CTCAACTGGTGTCGTGGA-3′.
U6: Forward: 5′-CTCGCTTCGGCAGCACA-3′; Reverse: 5′- AACGCTTCACGAATTTGCGT-3′.
The qRT-PCR cycling conditions were 95 ℃ for 30 s, 40 cycles of 95 ℃ for 10 s, 56 ℃ for 30 s, and 72 ℃ for 30 s. Relative miR-221 expression levels were calculated using the 2-ΔΔCT method.
-
We previously found that the LD50 of HQ was about 38 μmol/L[24]. Therefore, we induced apoptosis in 16HBE cells using 25 μmol/L HQ. Apoptosis was assessed using Annexin V-fluorescein isothiocyanate (V-FITC)/propidium iodide (PI) Apoptosis Detection Kits (KeyGEN BioTECH Co., Ltd., Nanjing, China). Cells (5 × 105) suspended in 500 μL of binding buffer were mixed with 5 μL each of Annexin V-FITC and PI and incubated for 15 min at room temperature in darkness, and then apoptosis was analyzed using a Guava® easyCyte flow cytometer (Merck KGaA, Darmstadt, Germany).
-
Data were presented as mean ± standard deviation. All data were statistically analyzed using SPSS 19.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 7.0.0 software (GraphPad Software, San Diego, CA, USA). Quantitative differences between groups were analyzed using Student t-tests, and apoptosis rates were compared among different groups using chi-square tests. The cutoff for statistical significance was P < 0.05.
-
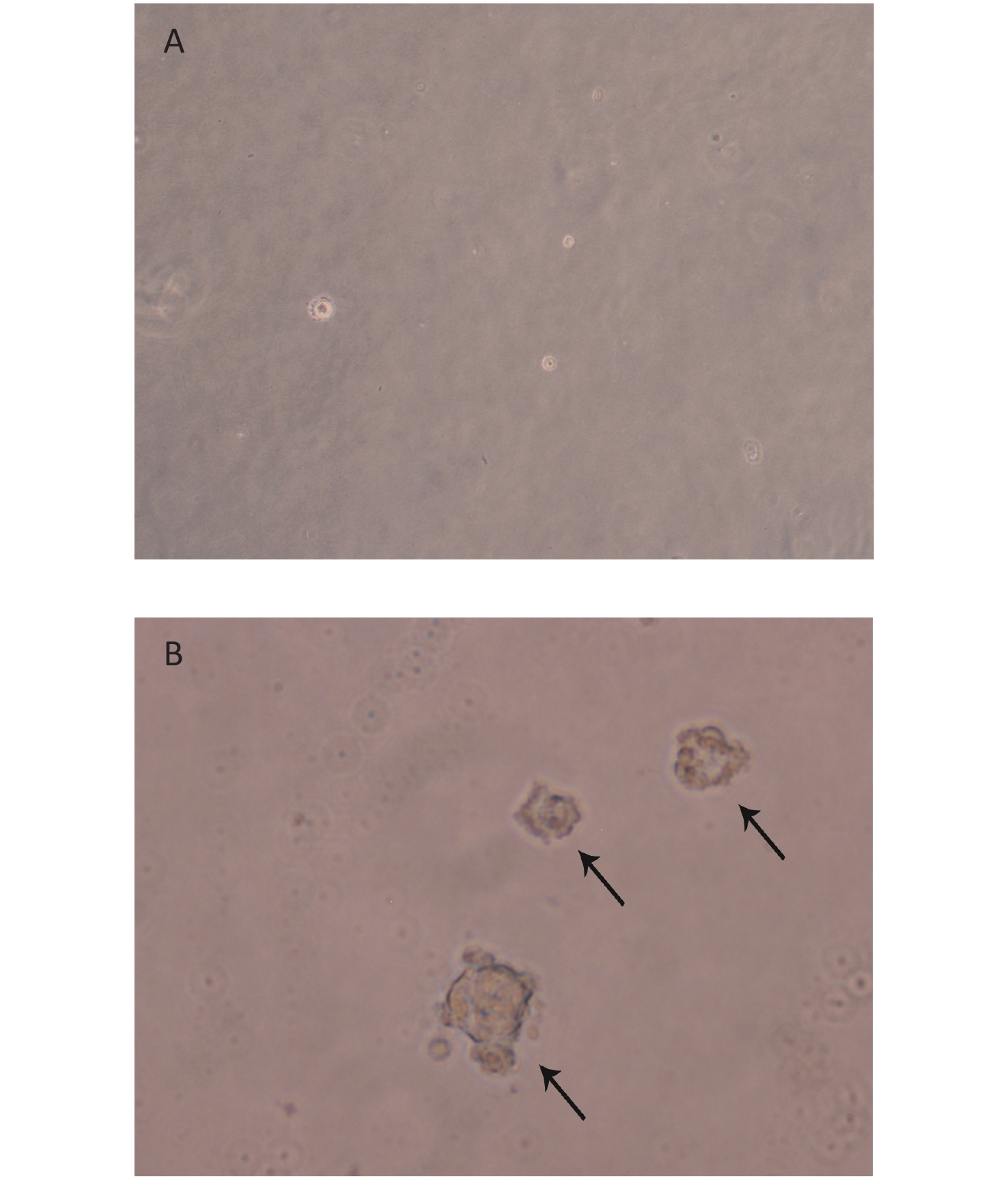
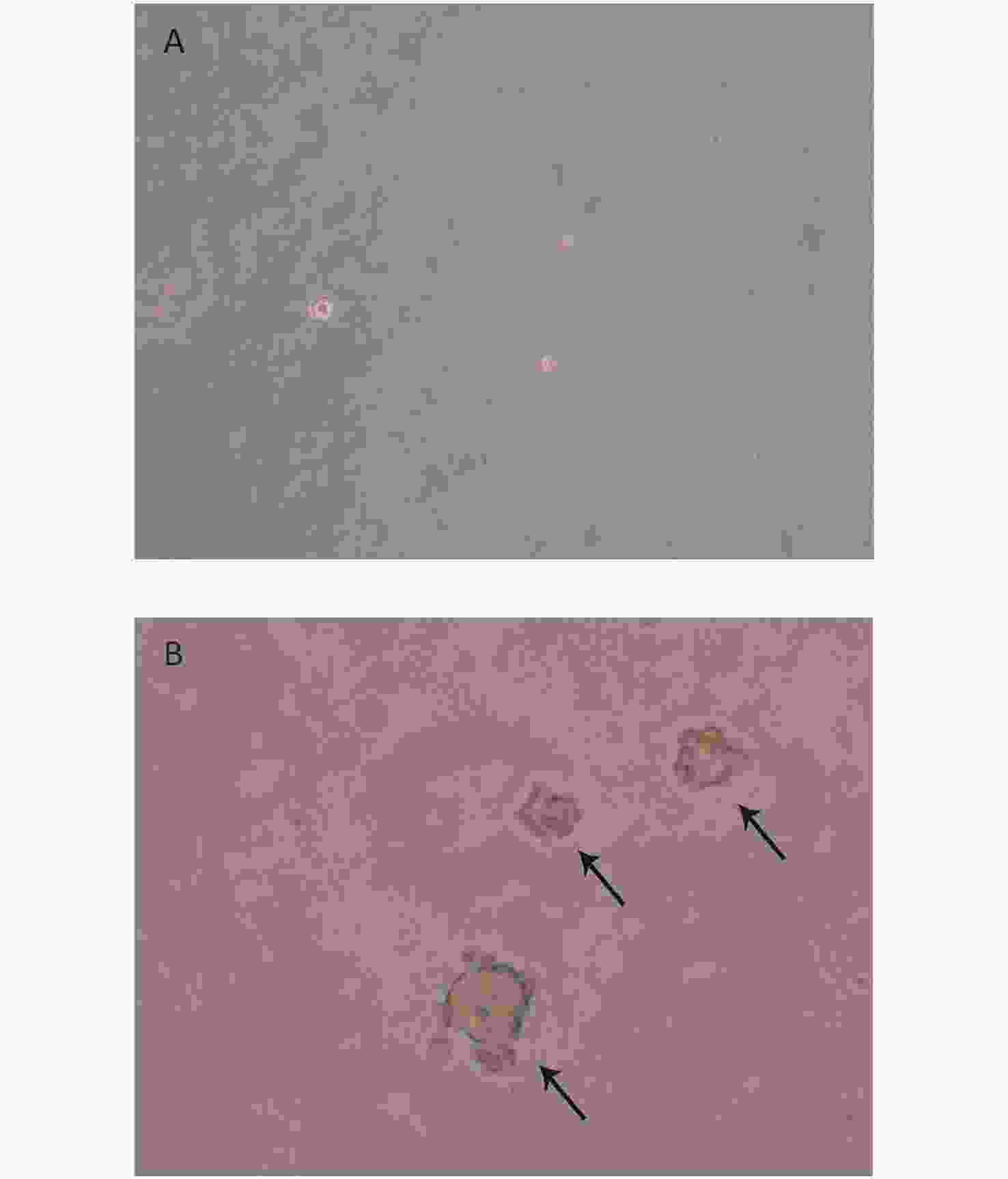
We created 16HBE-t cells as described[24]. The soft agar assay, which is a stringent test for malignant transformation of cells, and is semi-quantitative in nature to evaluate the response to treatment conditions[25], was used in malignant cell characterization. The results showed that 16HBE-t cells formed typical cell colonies in bilayers soft agar with about 6.85% efficiency. The colonies had the obvious morphological characteristic of anchorage-independent growth. While control group of 16HBE cells did not form colonies in bilayers soft agar (Figure 1).
-
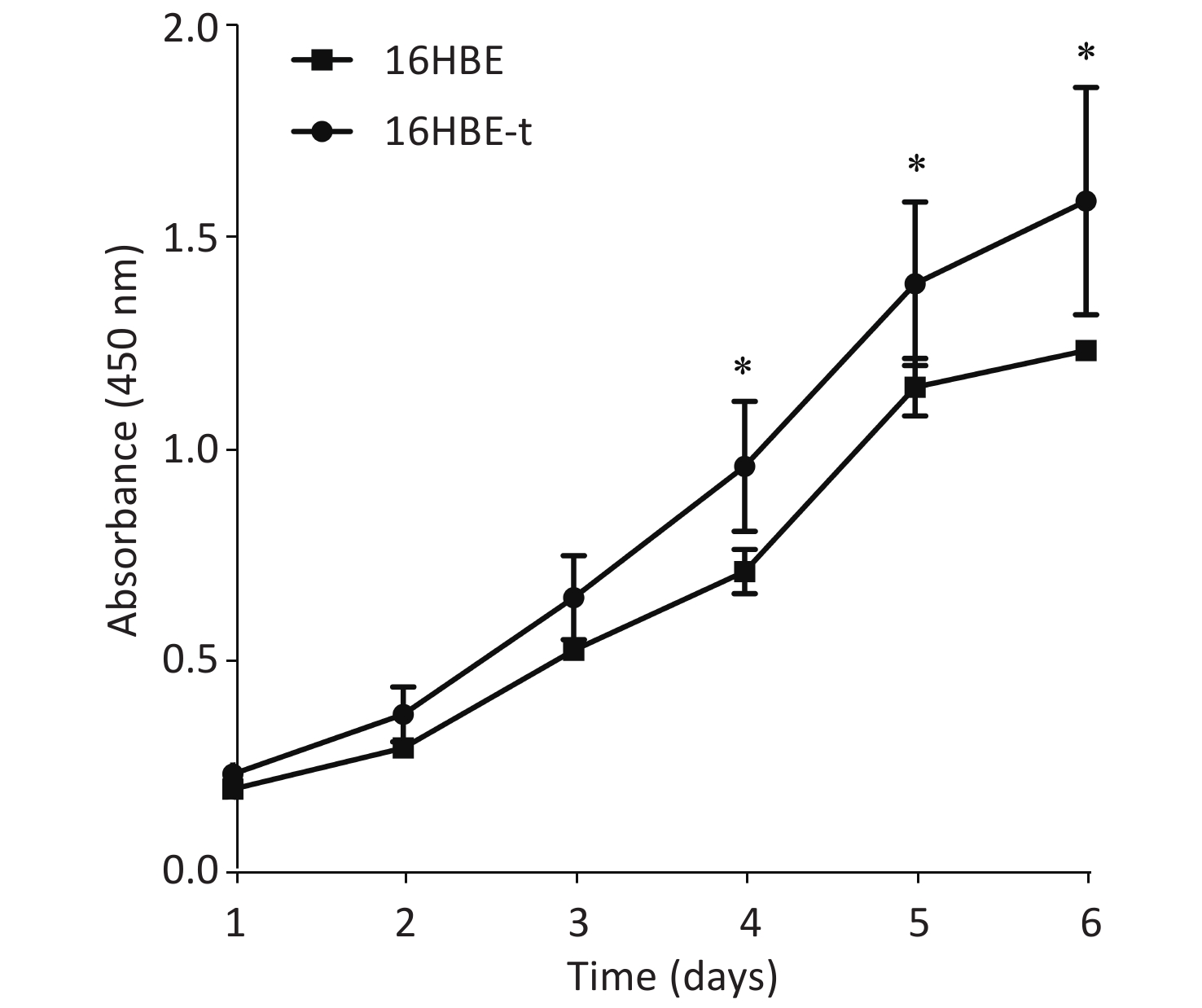
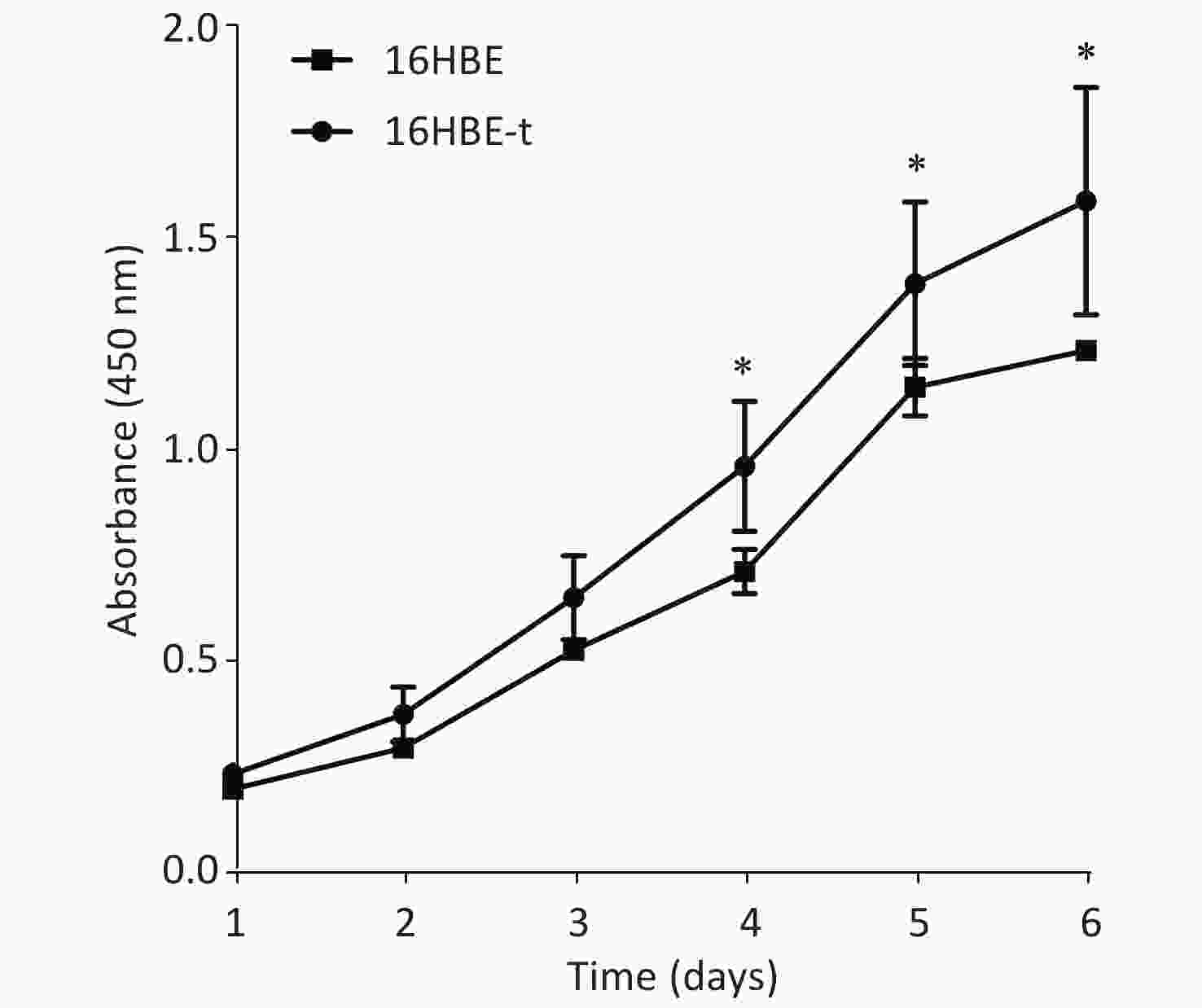
The proliferation of 16HBE and 16HBE-t cells was analyzed using CCK-8. Absorbance significantly differed (P < 0.05) between the two cell lines after 4–6 d in culture. The growth curve characteristics of 16HBE-t cells was typical of malignant cells (Figure 2).
-
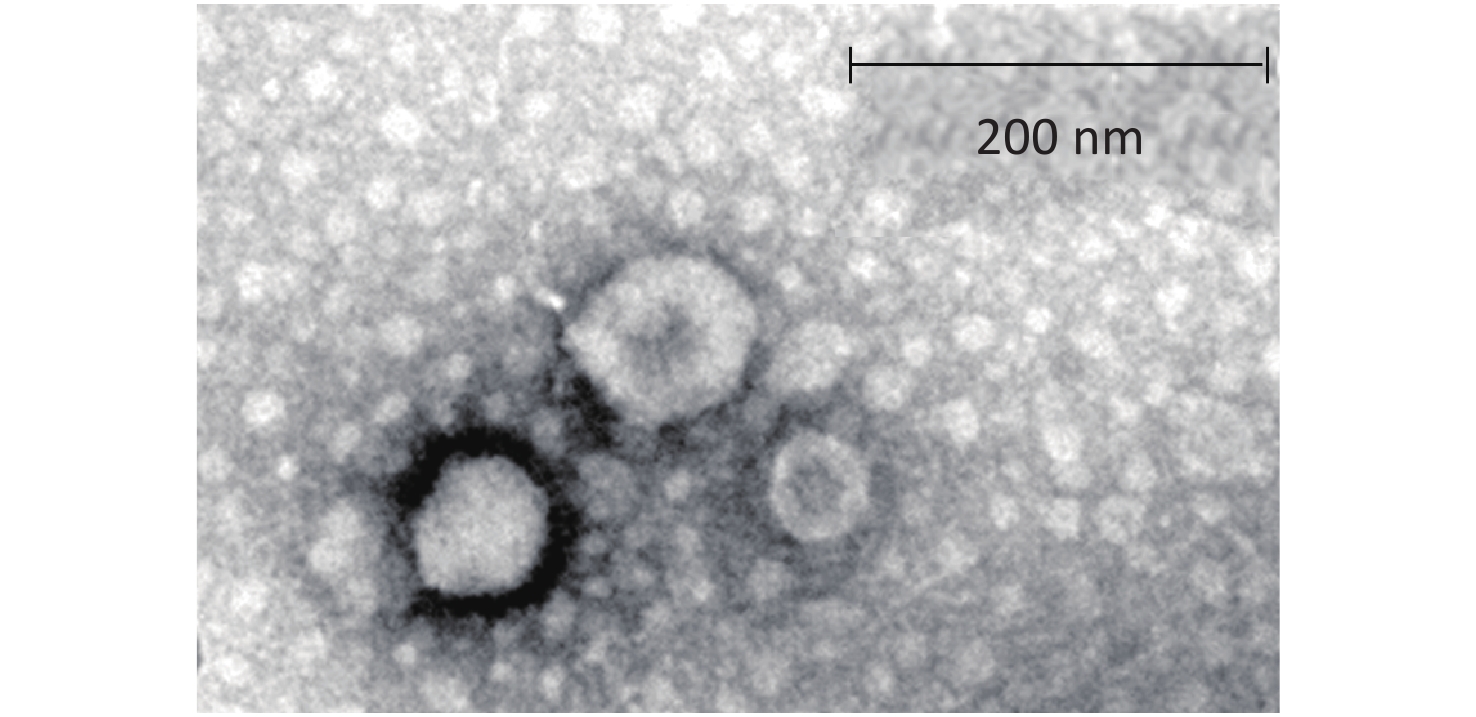
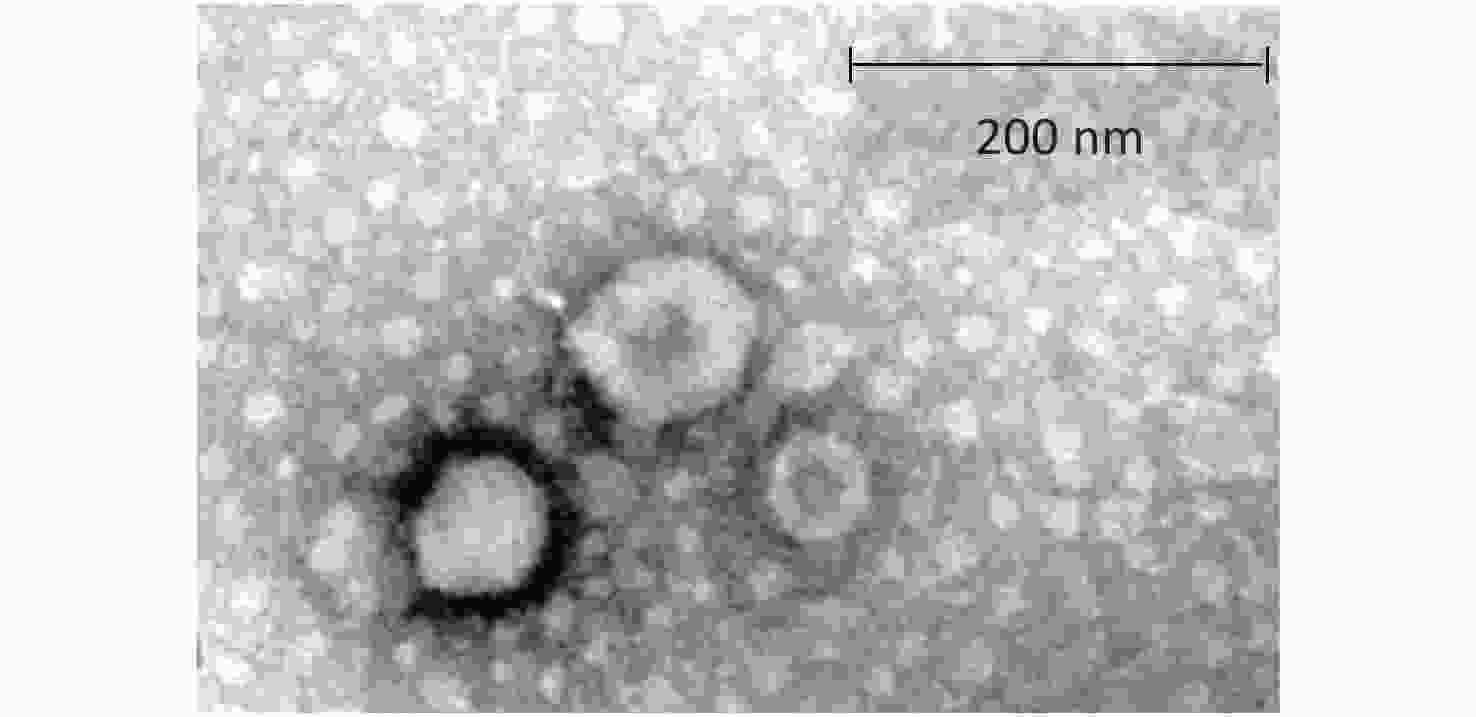
The TEM findings revealed exosomes with morphological characteristic of cup-shaped and typical double-layer membrane structure. As compared with the 200 nm scale bar, all the exosomes were within 60–120 nm in size, indicating that exosomes were isolated successfully (Figure 3).
-
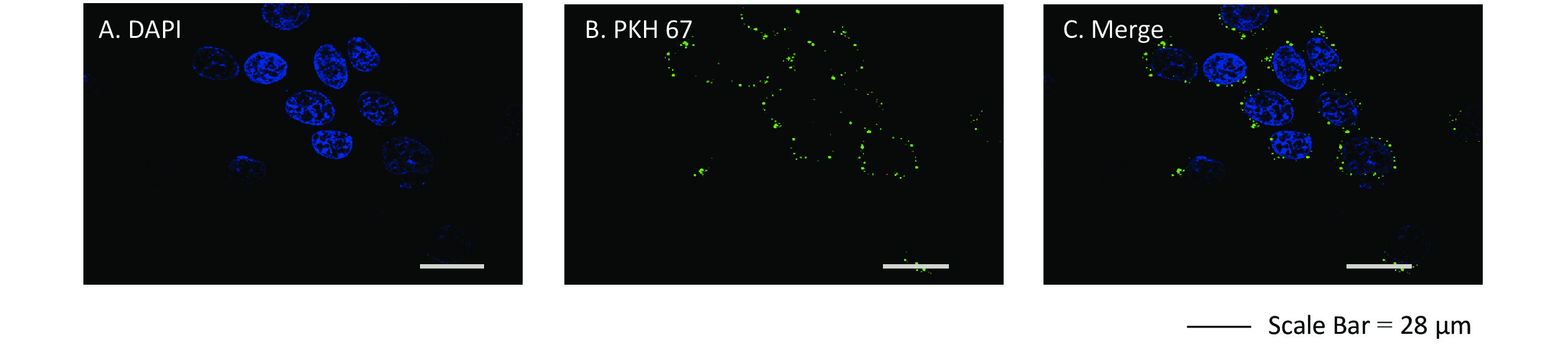
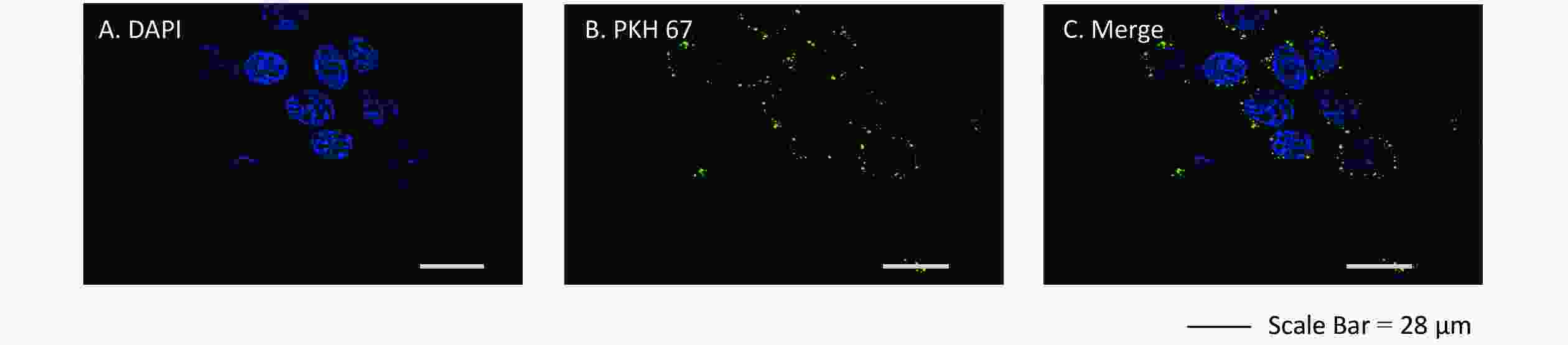
We assessed whether exosomes derived from 16HBE-t cells could be ingested by recipient 16HBE cells. The nuclei of 16HBE cells were stained blue using DAPI, and exosomes were stained in green with PKH 67. Laser scanning confocal microscope showed that recipient 16HBE cells ingested exosomes after 3 h of co-culture (Figure 4).
-
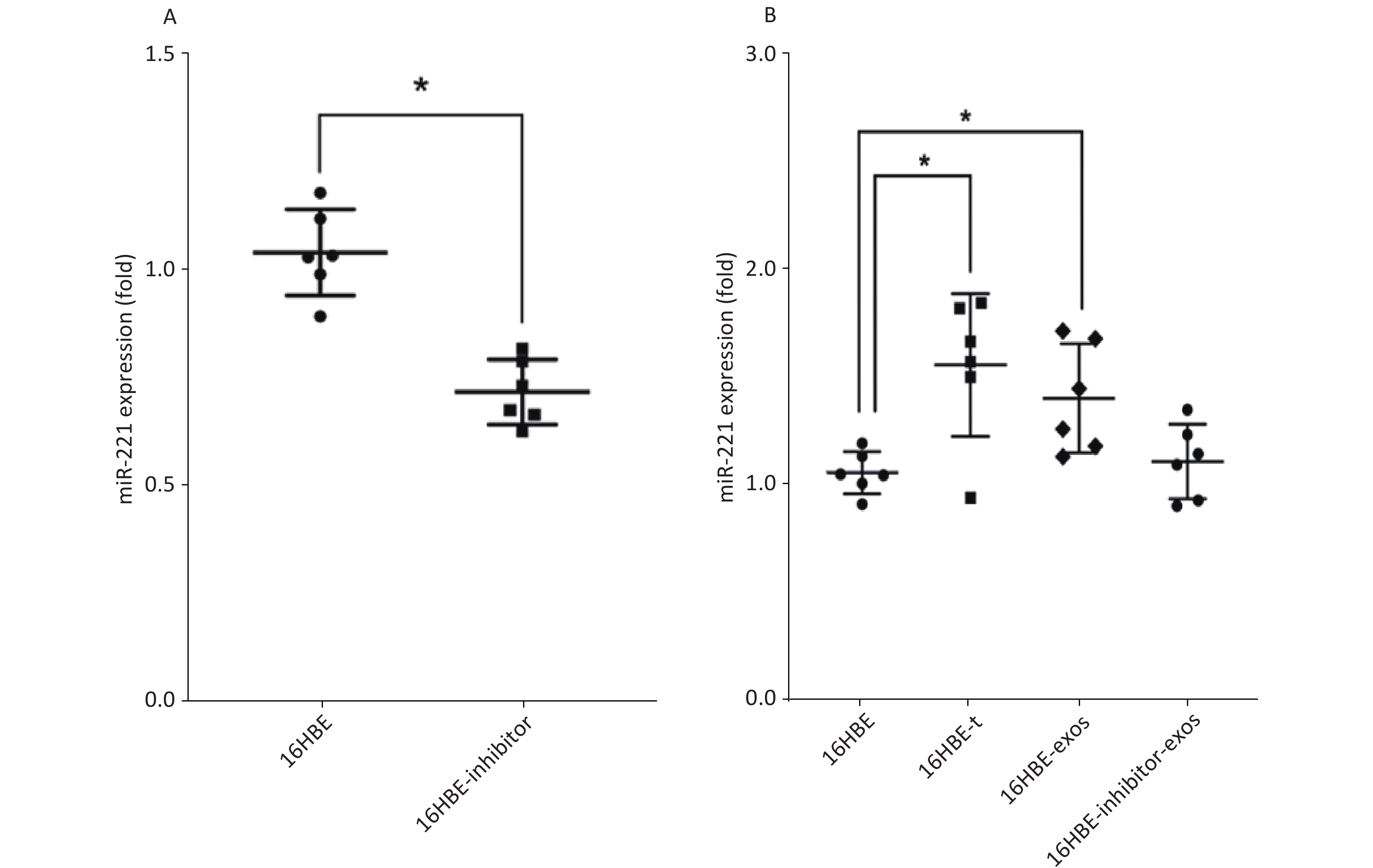
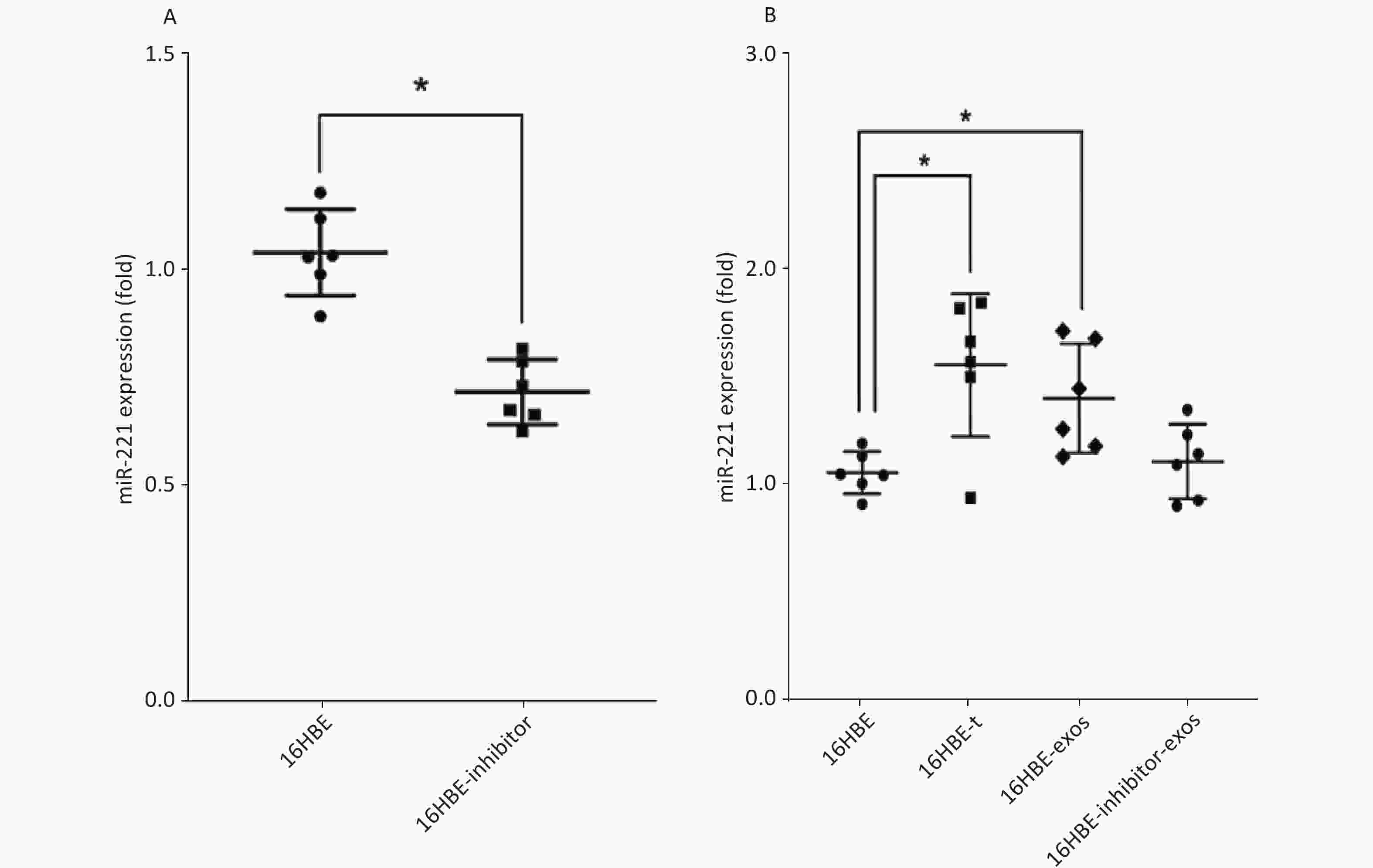
We detected the level of miR-221 using qRT-PCR. The data showed that miR-221 inhibitor could effectively inhibit the expression of miR-221 (P < 0.05; Figure 5A). Levels of miR-221 were significantly increased in 16HBE-t cells, compared with 16HBE cells (P < 0.05). After ingesting exosomes derived from 16HBE-t cells, miR-221 levels were significantly elevated in 16HBE-exos cells, compared with normal 16HBE cells. Levels of miR-221 in recipient cells did not significantly change after exosomal miR-221 in 16HBE-t cells was blocked using the inhibitor (Figure 5B).
-
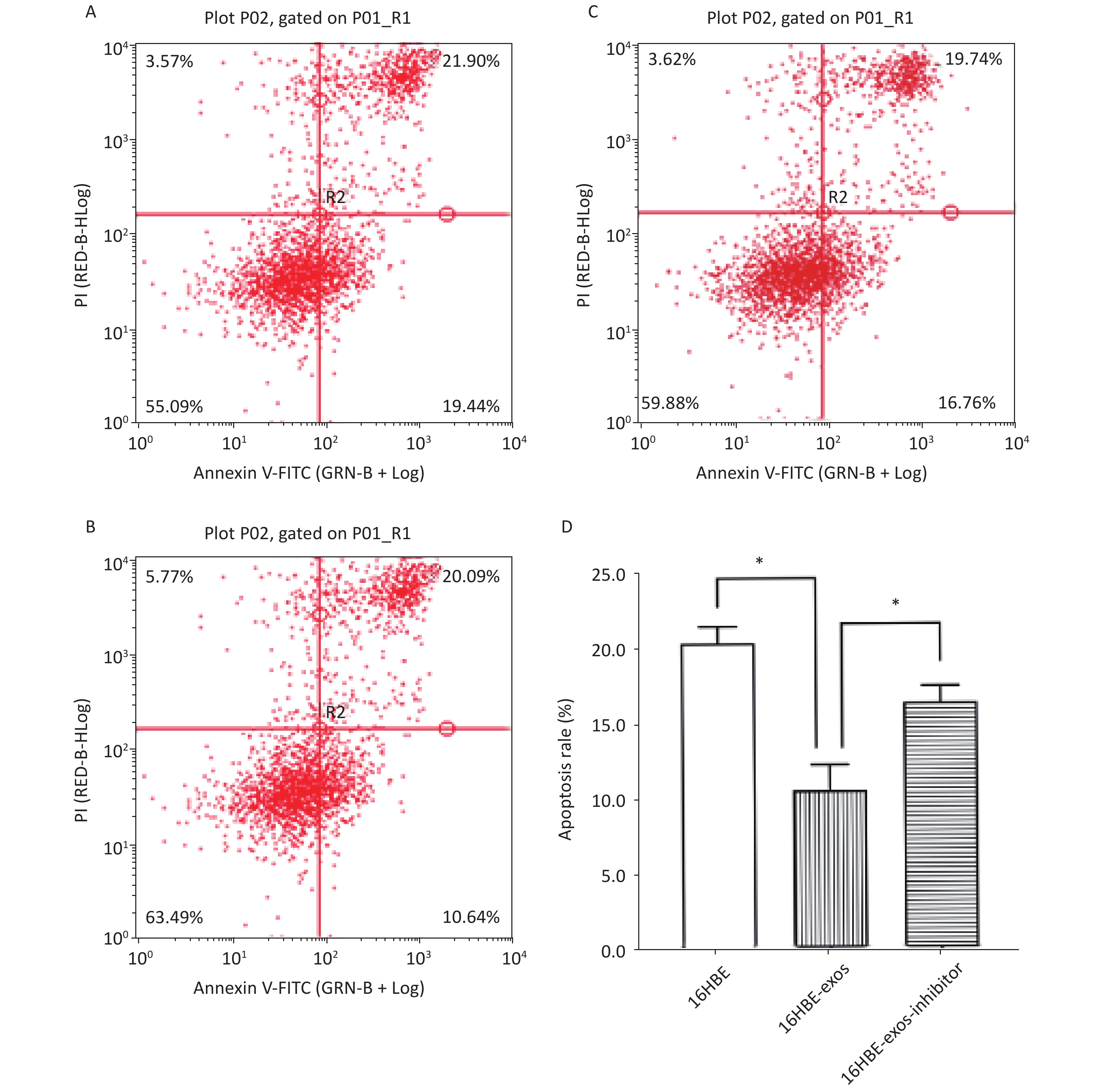
The effects of exosomal miR-221 on apoptosis induced by HQ were analyzed using flow cytometry. We found that HQ (25 μmol/L) induced obvious apoptosis in 16HBE cells. The apoptosis of 16HBE cells with ingested exosomes derived from 16HBE-t cells (16HBE-exos cells) decreased compared with normal 16HBE cells (P < 0.05). Notably, the apoptosis rate of 16HBE cells with ingested exosomes derived from 16HBE-t cells incubated with the miR-221 inhibitor did not significantly change (Figure 6).
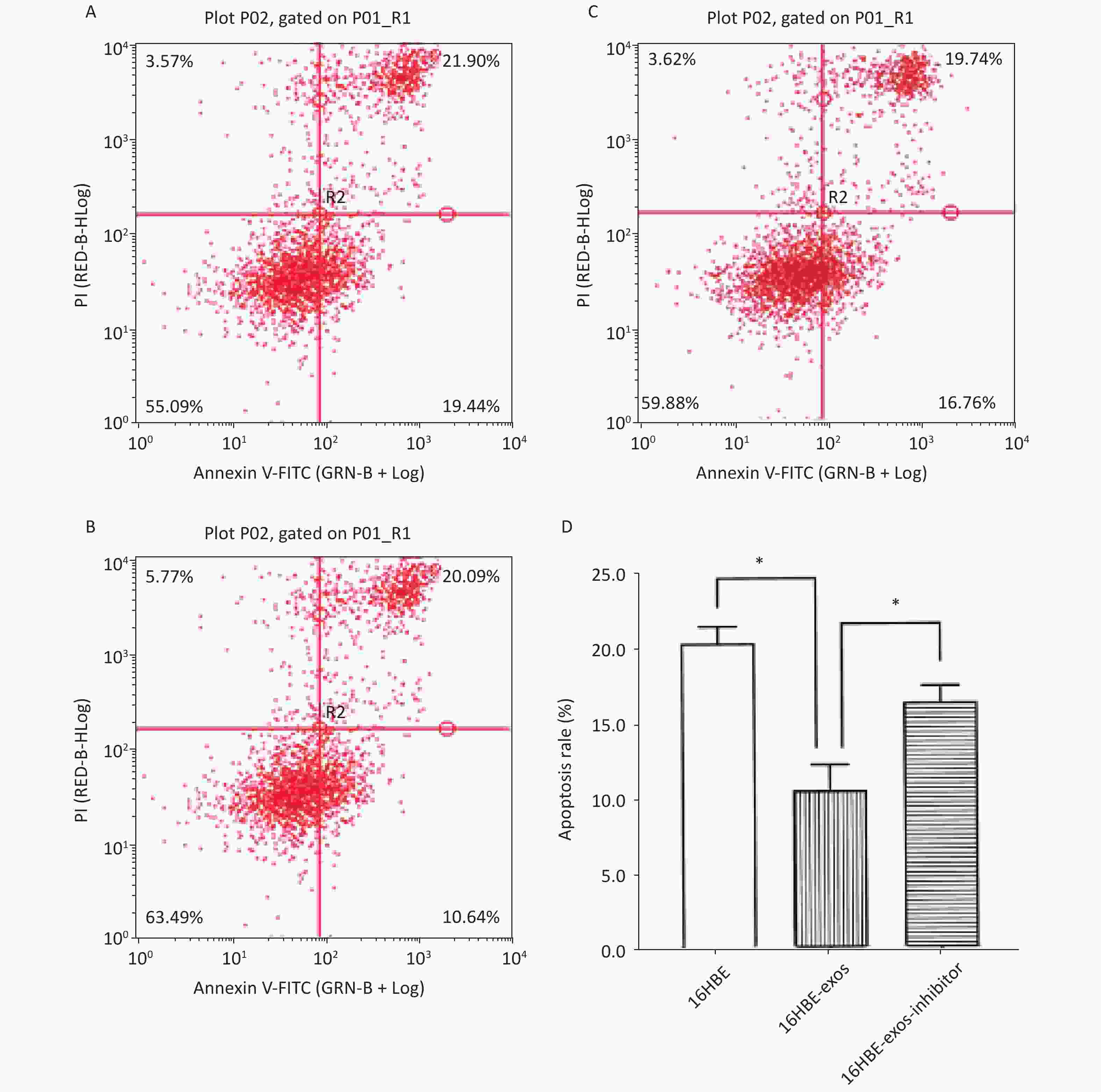
Figure 6. Inhibition effects of exosomal miR-221 on apoptosis induced by HQ in recipient cells. (A) Apoptosis induced by HQ in normal 16HBE cells; (B) Apoptosis induced by HQ in 16HBE-exos; (C) Apoptosis induced by HQ in 16HBE-exos-inhibitor; (D) Apoptosis rate of different groups of cells (* shows P < 0.05).
-
The mechanism of benzene-induced carcinogenesis remains unknown. The toxic effects of benzene on humans body is mainly mediated by its bioactive metabolites, among which HQ is the major representative and has frequently been applied in toxicological investigations of benzene[26-28]. Exosomes are associated with the occurrence and progression of various cancers[12, 13, 29]. This study demonstrated that HQ caused the malignant transformation of normal 16HBE cells. Significantly more miR-221 was expressed in 16HBE-t cells than in normal 16HBE cells, and miR-221 levels significantly enhanced in 16HBE cells after ingesting exosomes derived from 16HBE-t cells. Therefore, intercellular communication mediated by exosomes is involved in the toxic process of benzene. Furthermore, when 16HBE cells ingested the exosmes derived from 16HBE-t cells inhibited HQ-induced cell apoptosis. Apoptosis rates did not significantly change when exosomal miR-221 was inhibited, indicating that exosomal miR-221 reprogrammed the phenotype of the recipient cells, which might be a key event in the carcinogenesis of benzene. However, the tangible mechanism remains to be defined. For example, did exogenous miR-221 from exosomes itself increase the level of miR-221 in recipient cells? Or exogenous miR-221 from exosomes induced increase transcription of endogenous miR-221 in the recipient normal 16HBE cells? Or did both exist? Were there other pathways?
Apoptosis is programmed cell death that usually occurs via several mechanisms in response to internal and external stimuli such as exposure to environmental contaminants and radiation[30, 31]. The main role of apoptosis is to maintain the quantity of normal cells and control that of dysfunctional cells[32, 33]. Apoptosis involves obvious changes in morphological characteristics, including cell shrinkage, cytoplasm condensation, chromatin pyknosis, and nuclear fragmentation, it is considered to be strictly regulated by complex signaling networks [34]. Abnormal apoptosis often triggers pathological processes of various diseases, including tumors[35-37]. However, the tangible mechanisms of apoptosis remain unclear. The present study found that exosomal miR-221 derived from 16HBE-t cells inhibited apoptosis induced by HQ in normal recipient cells, indicating that it plays an important role in the progress of toxicity of benzene. However, the specific signaling pathways involved require further investigation.
Phosphatase and tensin homolog on chromosome 10 (PTEN), a typical tumor suppressor gene, can negatively regulate the PI3K/Akt signaling pathway and act as a molecular switch of the PTEN/PI3K/Akt axis that is involved in cell proliferation, apoptosis, migration, and invasion and plays an important role in carcinogenesis induced by exogenous chemicals[38-41]. Furthermore, PTEN is a direct target for the epigenetic regulation of miR-221[20, 42, 43], and the inactivation and dysfunction of PTEN in somatic cells are common in a variety of cancer patients, such as colon cancer and endometrial cancer, etcs.[31, 44]. The present study revealed that exosomes secreted by 16HBE-t cells inhibited HQ-induced apoptosis in recipient cells via miR-221 transfer. Therefore, we speculate that these biological phenomena mediated by exosomes are associated with the PTEN/PI3K/Akt pathway. Specifically, a novel exosomal-miR-221/PTEN/PI3K/Akt signaling pathway might be involved in the progress of the toxicity of benzene. We plan to follow up this speculation in our next study.
-
Taken together, the present investigation suggests that exosomal miR-221 secreted by 16HBE-t cells can be ingested by normal cells, thus results in inhibition of the apoptosis induced by HQ, which may be an important event in the toxic process of benzene and may be a consequence of human exposure to benzene at levels found in the environment, such as newly decorated houses, gas stations, shoe factories, paint factories and furniture factories, etc.
-
The authors have no conflicts of interest to report.
-
XIAN Hong Yi, CHEN Ying, ZHANG Jia Ying performed experimental study and paper writing; TANG Mei Lin, LIAN Zhen Wei, JIANG Ran, HU Zu Qing, LI Yang Feng performed experimental study, provided important suggestions and revised the paper; HU Da Lin is the corresponding author, who responsible for design, organizing, implementation and supervision of this project.
Exosomes Derived from Hydroquinone-transformed Human Bronchial Epithelial Cells Inhibited Recipient Cell Apoptosis by transferring miR-221
doi: 10.3967/bes2021.072
- Received Date: 2020-08-12
- Accepted Date: 2021-01-08
-
Key words:
- Benzene /
- Toxic mechanism /
- Apoptosis /
- Exosomes /
- miR-221 /
- Intercellular communications
Abstract:
| Citation: | XIAN Hong Yi, CHEN Ying, ZHANG Jia Ying, TANG Mei Lin, LIAN Zhen Wei, JIANG Ran, HU Zu Qing, LI Yan Feng, HU Da Lin. Exosomes Derived from Hydroquinone-transformed Human Bronchial Epithelial Cells Inhibited Recipient Cell Apoptosis by transferring miR-221[J]. Biomedical and Environmental Sciences, 2021, 34(7): 520-527. doi: 10.3967/bes2021.072 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: