-
Ticks function as vectors for a variety of etiological agents of zoonotic diseases, including viruses, bacteria, and protozoa[1]. Some of these tick-borne diseases, such as rickettsiosis, anaplasmosis, and babesiosis, are of substantial concern for both humans and animals all over the world. In China, tick-borne pathogens pose a great threat to residents, especially those in rural areas and forests[1]. However, most of these pathogens are still undetermined because of the limited epidemiological and clinical information about them. In addition, misdiagnosis is very common, as their clinical manifestation is often similar to other syndromes such as hemorrhagic fever with renal syndrome (HFRS)[1]. Importantly, ticks also act as vectors for a variety of pathogens that infect companion animals and livestock[1]. These include bovine anaplasmosis (Anaplasma marginale and Anaplasma bovis), bovine babesiosis (Babesia bigemina and Babesia bovis), canine babesiosis (Babesia canis), canine ehrlichiosis (Ehrlichia canis), caprine anaplasmosis (Anaplasma capra), and many more[2]. These pathogens have significant economic impacts on livestock production and causing great losses each year. On the other hand, infection in these animals also increases the risk of developing tick-borne disease in their owners[1].
Hainan Island, the second largest island of China, is located in the South China Sea. It has a typical tropical climate and plentiful wildlife. It has an area of 33,900 km2 and a population of 9.34 million (2018 estimations). In the recent decades, it has become a tourist attraction because of its beautiful scenery and geographical position. A previous study indicated that Hainan Island is the natural epidemic focus of North Asia tick-borne spotted fever (NASF), which is caused by Rickettsia sibirica belongs to spotted fever-causing group Rickettsiae (SFGR)[3]. Investigation of NASF antibodies in locality, revealed an incidence of 38.3% and 53.0% in local human and mouse, separately[3]. However, there are very few reports on other tick-borne pathogens in Hainan Island. In this study, to evaluate the potential risk of tick-borne pathogens to local residents, tourists, and domestic animals, we analyzed the occurrence and prevalence of bacteria and protozoans in ticks collected from Hainan Island, China.
A total of 285 adult tick samples were collected from 12 stray dogs, 10 goats, and 15 cattle in Fushan town, located in the northwest of Hainan Island in September and October 2019. We chose domestic animals that had extensive contact with humans, and 5–10 ticks were obtained from each animal. Ticks were brought to China CDC (Chinese center for disease control and prevention) alive and stored individually at −80 °C before DNA extraction. All the animal experiments were approved by the ethics committee of the National Institute of Communicable Disease Control and Prevention of the China CDC, under the permit number ICDC: 2020-018.
Before DNA extraction, the ticks were washed with 75% ethyl alcohol and 0.01 mol/L phosphate buffer solution (PBS) followed to be dried. They were divided into pools (3 ticks each) and homogenized in PBS. Genomic DNA was extracted using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the instructions of the manufacturer. All DNA extracts were stored at −20 °C.
These ticks were identified by morphological observation and sequence analysis of their cytochrome oxidase subunit 1 (COI) gene, and they were assigned to two tick species, Rhipicephalus sanguineus sensu lato (183, 64.21%) and Rhipicephalus microplus (102, 35.79%). The taxonomical keys used for tick identification mainly included the shape of basis capitulum, scutum, palp, coxae, eyes, anal groove, festoons and adanal plates, spiracle, and hypostomal teeth[4]. All the 132 ticks from dogs and 51 ticks from goats were R. sanguineus sensu lato. They were divided into 44 pools and 17 pools, respectively, with 3 ticks for each pool. The 102 ticks from cattle were all R. microplus and they were divided into 34 pools (Supplementary Table S1, available in www.besjournal.com).
Species Dogs Goats Cattle Total R. sanguineus sensu lato 6/44/132a 3/17/51 0/0/0 9/61/183 R. microplus 0/0/0 0/0/0 5/34/102 5/34/102 Total 6/44/132 3/17/51 5/34/102 14/95/285 Note. a positive pool/total pool/total tick. Table S1. Detection of tick-borne pathogens from domestic animals in Hainan island, China, 2019
All the tick samples were tested for the presence of Rickettsiales bacteria, Coxiellaceae bacteria, and parasites belonging to the families Babesiidae and Hepatozoidae. PCR targeting the 18S rRNA gene for the detection of Babesiidae and Hepatozoidae species was performed as described previously[5]. The rrs gene of Rickettsiales bacteria and Coxiellaceae bacteria species were amplified by nested PCR using primers as described[6,7]. For further exploration of the phylogenetic positioning of the bacterial strains, the 760 bp fragment of the gltA gene encoding citrate synthase and the 614 bp fragment of the groEL gene encoding the heat shock protein, were amplified for samples that were positive for these bacteria. Both negative and positive controls were used.
PCR amplicons were analyzed by electrophoresis in 1.0% agarose gels. The amplicons shorter than 600 bp were directly subjected to Sanger sequencing by Sangon Biotechnology Company (Shanghai, China). The PCR products longer than 600 bp were cloned into the pMD19-T cloning vector (TaKaRa, China), transformed into E. coli and plated onto a culture dish. Then the obtained clones were picked and sent for bi-directional sequencing.
Nucleotide sequence similarities between the obtained 16S/18S rRNA, groEL, and gltA sequences and those from GenBank were calculated by DNAStar (ver. 7.0). For phylogenetic analysis, the sequences were aligned with reference sequences using ClustalW (default parameters) within MEGA 5.2[6,8]. Phylogenetic trees were then estimated using the Maximum Likelihood (ML) method implemented in PhyML, version 3[6]. A total of 1,000 bootstrap replicates were used under the same procedure to estimate the support for each node. All trees were mid-point rooted.
The present study revealed that several species of ticks parasitize many wild animals and livestock in Hainan Island, China. We mainly found R. sanguineus sensu lato and R. microplus in Chengmai. Many kinds of tick species can be found around Hainan Island including Ixodes granulatus, R. sanguineus, R. microplus, Haemaphysalis longicornis, R. haemaphysaloides haemaphysaloides, Dermacentor auratus, Amblyomma javanense, A. testudinarium, H. lagrangei, H. hystricis, H. yeni, and H. doenitzi, and the main species are Ixodes granulatus, R. sanguineus, R. microplus, and R. haemaphysaloides haemaphysaloides in Chengmai County. In the current study we evaluated the presence of several pathogens like Rickettsiales bacteria, Coxiellaceae bacteria, Babesiidae, and Hepatozoidae in these two ticks. The results revealed the presence of four recognized species and two Candidatus spp. of Anaplasmataceae and Coxiellaceae. Importantly, A. marginale, A. platys, H. canis, B. canis, and Coxiella burnetii, which were identified in this study, are known to be animal and/or human pathogens[1]. Hence, our data clearly indicated that multiple bacteria and protozoa co-circulate in hard ticks in Hainan Island. As more tick species are present in Hainan Island, it is likely that more tick-associated pathogens are to be discovered.
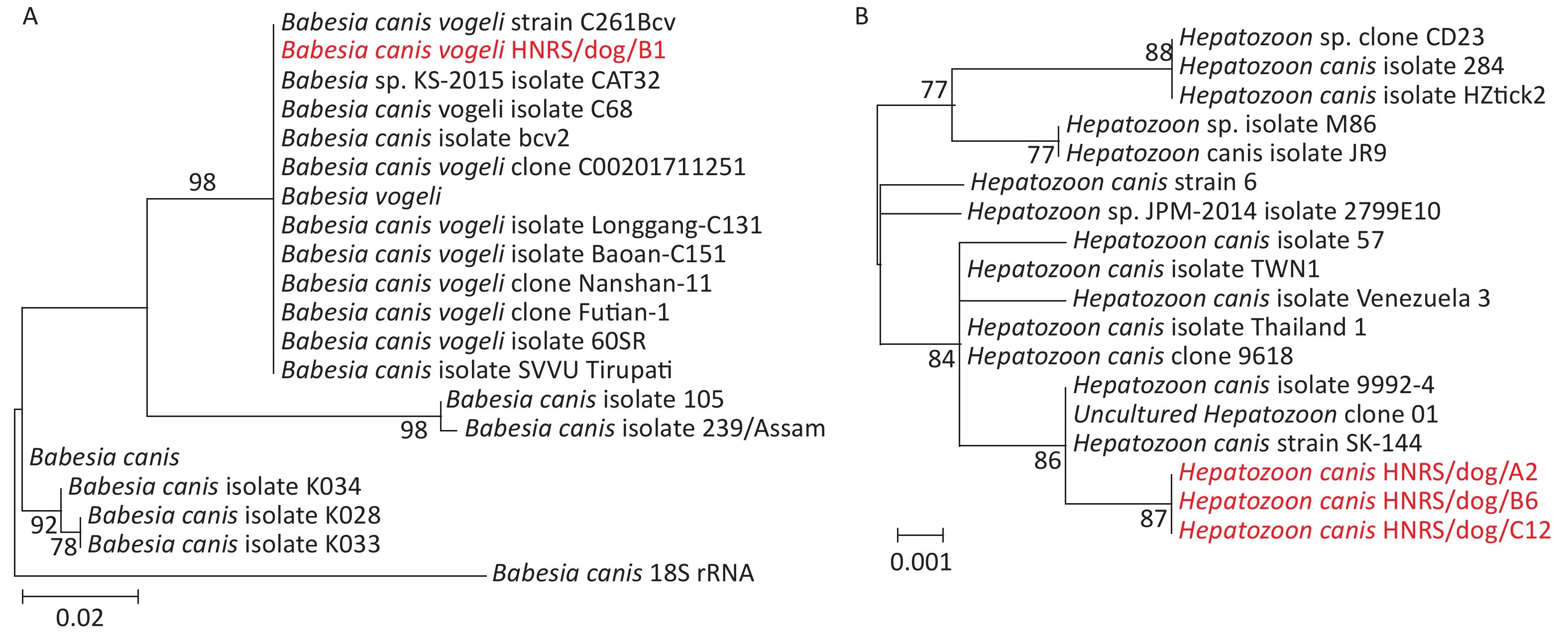
Genetic analysis of the recovered 18S rRNA gene sequences using the BlastN with Nucleotide collection (nr/nt) revealed that they were most closely related to those of Babesia canis vogeli (100.00%) and Hepatozoon canis strain SK-144 (99.77%) (Table 1). Phylogenetic analysis of 18S RNA gene sequences revealed the co-circulation of B. canis and H. canis in the 1 and 5 R. sanguineus sensu lato pools from dogs, respectively (Table 2, Supplementary Figure S1, available in www.besjournal.com). The sequences of B. canis vogeli HNRS/dog/B1 were closely related to those of the known B. canis vogeli found in R. sanguineus sensu lato from domestic and foreign[2,6] in the 18S RNA tree (Supplementary Figure S1A). Additionally, the sequences H. canis HNRS/dog/A2, H. canis HNRS/dog/B6, and H. canis HNRS/dog/C12 were closely related to each other, and they were also related to H. canis strain SK-144 and H. canis strain 9992-4[9] in Canis lupus specimens from Israel, Saint Kitts, and Nevis, forming a distinct lineage in the 18S RNA tree (Supplementary Figure S1B). Canine hepatozoonosis is a tick-borne disease distributed worldwide triggered by H. canis in dogs[9]. Canine babesiosis is a vector-borne disease caused by Babesia spp. like B. canis vogeli[9]. Our data revealed that these two agents are co-circulating in arthropods, and the infection risk is high for dogs, as well as other animals in certain regions.
Strains Genes (nt), n (%) Bacteria or protozoon rrs or 18s rRNA groEL gltA R. sanguineus sensu lato Dogs HNRS/dog/B1 368 (100.00)a −b − Babesia canis vogeli HNRS/dog/A2/B6/C12 436 (99.77) − − Hepatozoon canis strain SK-144 HNRS/dog/C6/C23 783 (99.87) 925 (99.89) 809 (99.86) Anaplasma platys strain S3 Goats HNRS/goat/Y5/Y9/Y10 1,347 (100.00) − − Coxiellaceae bacterium PH06 R. microplus Cattle HNRM/cattle/D6 782 (100.00) − − Anaplasma marginale str. Dawn HNRM/cattle/D26 782 (100.00) − 845 (99.76) Anaplasma marginale str. Dawn HNRM/cattle/D29 782 (100.00) 894 (100.00) 845 (99.76) Anaplasma marginale str. Dawn HNRM/cattle/E8/E24 781 (100.00) 971 (99.88) 848 (99.28) Ehrlichia sp. strain WHBMXZ-40 Note. aThe length of the sequence amplified from the samples (nucleotide sequence identity compared to the reference sequences from GenBank). b “−”, not available. Table 1. Bacterial and protozoal sequences obtained from ticks in Hainan island, China
Species of tick-borne pathogens R. sanguineus sensu lato R. microplus Anaplasmataceae Anaplasma marginale 0/0/0 4/34/102 Anaplasma platys 5/61/183a 0/0/0 Ehrlichia sp. 0/0/0 2/34/102 Coxiellaceae Coxiella-like bacteria 15/61/183 0/0/0 Babesiidae Babesia canis 1/61/183 0/0/0 Hepatozoidae Hepatozoon canis 5/61/183 0/0/0 Total 26/61/183 6/34/102 Note. aPositive pool/total pool/total tick. Table 2. Prevalence of tick-borne pathogens in ticks in Hainan Island, China
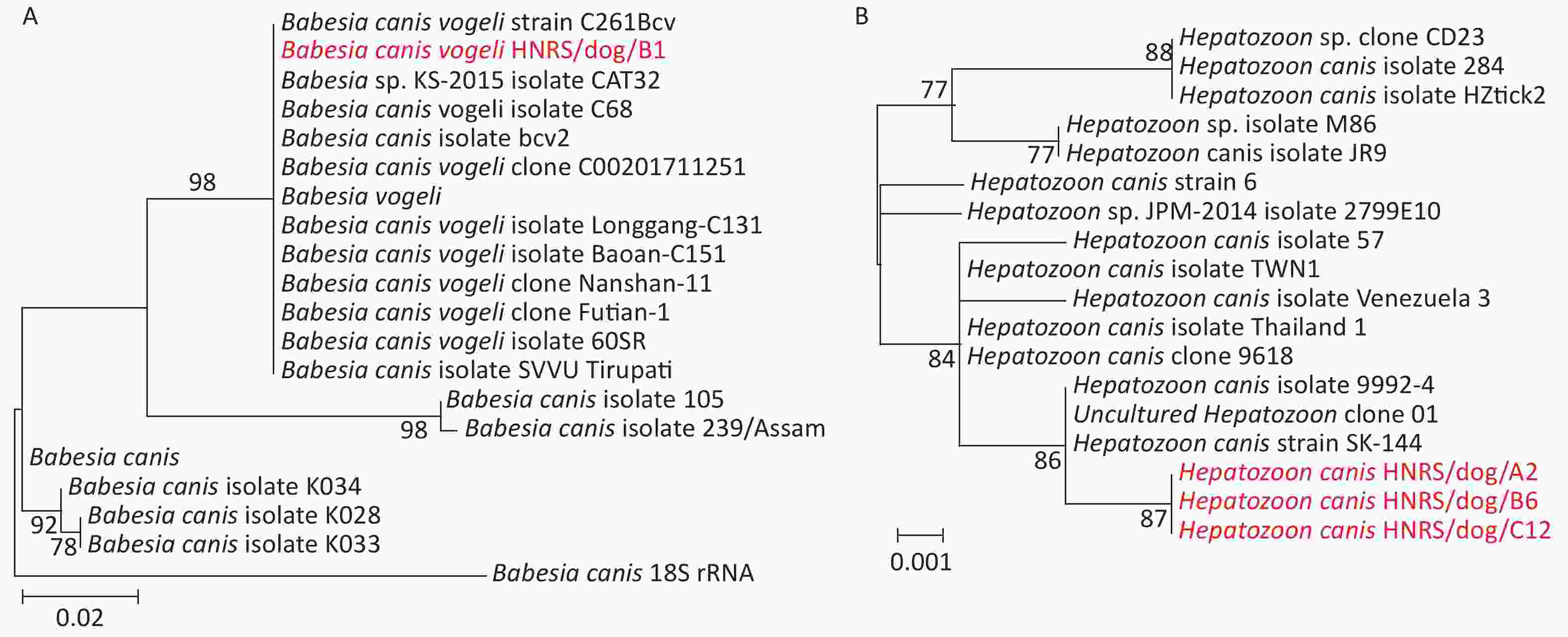
Figure S1. Phylogenetic trees based on the partial 18S rRNA gene sequences of Babesia canis (A) and Hepatozoon canis (B). Both trees are mid-point rooted for clarity only. Bootstrap values (> 70%) are shown for appropriate nodes. The scale bar represents number of nucleotide substitutions per site. The sequences revealed in the present study are marked in red.
Genetic analysis of the rrs, groEL, and gltA gene sequences from the bacterial pathogens revealed that they were closely related to those of Coxiellaceae and Anaplasmataceae bacteria. Briefly, the rrs sequences recovered from the tick pools sampled from Hainan Island exhibited high sequence similarities to those from species of Coxiellaceae bacterium (100%), A. marginale (100%), A. platys (99.87%), and Ehrlichia sp. (100%) (Table 1). The similarities between the sequences recovered from this study and known reference sequences from GenBank varied from 99.28% to 99.86% for the gltA gene sequences, and from 99.88% to 100.0% for the groEL gene sequences (Table 1). Hence, these data revealed the co-circulation of Ehrlichia, Anaplasma, and the proposed Candidatus Coxiellaceae bacterium in ticks collected from Hainan Island (Figure 1, Table 1, Supplementary Figure S2, available in www.besjournal.com).
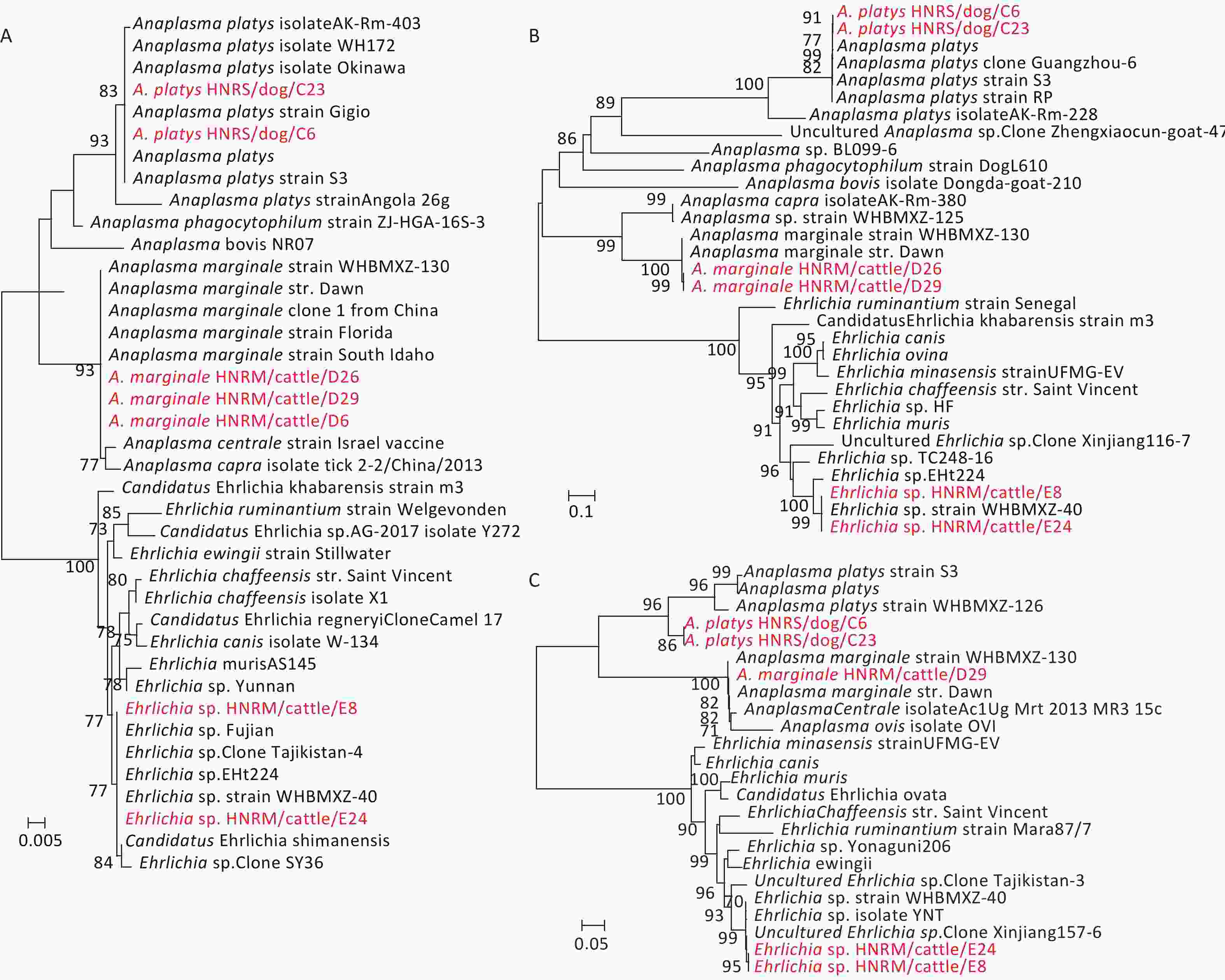
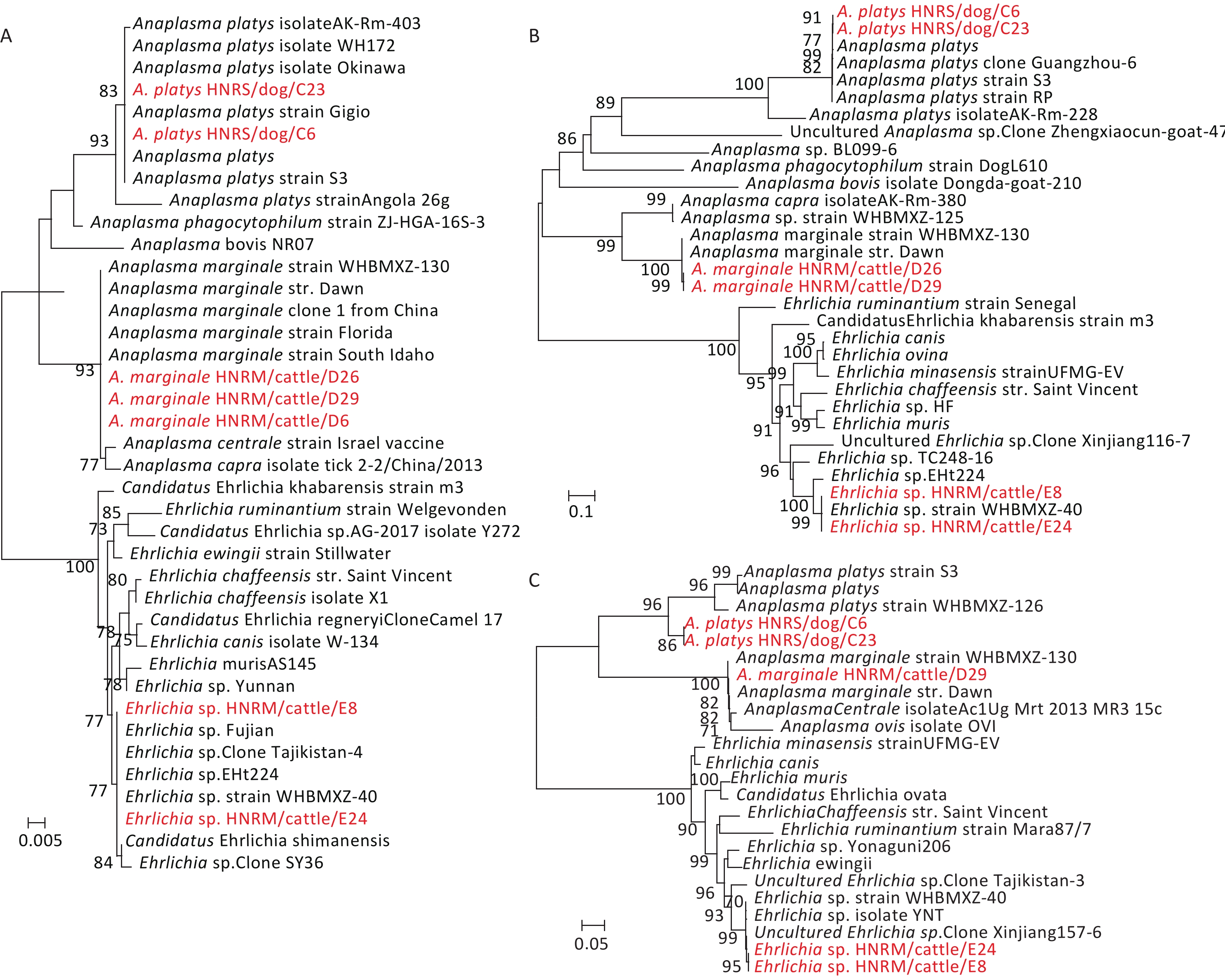
Figure 1. Phylogenetic trees based on partial Anaplasmataceae rrs (A), gltA (B), and groEL (C) gene sequences. Trees were mid-point rooted for clarity only. Bootstrap values (> 70%) are shown for appropriate nodes. The scale bars represent number of nucleotide substitutions per site. The sequences revealed in the present study are marked in red.
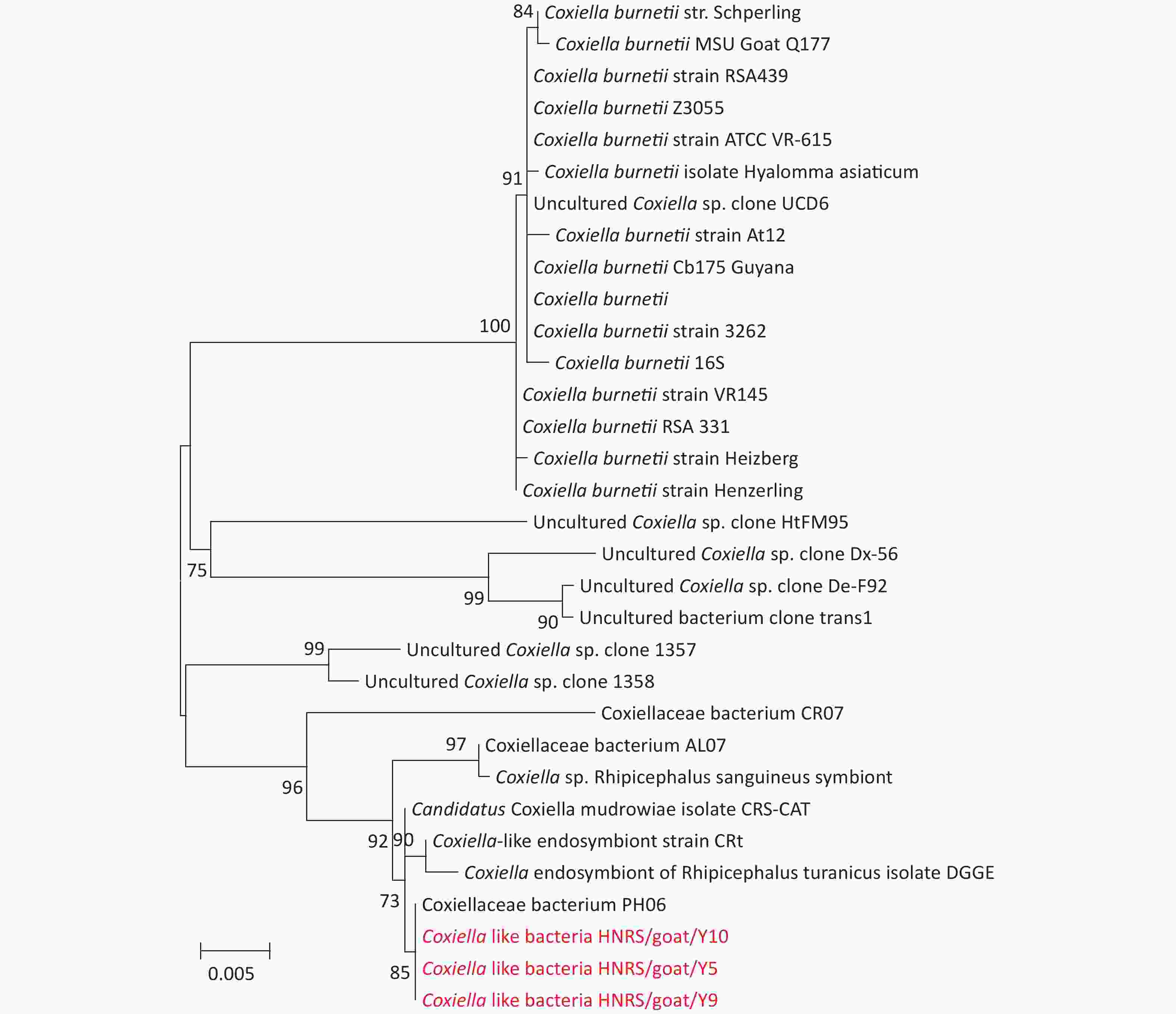
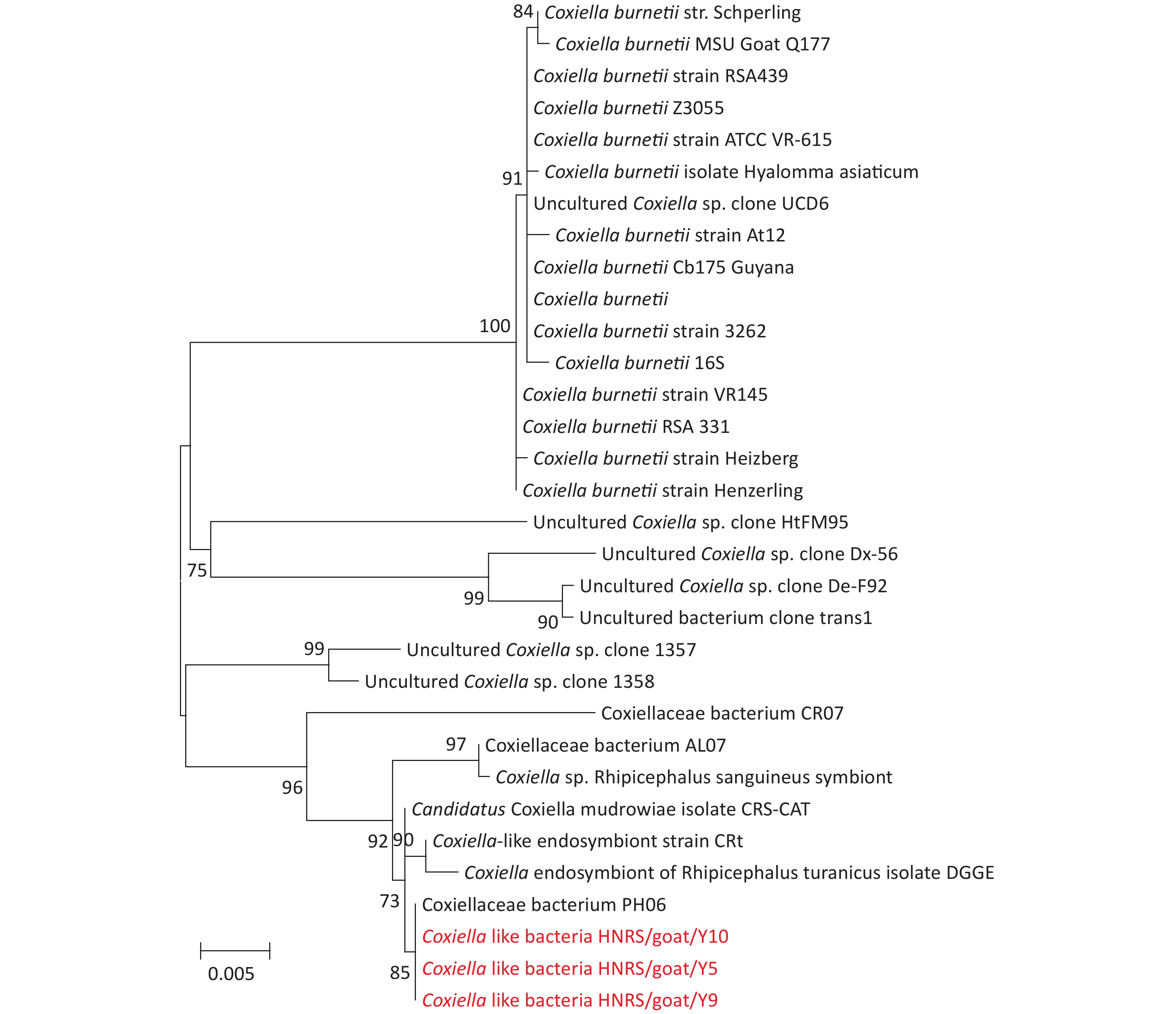
Figure S2. Phylogenetic trees based on partial Coxiellaceae bacteria rrs gene sequences. Trees are mid-point rooted for clarity only. Bootstrap values (> 70%) are shown for appropriate nodes. The scale bars represent number of nucleotide substitutions per site. The sequences revealed in the present study are marked in red.
Anaplasmataceae bacteria were identified in 5 R. sanguineus sensu lato pools, and 6 R. microplus pools (Table 2). Phylogenetic analysis of the sequences of rrs, gltA, and groEL genes revealed the circulation of three species of Anaplasmataceae bacteria in the ticks from Hainan Island. In the rrs, gltA, and groEL gene trees (Figure 1), the sequences of A. marginale HNRM/cattle/D6, A. marginale HNRM/cattle/D26, and A. marginale HNRM/cattle/D29, were closely related to those of the known A. marginale found in ticks from cattle[6]. Notably, A. platys HNRS/dog/C6 and A. platys HNRS/dog/C23 clustered with those of A. platys discovered in ticks, dogs, and camels from China, Portugal, Italy, and Japan[6] in all three gene trees (Figure 1). Finally, the sequence Ehrlichia sp. HNRM/cattle/E8 and Ehrlichia sp. HNRM/cattle/E24 recovered from two R. microplus tick pools exhibited a close relationship to those of the candidatus Ehrlichia sp. identified in ticks from China (Fujian, Wuhan, Xinjiang, and Shenyang provinces), Niger, and Thailand[6] (Figure 1).
Many members in the family Anaplasmataceae cause tick-borne diseases (i.e. Anaplasmosis and Ehrlichiosis) with a remarkable impact on human and animal health[2,10]. For example, A. phagocytophilum, A. marginale, and A. platys are the important disease-producing pathogens in the genus Anaplasma[2]. A. marginale is a common pathogen among ruminants infecting buffalo and cattle distributed on six continents, especially in tropical and subtropical regions[10]. In contrast, A. platys causes cyclic thrombocytopenia in dogs and is the only classified Rickettsiales species known to infect platelets[2]. Furthermore, A. platys infection has been reported in cats, foxes (Vulpes vulpes), cattle, goats, camels, red deer, and humans[2]. In our study, A. marginale and A. platys were identified in R. microplus and R. sanguineus sensu lato ticks, respectively. The cattle, which are the major hosts of R. microplus, showed the high prevalence of A. marginale. Therefore, these results suggest that more cattle surveillance in this area is necessary. R. sanguineus sensu lato is considered the primary vector of A. platys. Due to the close exposure between the dogs and others animals, the high infection rate of A. platys in local dog ticks indicated high risk of cyclic thrombocytopenia affecting animals and humans. Therefore, more surveillance and research on dogs and its pathogens should be carried out in Hainan Province.
In the past decades, several novel Ehrlichia species have been discovered in ticks and vertebrate hosts[9]. Herein, a tentative species was identified in R. microplus ticks from cattle, which is mainly found in R. microplus ticks from several provinces and countries. The genome sequence of this candidatus species is close to those of E. chaffeensis, a widespread human pathogen. Therefore, more attention should be paid to its pathogenicity and potential public health risk.
Coxiella-like bacterial DNA was found in 15 R. sanguineus sensu lato tick pools (Table 2). In the rrs gene tree (Supplementary Figure S2), three positive samples (Coxiella-like bacteria HNRS/goat/Y5, Coxiella-like bacteria HNRS/goat/Y9, and Coxiella-like bacteria HNRS/goat/Y10) were analyzed, which were 100% similar to Coxiellaceae bacterium PH06 (KM079622), found in Pediculus humanus in Marseille, France in 2014. In this study, the positive sequences were clustered with those of Coxiellaceae bacterium discovered in R. sanguineus sensu lato and R. bursa ticks in Marseille, France, and Israel. Coxiella burnetii is the causative agent of Q fever, which is a worldwide zoonotic disease. Normally, the infection is persistent in animals, whereas humans are often asymptomatic. It can manifest as a flu-like illness or pneumonia in its acute form, or in a chronic form like endocarditis[7]. There are no typical symptoms when infection occurs in animals and humans except during pregnancy. The primary reservoirs of C. burnetii are goats, sheep, and cattle[7]. In nature, ticks play an important part in the maintenance and transmission of the bacteria, whereas cattle and goats play a very important role in human infections. In this study, Coxiella-like bacteria were identified in 15 R. sanguineus sensu lato ticks sampled from Hainan Island. More attention should be paid on this pathogen due to its close relationship with the known human pathogen Coxiella burnetii.
In conclusion, two species (R. microplus and R. sanguineus sensu lato) of hard ticks were sampled from dogs, cattle, and goats and four recognized species and two Candidatus spp. of Anaplasmataceae and Coxiellaceae were discovered from these two hard ticks, indicating that this specific geographic region harbors a considerable diversity of Anaplasmataceae bacteria, Coxiellaceae bacteria, Babesiidae, and Hepatozoidae. The data from this study highlight the necessity for the surveillance of local arthropods, mammals, and humans, which may provide the evidence of the presence of several bacterial and protozoan pathogens.
Conflicts of Interest The authors declare no competing interests.
Author Contribution LI Kun designed the research and supervised the experiments; TANG Guang Peng, BAI Xiao Song, LI Kun, and QIN Xin Cheng collected the samples and performed the experiments; LI Kun, LU Miao, and WANG Wen analyzed the data; LI Kun, LU Miao, and GUO Wen Ping wrote the manuscript.
Molecular Detection of Tick-borne Pathogens in Ticks Collected from Hainan Island, China
doi: 10.3967/bes2021.081
- Received Date: 2020-11-30
- Accepted Date: 2021-02-10
-
Key words:
- Ticks /
- Rickettsiales bacteria /
- Protozoa /
- Coxiellaceae bacteria /
- Tick-borne disease /
- China
Abstract: Pathogens like bacteria and protozoa, which affect human and animal health worldwide, can be transmitted by vectors like ticks. To investigate the epidemiology and genetic diversity of bacteria and protozoans carried by ticks in Chengmai county of Hainan province, China, 285 adult hard ticks belonging to two species [Rhipicephalus sanguineus (sensu lato): 183, 64.21% and Rhipicephalus microplus: 102, 35.79%] from dogs, cattle, and goats were collected. Microbial families were identified in these ticks by amplifying the 18S rRNA, 16S rRNA (rrs), citrate synthase (gltA), and heat shock protein (groEL) genes. Our data revealed the presence of four recognized species and two Candidatus spp. of Anaplasmataceae and Coxiellaceae. In sum, these data reveal an extensive diversity of Anaplasmataceae bacteria, Coxiellaceae bacteria, Babesiidae, and Hepatozoidae in ticks from Hainan Island, highlighting the need to understand the tick-borne pathogen infection in local animals and humans.
| Citation: | LU Miao, TANG Guang Peng, BAI Xiao Song, QIN Xin Cheng, WANG Wen, GUO Wen Ping, LI Kun. Molecular Detection of Tick-borne Pathogens in Ticks Collected from Hainan Island, China[J]. Biomedical and Environmental Sciences, 2021, 34(7): 581-586. doi: 10.3967/bes2021.081 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: