-
Cooking oil fumes (COF) are one of the most common air pollutants in indoor living environments in China. When oil is heated above 250 °C, at least 220 types of products are produced in the lampblack, including carcinogenic heterocyclic amines and B[α]P[1]. These agents can lead to the occurrence and development of cancer[2]. Previous studies have reported that exposure of lung epithelial cells to COF increases lipid peroxidation and oxidative stress[3]. A meta-analysis has indicated that COF exposure, notably in the absence of a fume extractor, may increase the risk of lung cancer among Chinese nonsmoking women[4]. Previous experiments have also shown that COF-derived components induce oxidative stress, apoptosis and inflammation in A549 cells and BEAS-2B cells, whereas this effect is prevented by vitamin C and N-acetylcysteine[5]. The antioxidant effects of vitamin C have been demonstrated in several in vitro models. The aim of the present study was to investigate the adverse effects of COF in lung tissues and the protective effects of vitamin C in mice after sub-acute exposure to different doses of COF, to provide chronic toxicity testing data and a basis for further mechanistic studies. Collectively, this information could be used as a reference for the prevention and treatment of lung injury caused by COF.
Cooking fume condensate (10 mL) was obtained from waste oil boxes from the kitchens of five urban families and used to prepare a mixed COF sample solution. Suspensions with low, moderate or high concentrations were prepared (Supplementary Table S1 available in www.besjournal.com). Before use, the COF suspension was sonicated with an ultrasonic cleaner (kq-500dv) at low temperature for approximately 10 minutes to ensure the uniformity of the mixture. Then, 30 μL COF of sample solution per mouse was administered via tracheal instillation with a lung liquid quantitative nebulizer (hrh-mag4, Beijing huironghe Technology Co., Ltd.). The concentration of vitamin C was 10 mg/mL, and the intraperitoneal injection was 200 mg/(kg·bw) per day.
COF groups Cooking oil fume (mL) Ethanol (mL) Distilled water (mL) Crude oil (%) Ethanol (%) Low Cof group 1 2 7 10 20 Mid Cof group 4 2 4 40 20 High Cof group 8 2 0 80 20 Table S1. Preparation of cooking oil fume sample
Specific pathogen Free (SPF) male mice weighing 25–30 g were purchased from the Qingdao Animal Experimental Center [license number: scxk (Lu) 20140007]. A total of 70 male mice were divided randomly into the following seven groups: control, ethanol, vitamin C, low dose COF (low COF), moderate dose COF (mid COF), high dose COF (high COF) and vitamin C + high dose COF (vit C + high COF). In the control group, a certain amount [25 mL/(kg·bw) per day] of normal saline was injected intraperitoneally, and 30 μL of sterilized normal saline was administered through tracheal instillation with a lung liquid quantitative nebulizer. In the ethanol group, a certain amount of normal saline was injected intraperitoneally, and 30 μL 20% ethanol solution was administered via tracheal instillation with a lung liquid quantitative nebulizer. In the vitamin C group, a certain amount of vitamin C was injected intraperitoneally, and 30 μL sterilized normal saline was administered by tracheal instillation with a lung liquid quantitative nebulizer. The low, moderate and high dose COF groups received intraperitoneal injection of saline and administration of 30 μL of 10%, 40%, and 80% COF suspension via tracheal instillation with a lung liquid quantitative nebulizer. In the vit C + high-dose COF group, a certain amount of vitamin C was injected intraperitoneally, and 30 μL 80% COF suspension was administered via tracheal instillation with a lung liquid quantitative nebulizer at 8:00 am every day.
After continuous treatment for 30 days, the mice were fasted for 12 h, weighed and sacrificed by cervical vertebral dislocation. The trachea was exposed quickly, and an oblique opening was cut. A lung lavage needle with a polyethylene tube was inserted, ligated and fixed, 0.6 mL normal saline was slowly injected, and the lavage solution was removed. Lavage was performed three or four times for each mouse, the total amount of lavage fluid was measured, and the fluid was stored separately for subsequent use. The lung tissue without lavage was excised, and an appropriate amount was fixed in 4% paraformaldehyde solution for 24 hours. After regular paraffin embedding and sectioning (thickness of approximately 1–2 μm), HE staining was used to observe the morphological characteristics of lung tissue under a light microscope (Olympus Co., Ltd., Tokyo, Japan). Lung tissue homogenate was prepared and centrifuged at low temperature for 10 min. Part of the supernatant was used for protein quantification and detection of select indicators, including superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), reduced glutathione (GSH), myeloperoxidase (MPO) and malondialdehyde (MDA), which were detected with specific kits (Nanjing Jiancheng Biotechnology Co., Ltd.). The levels of TNF-α, IL-6, caspase 3 and caspase 8 were detected with ELISA (Shanghai Beyotime Biotechnology Co., Ltd.). The remainder of the samples were stored in a refrigerator at –80 ℃. After centrifugation (Sigma, Germany) of the bronchoalveolar lavage fluid (BALF) samples, the cells were resuspended in 1 mL PBS buffer. A total of 10 μL cell suspension was obtained, the cells were counted with a hemocytometer, and the total number of cells was calculated. Total protein in the BALF was determined with a BCA protein quantitative kit. Levels of LDH and AKP were detected with LDH and AKP test kits (FC enzyme label instrument, Thermo Fisher Instrument Co., Ltd.).
Reactive oxygen species (ROS) were detected with DCFH-DA (Nanjing Kai ji biological Co., Ltd.). The cells in the BALF were collected and suspended in diluted DCFH-DA (10 μmol/L) at a concentration of 106/mL, then incubated at 37 ℃ for 30 min. The centrifugation tube was mixed by inversion every 10 min to ensure that the probe fully contacted the cells. Finally, the cells were washed with PBS three times and resuspended. ROS levels were immediately detected with flow cytometry (BD Accuri C6, USA, 488 nm excitation wavelength, 525 nm emission wavelength).
The experimental data are presented as the mean ± standard deviation (SD). Statistical analysis was performed with SNK-q tests, with a significance threshold of *P < 0.05 compared to the control group and #P < 0.05 compared to the high COF group.
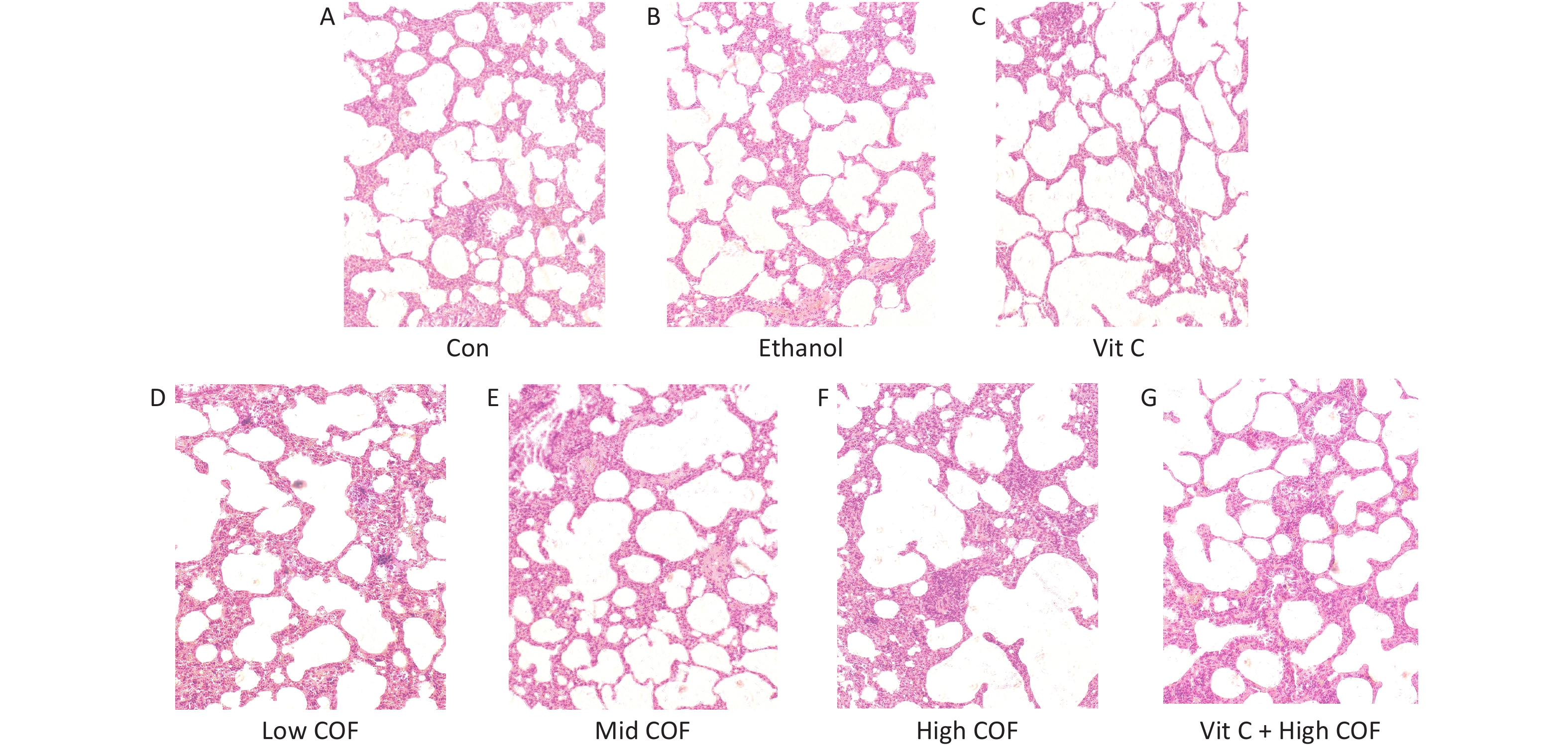
The pathological characteristics of lung tissue were normal; the size and connections among alveoli were normal; and we observed no abnormalities in the alveolar cavity and no inflammatory cells in the lung interstitium in the control group, ethanol group and vitamin C group (Figure 1). In the low COF group, no clear abnormalities in lung tissue were observed, the alveolar connections were essentially normal, a small amount of red blood cell exudate was observed in the alveolar cavity, and no inflammatory cells were found in the lung interstitium. In the mid COF group, we observed lung tissue injury, alveolar junction rupture, increased red blood cells in the alveolar cavity and interstitium, a widened interstitium, a small amount of edema fluid in the alveolar cavity, and accumulation and infiltration of inflammatory cells. In the mice exposed to high COF, the lung tissue was severely damaged, many alveolar junctions were broken, red blood cell exudate was observed in the alveolar cavity and pulmonary interstitium, the pulmonary interstitium was significantly widened, edema was significant, and many inflammatory cells had accumulated and infiltrated. Vitamin C pretreatment improved the lung pathological changes caused by high-dose COF, the alveolar connections were not broken, the red blood cell exudate in the alveolar cavity decreased, the lung interstitial width was reduced, and the inflammatory cell infiltration was not clear, which compared with high-dose COF.
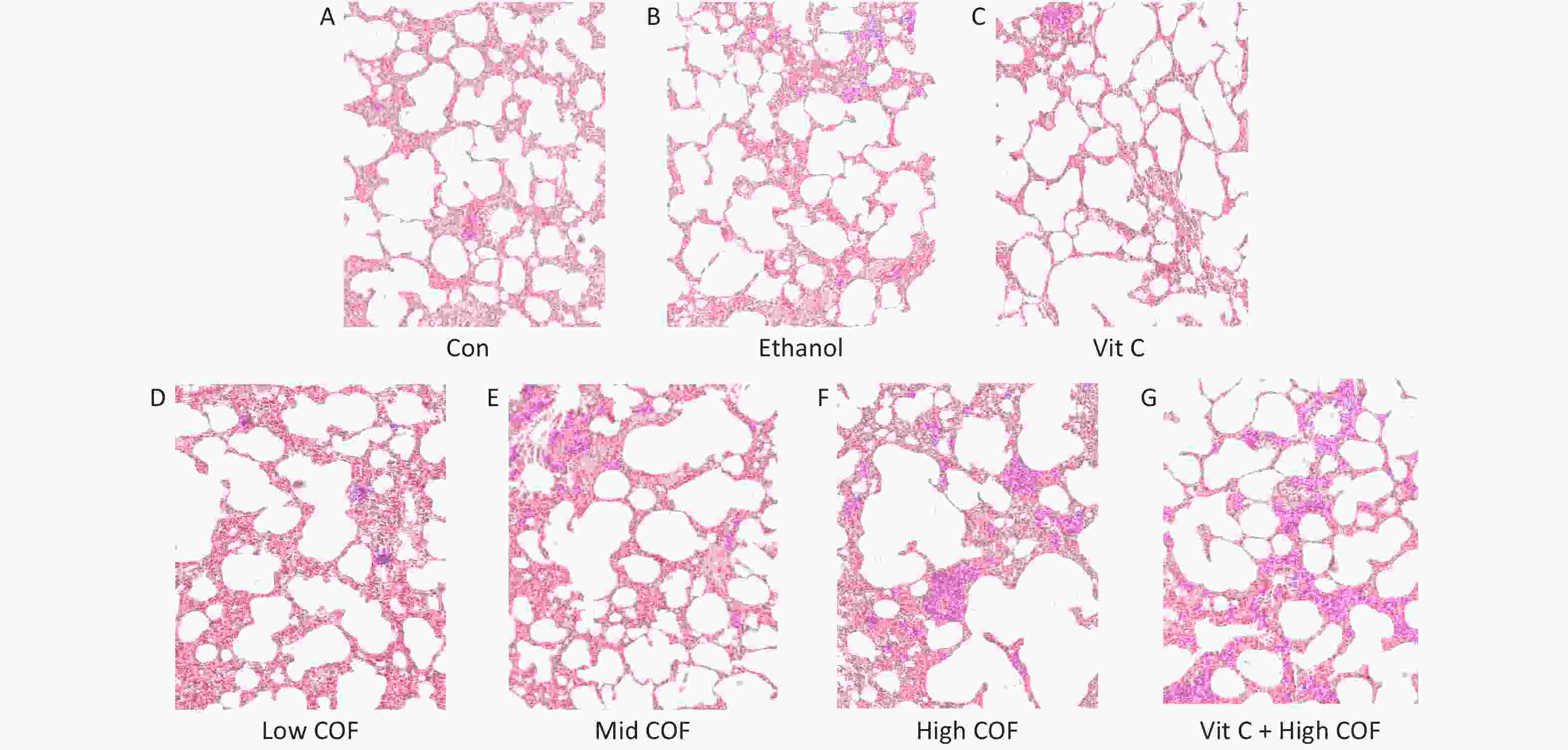
Figure 1. Pathological changes of lung tissue in mice (10 ×). (A, B, D–F) A certain amount of normal saline was injected intraperitoneally and then 30 μL sterilized normal saline, 30 μL 20% ethanol solution, 30 μL 10%, 40%, 80% cooking oil fumes (COF) suspension was provided by a tracheal instillation by lung liquid quantitative nebulizer respectively. (C, G) A certain amount of vitamin C was injected intraperitoneally and then 30 μL sterilized normal saline, 30 μL 80% COF suspension was provided by a tracheal instillation by lung liquid quantitative nebulizer respectively
Oxidative stress is a major mechanism in COF-induced lung injury[3]. A previous study has demonstrated that COF decrease epithelial cell viability, increase MDA levels, and decrease SOD and GSH activity[6]. The level of MPO reflect the number and activity of neutrophils. Vitamin C is a water-soluble and antioxidant vitamin that can decrease level of ROS[6] in cells, and "clear" pathological or physiological-related free radicals and ROS. Our findings suggested that the levels of ROS in BALF samples and MPO in the lung tissue in mice exposed to COF at moderate and high doses were significantly elevated (P < 0.05). The levels of SOD, GSH-Px and GSH in the high-dose COF group were significantly lower than those in the control group (P < 0.05). The levels of MDA in each dose of COF group were significantly higher than these in the control group (P < 0.05). All these indicated that COF exposures induced lung oxidative stress. However, vitamin C pretreatment significantly decreased the levels of ROS, MDA and MPO, increased the levels of SOD, GSH-Px (P < 0.05), as shown in Table 1.
Group SOD (U/mg) GSH-Px (U/mg) GSH (μmol/mg) MPO (U/mg) MDA (nmol/mg) ROS level (fold of control) Control 68.6 ± 3.34 60.1 ± 3.33 2.06 ± 0.16 4.96 ± 0.25 1.09 ± 0.06 1.00 ± 0.04 Ethanol 65.3 ± 3.57 59.3 ± 3.65 2.03 ± 0.63 5.01 ± 0.23 1.12 ± 0.09 1.02 ± 0.06 Vit C 67.5 ± 3.52 62.1 ± 4.01 2.10 ± 0.23 4.89 ± 0.26 1.10 ± 0.07 1.01 ± 0.04 Low COF 51.6 ± 2.33 50.4 ± 3.16 2.01 ± 0.11 8.99 ± 0.49 3.01 ± 0.16* 1.26 ± 0.10 Mid COF 42.3 ± 2.12 44.6 ± 2.41 1.74 ± 0.25 11.69 ± 0.57* 5.19 ± 0.27* 1.68 ± 0.16* High COF 35.6 ± 1.79* 31.4 ± 1.99* 1.32 ± 0.06* 16.79 ± 0.83* 6.03 ± 0.31* 1.88 ± 0.17* Vit C + High COF 62.6 ± 3.19# 54.3 ± 2.87# 1.91 ± 0.89 5.36 ± 0.27# 2.02 ± 0.11# 1.16 ± 0.11# Note. *P < 0.05, compared with the control group, #P < 0.05, compared with the high COF group. COF: cooking oil fumes; SOD: superoxide dismutase; GSH-Px: glutathione peroxidase; GSH: reduced glutathione; MPO: myeloperoxidase; MDA: malondialdehyde; ROS: reactive oxygen species. Table 1. Changes in SOD, GSH-Px, GSH, MPO and MDA in lung tissues
$(\overline X \pm {\rm{SD}})$ (n = 10)As shown in Table 2, the levels of LDH and AKP in the BALF in mice significantly increased after exposure to moderate and high doses of COF (P < 0.05), whereas vitamin C pretreatment significantly decreased their levels (P < 0.05). LDH is a cytoplasmic enzyme that is released after cell membrane damage or cell death and dissolution. AKP is mainly produced by alveolar type II cells and is also produced by neutrophils. Its activity reflects the degree of lung injury and neutrophil infiltration[7]. The levels of TNF-α and IL-6 in the lung tissues of mice exposed to moderate or high doses of COF were significantly higher (P < 0.05) than those in the control group (P < 0.05). Vitamin C pretreatment significantly decreased the level of IL-6 (P < 0.05), in agreement with previous data[3], whereas a nonsignificant decrease was observed for TNF-α.
Group LDH (U/L) AKP (ng/mL) TNF-α (pg/mg) IL-6 (pg/mg) Control 206 ± 9.65 2.99 ± 0.16 0.79 ± 0.041 0.65 ± 0.033 Ethanol 209 ± 8.32 2.96 ± 0.17 0.76 ± 0.039 0.66 ± 0.035 Vit C 200 ± 10.01 2.89 ± 0.14 0.78 ± 0.043 0.63 ± 0.034 Low COF 249 ± 12.25 4.13 ± 0.21 0.99 ± 0.049 1.03 ± 0.054 Mid COF 279 ± 14.98* 6.87 ± 0.31* 1.39 ± 0.072* 1.43 ± 0.077* High COF 304 ± 15.83* 8.96 ± 0.39* 1.77 ± 0.091* 1.83 ± 0.089* Vit C + High COF 210 ± 11.06# 3.14 ± 0.15# 1.01 ± 0.053 0.86 ± 0.053# Note. *P < 0.05, compared to the control group; #P < 0.05, compared to the high COF group. COF: cooking oil fumes. Table 2. Changes in LDH, AKP, TNF-α and IL-6 levels
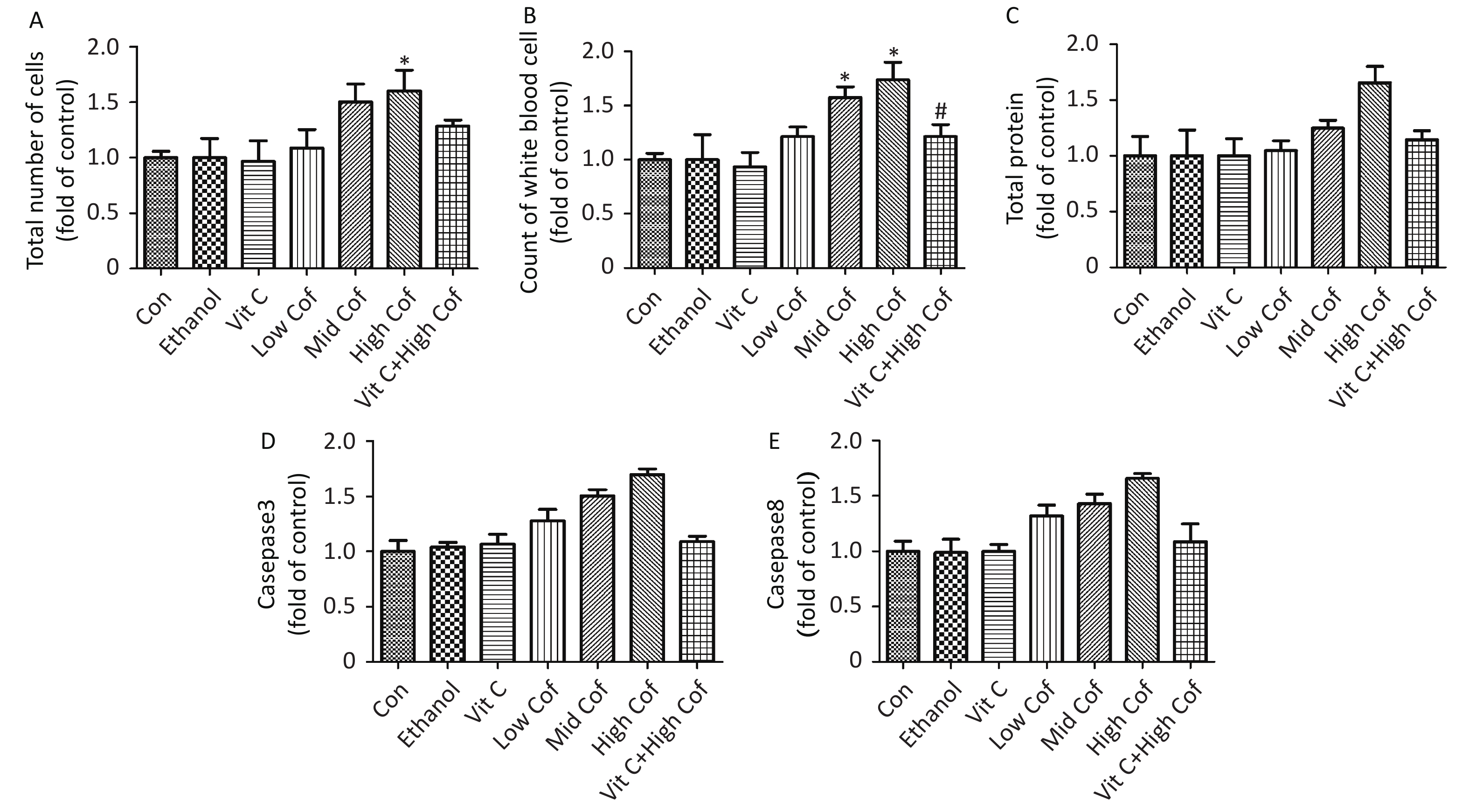
$(\overline X \pm {\rm{SD}})$ (n = 10)The total number of white blood cells in BALF has been suggested to be associated with pulmonary inflammation[8]. Total protein (TP) is associated with pulmonary vascular permeability, and the level of TP can be used as an indicator of the degree of damage to the alveolar epithelial capillary barrier. Supplementary Figure S1 (available in www.besjournal.com) shows a significant increase in the total number of cells and total protein in BALF samples after exposure to high-dose COF (P < 0.05). The number of white blood cells in BALF samples from mice increased significantly after exposure to moderate or high doses of COF (P < 0.05). Vitamin C has also been shown to diminish the risk of pneumonia by decreasing lung inflammation[9]. Our results showed that vitamin C pretreatment resulted in significantly lower TP and numbers of white blood cells than were observed in the high dose COF group (P < 0.05), thus, indicating that vitamin C might ameliorate lung inflammation induced by COF.
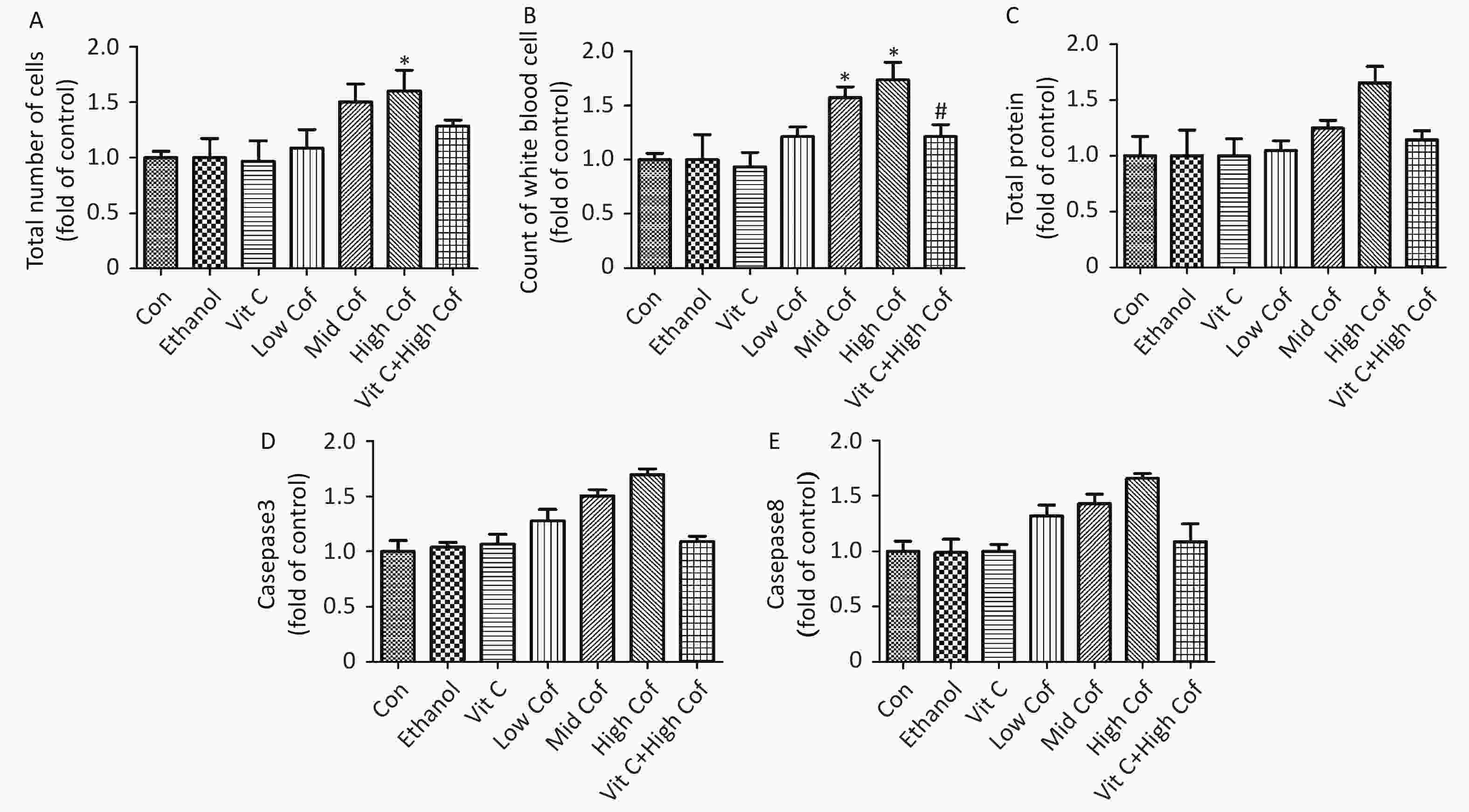
Figure S1. Total cell count, white blood cell count and total protein content in BALF and caspase3 and caspase 8 activities in lung tissue. (n = 10). (A) Total number of cells in BALF. (B) Count of white blood cells in BALF. (C) Total protein levels in BALF. (D) Activity of caspase 3 in lung tissues. E. Activity of caspase 8 in lung tissues. *P < 0.05, compared with control group, #P < 0.05, compared with High Cof group
Caspase 3 is a crucial member of the caspase family, which comprises a group of cysteine proteases that mediate apoptosis[10]. Caspase 3 functions as a major effector caspase through cleaving numerous cell death substrates, thus, leading to cellular dysfunction and destruction. A specific dose of vitamin C significantly inhibits apoptosis in mouse granulosa cells[10]. The levels of caspase 3 in the lung tissues of mice significantly increased after exposure to moderate or high doses of COF (P < 0.05). Caspase 8 levels in the high-dose COF group were significantly higher than those in the control group (P < 0.05). Vitamin C pretreatment significantly decreased the elevated levels of caspase 3 and caspase 8 caused by high-dose COF (P < 0.05). Therefore, vitamin C pretreatment might inhibit the apoptosis induced by COF (Supplementary Figure S1).
Disclosures The authors declare that they have no conflicts of interest.
Acknowledgement We thank the anonymous reviewers for commenting on earlier versions of this manuscript.
HTML
 21053Supplementary Materials.pdf
21053Supplementary Materials.pdf
|

|









 Quick Links
Quick Links
 DownLoad:
DownLoad: