-
In previous decades, people were concerned about their health because of growing environmental influences. The advancements in pulsed power technologies have increased the availability of microwave transmitters. The possible health problems caused by the widespread use of microwaves have attracted attention in recent years. This includes wireless communication technology, radar, and microwave ovens. Many studies have assessed the health risks and investigated the biological consequences of microwaves on the nervous system[1]. Studies have also demonstrated that microwave induces learning and memory deficits, possibly because the hippocampus is particularly sensitive to microwave exposure[2, 3]. The swollen mitochondria could be detected in hippocampal neurons of microwave-exposed rats and neuron-like cells[2, 4]. Defective mitochondria have a potential for futile adenosine triphosphate (ATP) hydrolysis and accelerated reactive oxygen species (ROS) production, resulting in neurological damage or death.
As a regulator of oxidative stress, the Nrf2/ARE pathway is tightly regulated in normal conditions, but this pathway is deregulated in different diseases, such as neurodegenerative diseases caused by mitochondrial dysfunction status and oxidative damage. However, its semantic expression is not clear. In Parkinson’s disease, Nrf2 is detected in the nucleus, but antioxidant genes are not sufficient to protect the neurons [5]. However, there is little concern about the condition of the Nrf2/ARE pathway and its protective mechanism against microwave-induced neuronal injuries. In this study, cells were exposed to a 2.856-GHz microwave at an average power density of 30 mW/cm2 for 6 min. The microwave had a high-peak intensity of 200 W/cm2, with relatively short pulse widths. The average specific absorption rate (SAR) was 19 W/kg.
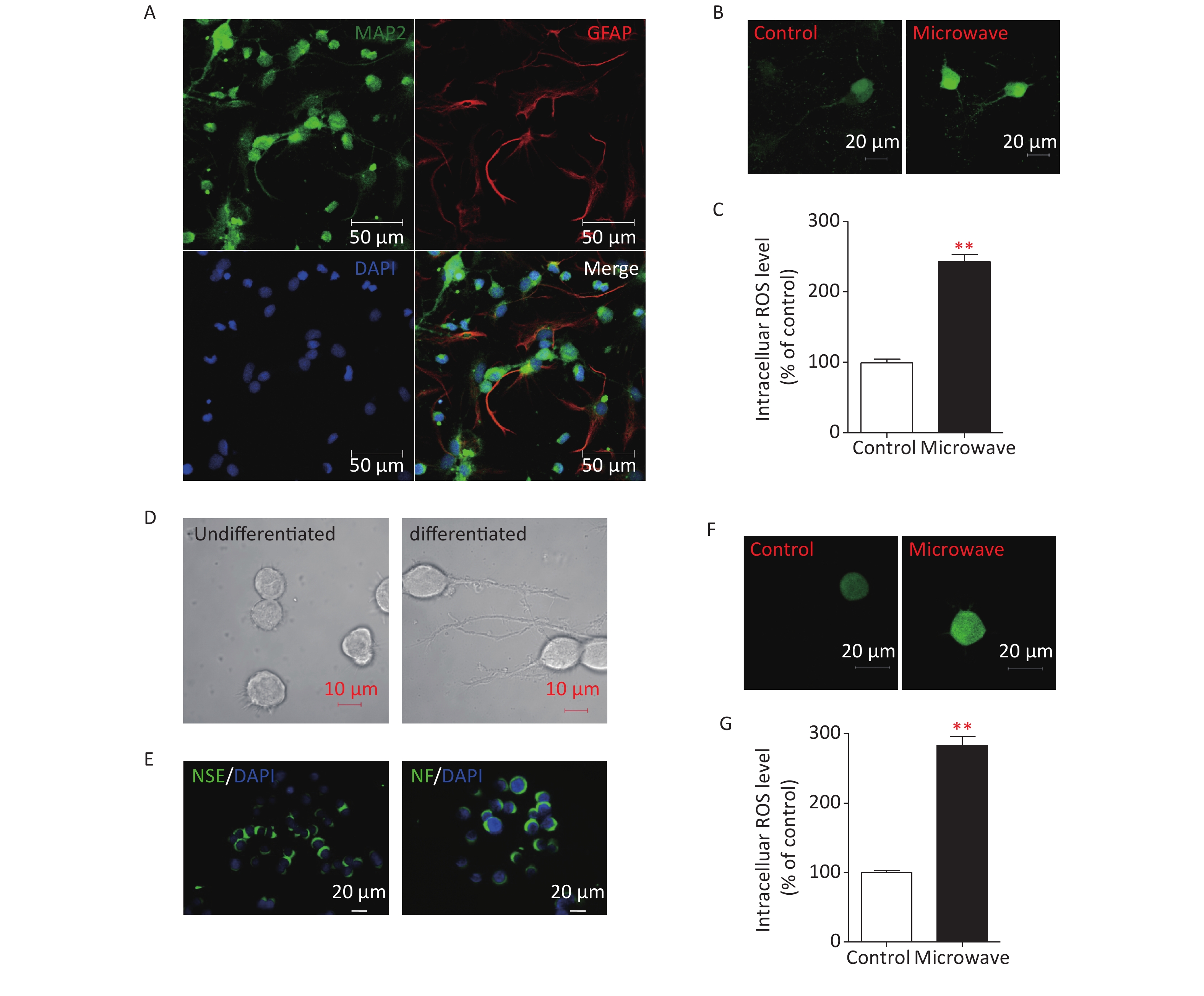
To determine if microwave-induced-mitochondrial damages could result in oxidative damage to hippocampal neurons, the hippocampal neurons of newborn rats were separated first (Supplementary Figure S1A, available in www.besjournal.com). Then, primary neurons were exposed to microwave, while some were not. Intracellular ROS were detected by staining with Dichlorodihydrofluorescein Diacetate (DCFH, Sigma-Aldrich, USA), which can be oxidized to dichlorofluorescein (DCF) by ROS. Microwave exposure significantly increased the intensity of green fluorescence compared to sham exposure (Supplementary Figure S1B, S1C). A PC12 cell line was initially derived from a rat pheochromocytoma, and completely differentiated PC12 cells possessed the phenotypic properties of sympathetic neurons, as they stop dividing, extend neural neuritis (Supplementary Figure S1D), and up-regulate several neural markers (Supplementary Figure S1E). The microwave was also reported to induce mitochondrial damage in differentiated PC12 cells, so we also determined whether ROS production could be up-regulated in differentiated PC12 cells. Data showed the same result, where intracellular ROS levels were significantly increased after microwave exposure (Supplementary Figure S1F, S1G). Notably, microwave exposure could accelerate the accumulation of intracellular ROS, leading to oxidative stress.
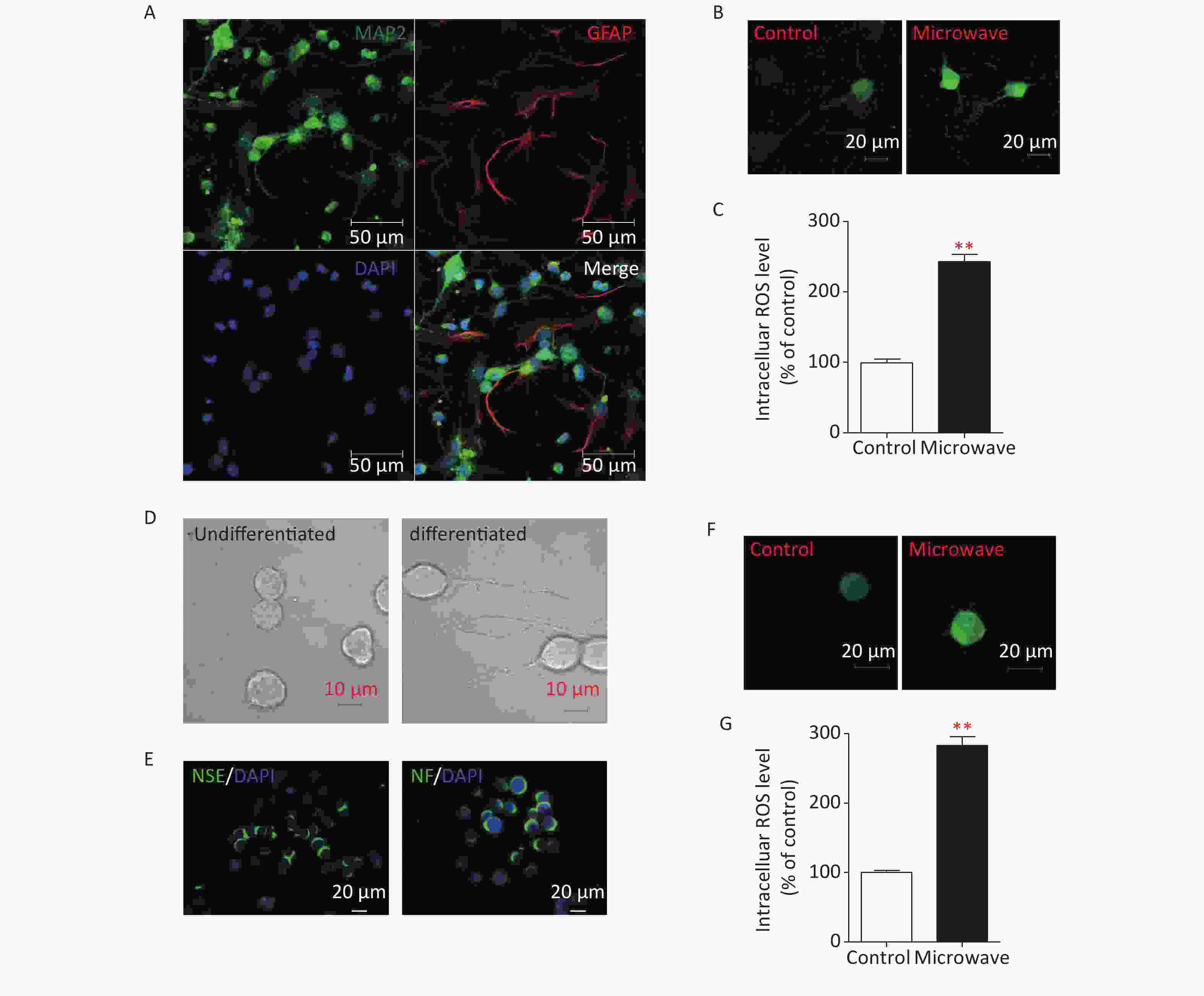
Figure S1. Microwave exposure increases levels of intracellular ROS in primary cultured rat hippocampal neurons and differentiated PC12 cells. (A) Immunostaining of DIV7 neurons (MAP2, green) and glial cells (GFAP, red) cultured without arabinofuranoside. Scale bar, 50 μm. (B) Hippocampal neurons were exposed at 30 mW/cm2 or sham exposures for 6 min at DIV7, and incubated with DCFH-DA for 30 min. Fluorescence images of neurons loaded with DCFH-DA were shown. Scale bars, 20 μm. (C) Intracellular ROS was measured by fluorospectrophotometer. (D) Morphological changes of NGF-induced differentiated PC12 cells. Scale bars, 10 μm. (E) Identification of NGF-induced differentiated PC12 cells. Differentiated PC12 cells were immunostained using anti-NSE and anti-NF antibodies. Scale bars, 20 μm. (F) The differentiated PC12 cells were exposed at 30 mW/cm2 or sham exposures for 6 min, and incubated with DCFH-DA for 30 min. Fluorescence images of cells loaded with DCFH-DA were shown. Scale bars, 20 μm. (G) Intracellular ROS was measured by fluorospectrophotometer. *P < 0.05, **P < 0.01, vs. control group.
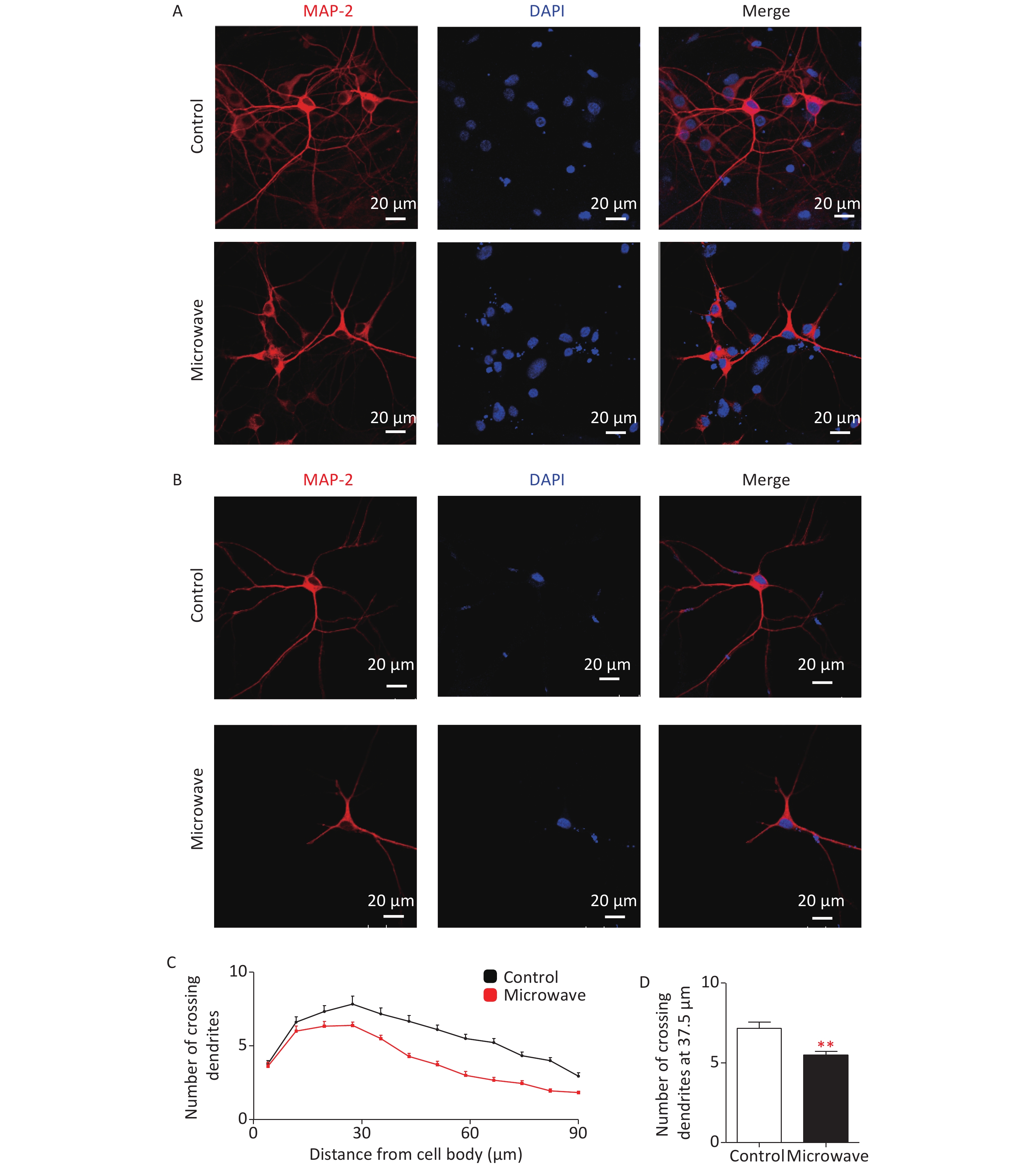
Dendrites are branched extensions of neurons that play a critical role in integrating synaptic inputs. Dendrite size and complexity are important determinants of how a neuron accepts, receives, integrates, and transmits inputs from other neurons. ROS have been implicated in the dysfunction of dendrite development [6]. Chronic exposure to 2.4 W/kg GSM 1,800 MHz microwaves during early developmental stages could affect dendrite development [7]. Thus, we investigated the possible correlation between microwave exposure and neuronal cell differentiation. The hippocampal neurons were exposed to microwave and immunostained for MAP2 (DAKO, Japan). The neurons exposed to microwave were characterized by less immature dendrites as compared to the sham-exposed cells (Supplementary Figure S2A, S2B available in www.besjournal.com). Sholl analysis was done to quantify this effect. The sham-exposed neurons showed the highest number of crossings at about 30-μm distance from the cell body, while microwave-exposed neurons showed the peak at about 22.5 μm (Supplementary Figure S2C). The number of crossing dendrites at a 37.5-μm distance from the cell bodies was significantly reduced after microwave exposure (Supplementary Figure S2D). These results showed that microwave-induced ROS upregulation might inhibit neuronal outgrowths, such as dendrite extension and arborization.
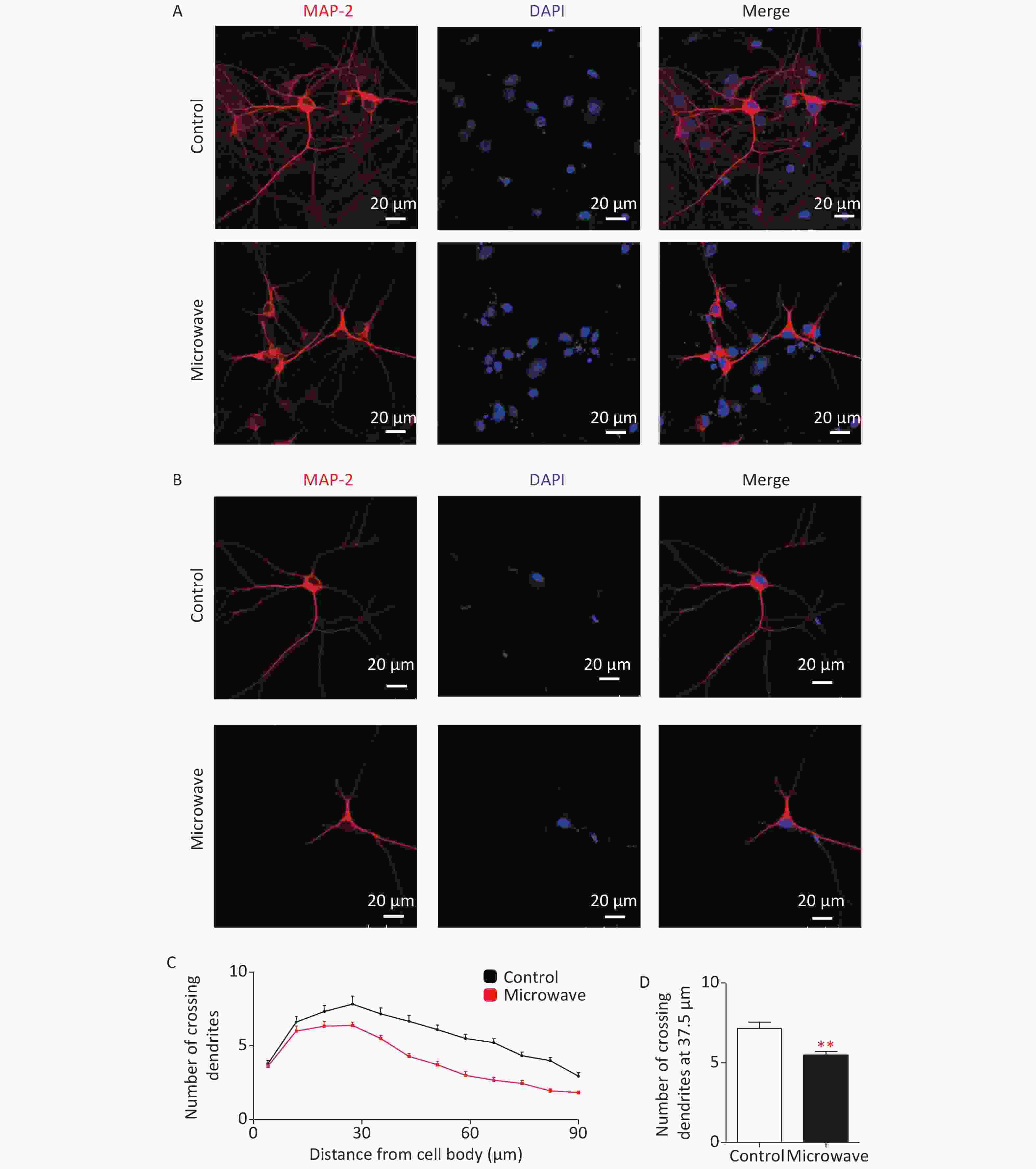
Figure S2. Impaired dendrite development in microwave exposed primary cultured rat hippocampal neurons. (A) Hippocampal neurons were exposed at 30 mW/cm2 or shame exposure for 6 min at DIV7, then cultured, fixed at DIV14 and dendrites were immunostained using an anti-MAP2 antibody. Scale bars, 20 μm. (B) The typical image of the immunostaining. (C) Sholl analysis of microwave exposed neurons (red line, n = 18) or sham-exposed neurons (black line, n = 18). (D) Numbers of crossing dendrites at 37.5 μm from the cell body in exposed and sham-exposed neurons. *P < 0.05, **P < 0.01, vs. control group.
Nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor, is involved in endogenous antioxidant response. Nrf2 binds to antioxidant response element (ARE) and regulates the expression of oxidative stress defensive genes, such as NAD(P)H quinone oxidoreductase-1 (NQO1). This is a mechanism essential for cellular protection against oxidative stress. The repressor protein Kelch-like ECH-associated protein-1 (Keap1) is a typical negative regulator of Nrf2, and it has been considered as the basic control of the Nrf2 signaling pathway. Under normal conditions, Keap1 promotes Nrf2 ubiquitination and subsequent degradation by binding to E3 ubiquitin ligase complex Rbx-1/cullin-3. Under electrophilic and oxidative stress, reactive KEAP1 cysteine residues result in the release of Nrf2 and its translocation to the nucleus. Then, Nrf2 binds to the ARE to activate the transcription of antioxidant genes [8].
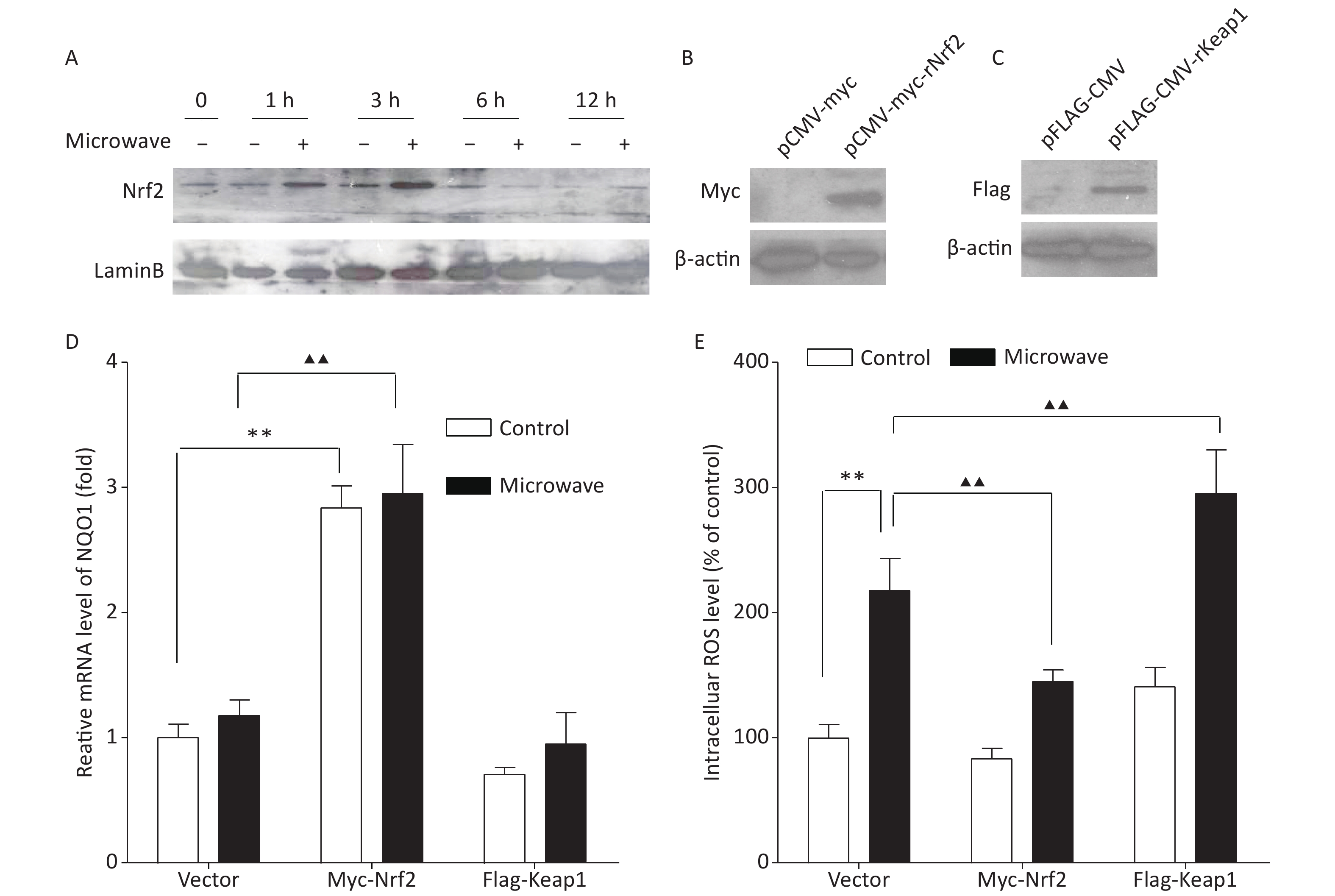
According to our data, the protein level of Nrf2 in the nucleus was up-regulated between 1 and 3 h after the microwave exposure, but the effect disappeared in 6 h after the exposure (Figure 1A). However, the mRNA expression of its downstream gene NQO1 showed no significant difference between the two groups, and oxidative injury still existed. Therefore, we thought that the transit activation of Nrf2 could not be enough to protect the cells. To understand the possible correlation between Nrf2/ARE signaling and the protective mechanism against microwave-induced oxidative stress, we established PC12 cells stably expressing Nrf2 and its negative regulator Keap1. Myc-Nrf2 and Flag-Keap1 were transfected into the PC12 cells, and the transfected cells, which stably expressed Nrf2 and Keap1, were selected by culturing with G418 for two weeks. Nrf2 and Keap1 expressions were demonstrated by western blot (Figure 1B, 1C). The overexpression of Nrf2 resulted in an enhanced expression of NQO1, indicating the up-regulation of Nrf2 and its downstream events, while the ectopic expression of Keap1 reduced the mRNA level of NQO1 (Figure 1D). Further detection showed that ROS accumulation was abrogated by Nrf2 overexpression, while Keap1 overexpression enhanced the accumulation of ROS (Figure 1E). These data indicated that the up-regulation of Nrf2 promoted ROS elimination, while its down-regulation restrained ROS elimination; thus, the inhibition of the Nrf2/NQO1 pathway leads to a decreased antioxidant capacity and makes cells vulnerable to microwave damage.
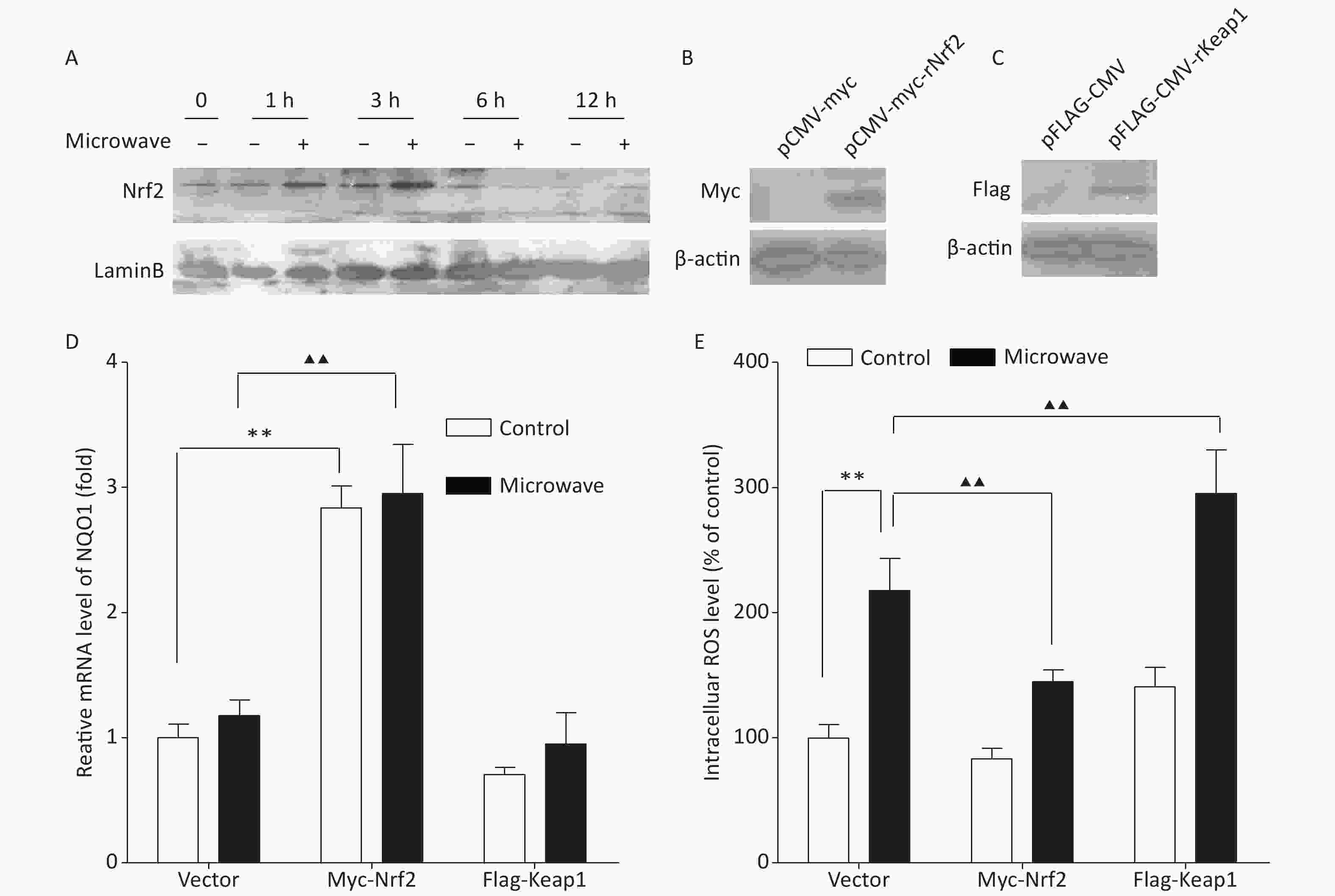
Figure 1. Ectopic expression of Nrf2 reduced the oxidative stress in PC12 cells. (A) Proteins were extracted at indicated time points after microwave exposure. Cell lysates were immunoblotted with antibodies, which detected the Nrf2 levels in nuclear fractions. (B) pCMV-vector and pCMV-Myc-Nrf2 were separately transfected into PC12 cells. After two weeks of culture with G418 for selection, the Nrf2 expression in PC12 cells was detected by western blot. (C) pCMV-vector and pCMV-Flag-Keap1 were separately transfected into PC12 cells. After two weeks of culture with G418 for selection, the Keap1 expression in PC12 cells was detected by western blot. (D) The relative mRNA expression of NQO1 was measured by qPCR in Nrf2- or Keap1-overexpressing cells or empty vector-expressing cells with or without microwave exposure. (E) PC12 cells overexpressing Nrf2, Keap1 or vector were exposed to microwave, while some were not. The cells were loaded with DCFH-DA and detected using a fluorospectrophotometer. *P < 0.05, **P < 0.01 vs. control group; ▲P < 0.05, ▲▲P < 0.01 vs. microwave group.
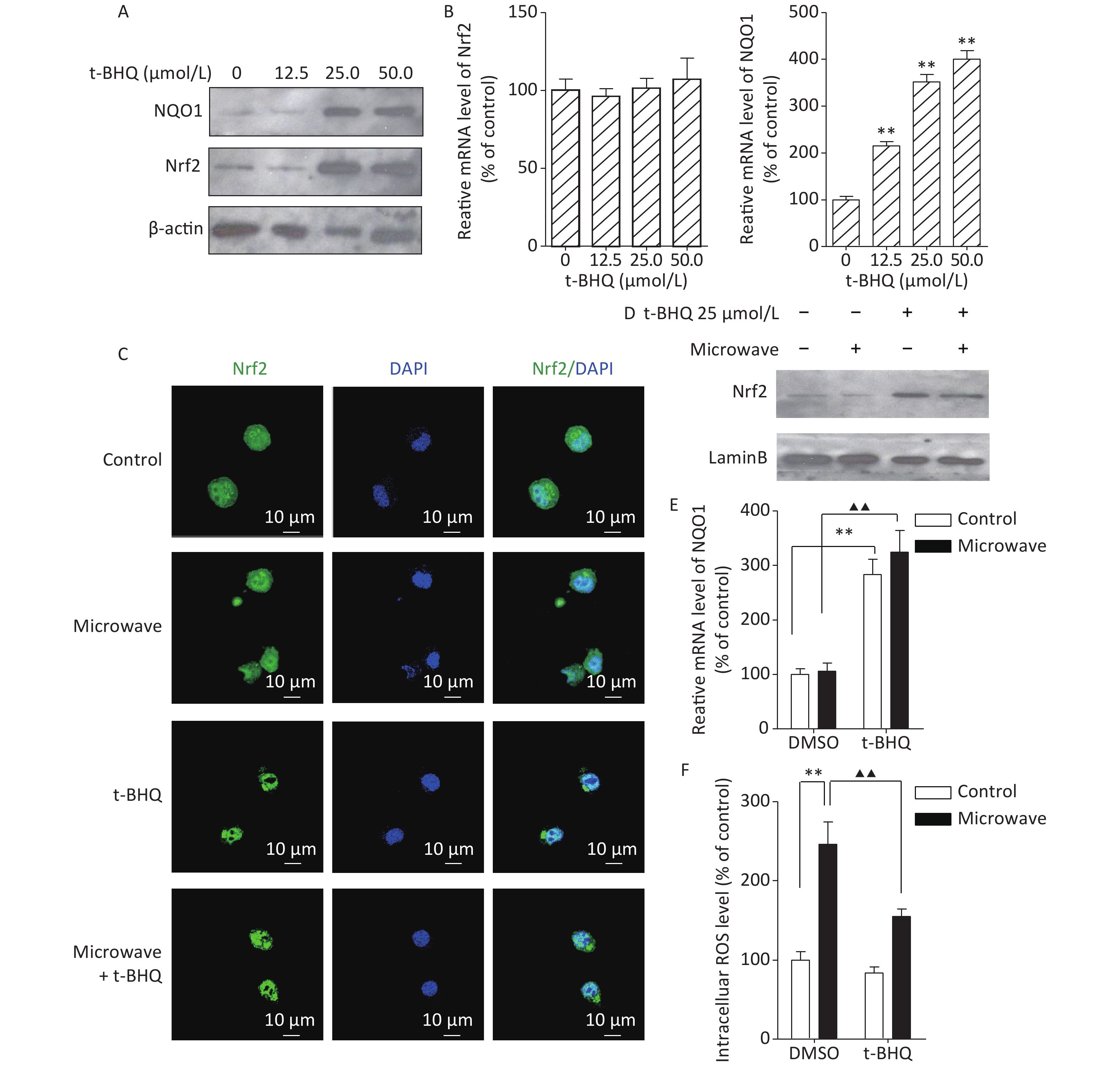
To confirm the Nrf2/NQO1 function in vivo, we used specific activators, such as tert-butylhydroquinone (t-BHQ), to activate endogenous Nrf2. First, PC12 cells were incubated with different concentrations (0, 12.5, 25, and 50 mol/L) of t-BHQ for 24 h prior to our experiments. Then, the protein and mRNA levels of Nrf2 and its prototypical target gene NQO1 were tested. The Nrf2 protein, but not its mRNA, was significantly induced at 25 mol/L. The NQO1 protein and mRNA were both observed at 25 mol/L (Figure 2A, 2B). These results indicated that t-BHQ up-regulated Nrf2 by decreasing Nrf2 degradation. Thus, 25 mol/L t-BHQ was chosen for the induction of Nrf2 and NQO1. Next, we verified whether t-BHQ could inhibit the increase in ROS under microwave exposure. PC12 cells were incubated with t-BHQ for 24 h, followed by microwave exposure for 6 min. Nrf2 translocation was measured by immunostaining and western blot. Data showed that Nrf2 accumulated in the nucleus after the t-BHQ treatment even after microwave exposure, indicating the stimulation of Nrf2/ARE signaling (Figure 2C, 2D). qPCR results showed that the pre-treatment with t-BHQ resulted in a dramatic increase in the mRNA expression of NQO1 (Figure 2E). It also attenuated the ROS upregulation induced by microwave exposure (Figure 2F). These data suggested that the activation of Nrf2/ARE signaling was required for protection against microwave-induced oxidative stress.
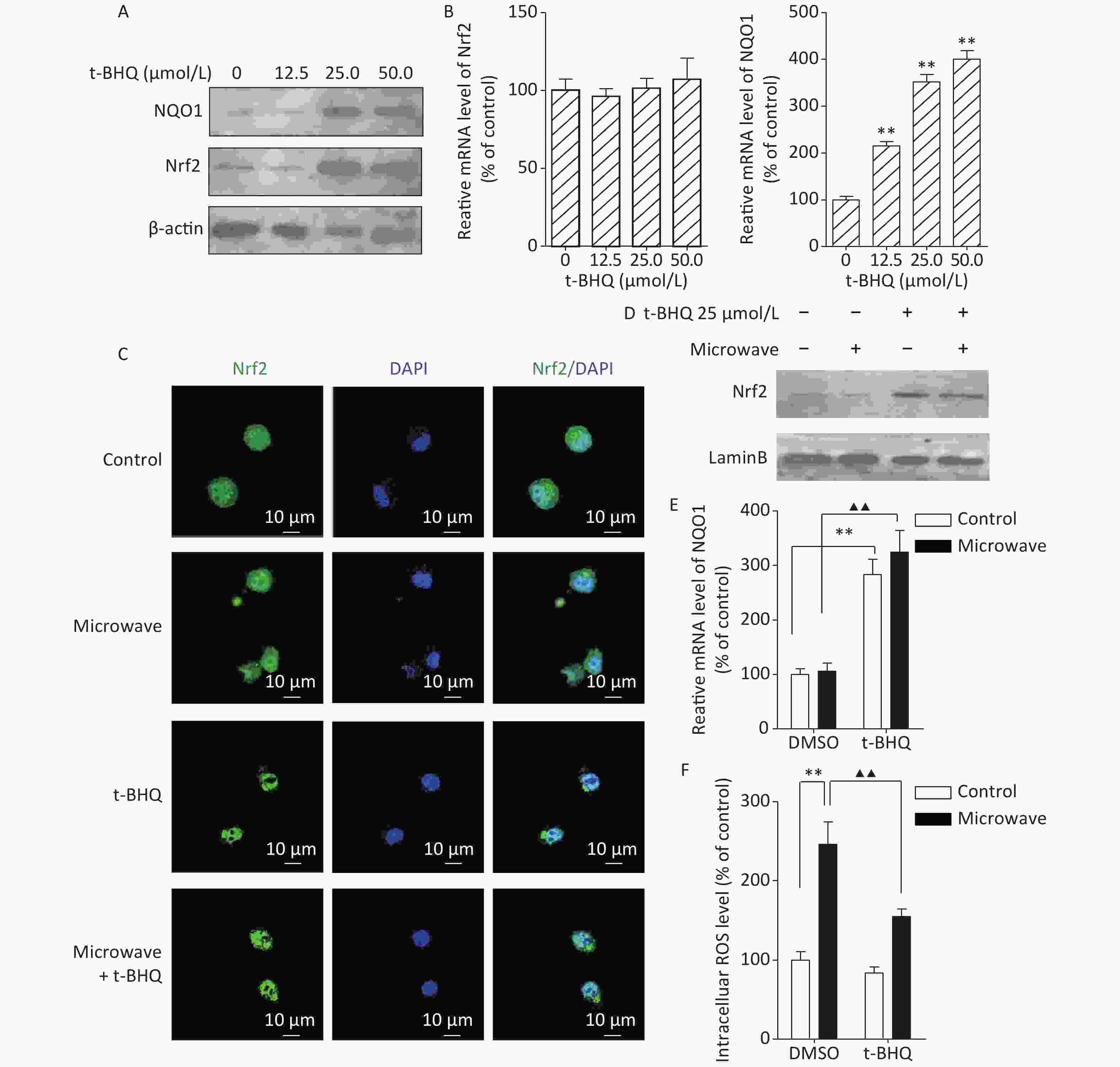
Figure 2. t-BHQ suppressed the microwave-induced oxidative stress in PC12 cells. (A) To detect the effect of different concentrations of t-BHQ on Nrf2 and NQO1 protein, PC12 cells were treated with 12.5, 25, and 50 μmol/L t-BHQ for 24 h, lysed, and detected by western blot. (B) Effect of different concentrations of t-BHQ on Nrf2 and NQO1 mRNA. (C) PC12 cells were grown on coverslips, treated with or without 25 μmol/L t-BHQ for 24 h, exposed or sham-exposed to microwave for 6 min, fixed, permeabilized, and probed with anti-Nrf2 antibodies, followed by FITC-tagged secondary antibodies. The cells were also stained with DAPI to visualize the nuclei (blue). Scale bars, 10 μm. (D) PC12 cells were lysed and detected by western blot with indicated protein antibodies. (E) The relative mRNA expression of NQO1 in t-BHQ-treated or untreated cells with or without microwave exposure was measured by qPCR. (F) PC12 cells were pre-treated with 25 μmol/L t-BHQ for 24 h before microwave exposure. The cells were loaded with DCFH-DA and detected using a fluorospectrophotometer. *P < 0.05, **P < 0.01 vs. control group; ▲P < 0.05, ▲▲P < 0.01 vs. microwave group.
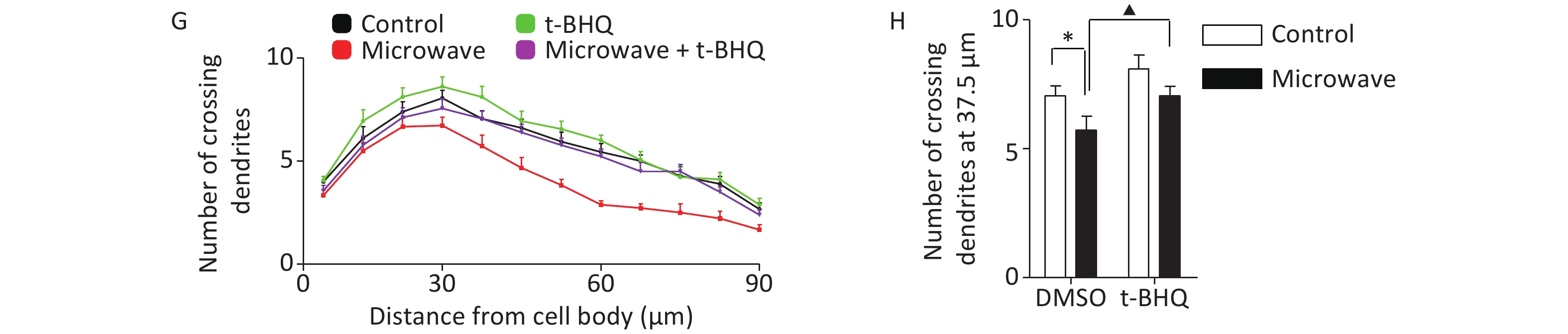
Then, we investigated whether t-BHQ pre-treatment could reduce hippocampal neuronal injury under oxidative conditions. t-BHQ induced the nuclear accumulation of Nrf2 in hippocampal neurons even after microwave exposure, along with the ectopic expression of NQO1 mRNA (Figure 3A, 3B, 3C). Based on the measurement of intracellular ROS by DCFH-DA staining, t-BHQ inhibited the accumulation of microwave-induced ROS in primary neurons (Figure 3D, 3E). For differentiation studies, the impaired dendrite development caused by microwave could be rescued by pre-treatment with t-BHQ; the group incubated with t-BHQ before microwave exposure had more immature dendrites, with an increased number of crossing dendrites as compared to the group that only underwent microwave exposure (Figure 3F, 3G, 3H). These results indicated that the stimulation of the Nrf2/NQO1 pathway contributed to the protection of primary neurons from oxidative injury.
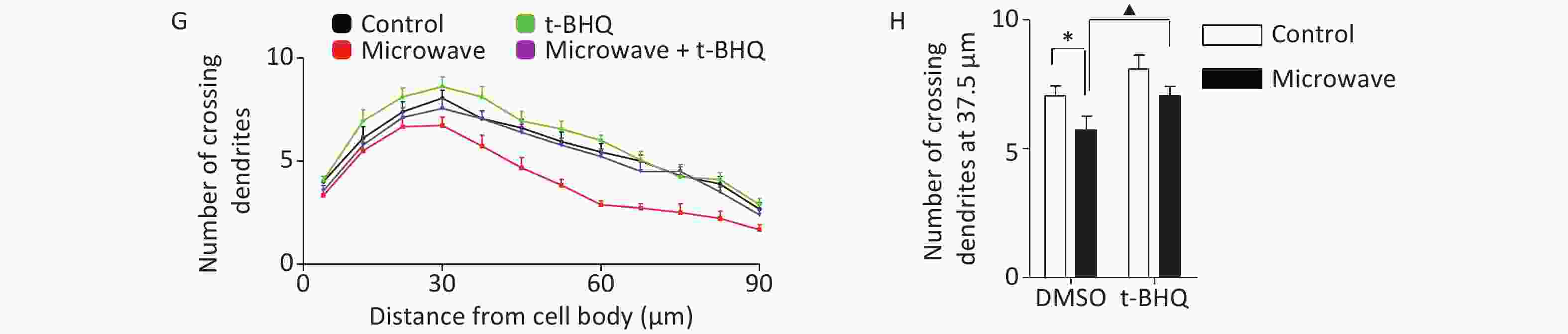
Figure 3. t-BHQ suppressed the microwave-induced oxidative injury in primary cultured rat hippocampal neurons. (A) Rat hippocampal neurons were treated with 25 μmol/L t-BHQ for 24 h at DIV7 and were exposed to microwave for 6 min. Then, the neurons were fixed, permeabilized, and probed with anti-Nrf2 antibodies, followed by FITC-tagged secondary antibodies. The cells were also stained with DAPI to visualize the nuclei (blue). Scale bars, 20 μm. (B) Primary neurons were lysed and detected by western blot with indicated protein antibodies. (C) The NQO1 mRNA level was detected by qPCR. (D) The hippocampal neurons were exposed at 30 mW/cm2 or sham-exposed with or without t-BHQ. The fluorescence images of neurons loaded with DCFH-DA are shown. Scale bars, 20 μm. (E) The neurons were loaded with DCFH-DA and detected using a fluorospectrophotometer. (F) The hippocampal neurons were exposed at 30 mW/cm2 or sham-exposed for 6 min with or without t-BHQ at DIV7, cultured, and fixed at DIV14. The dendrites were immunostained with anti-MAP2 antibodies. Scale bars, 20 μm. (G) Sholl analysis of control neurons (black line, n = 18), microwave-exposed neurons (red line, n = 18), unexposed and t-BHQ-treated neurons (green line, n = 18), microwave-exposed and t-BHQ-treated neurons (purple line, n = 18). (H) Numbers of crossing dendrites at 37.5 μm from the cell body. *P < 0.05, **P < 0.01 vs. control group; ▲P < 0.05, ▲▲P < 0.01 vs. microwave group.
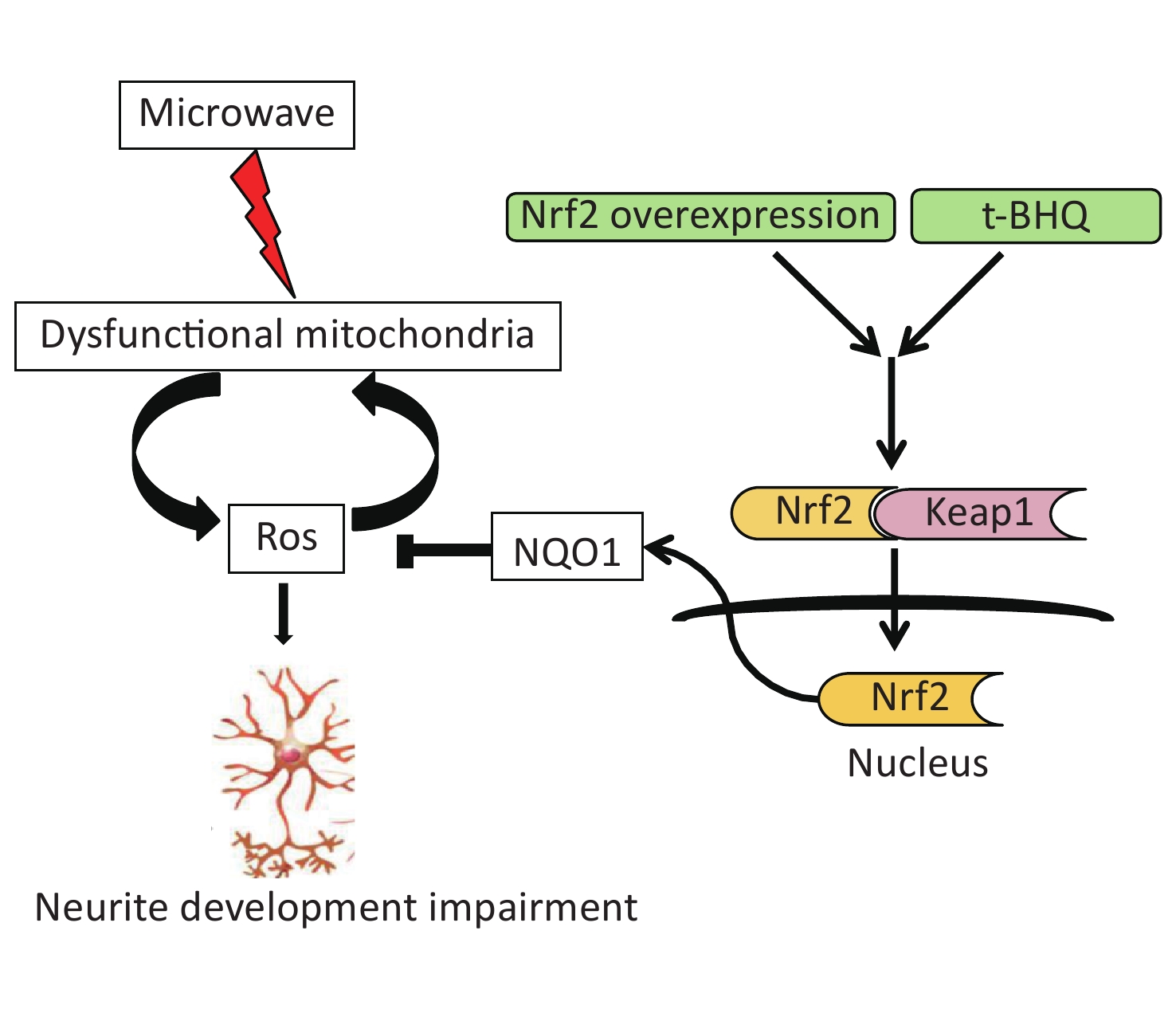
Therefore, we concluded that microwave exposure damaged the mitochondria and generated ROS and oxidative stress. Under ROS-stressed conditions, the accumulation of ROS contributed to secondary mitochondrial damage. The activation of the Nrf2 pathway in vitro or in vivo led to a decline in oxidative stress and protection of neuronal cells (Supplementary Figure S3 available in www.besjournal.com).
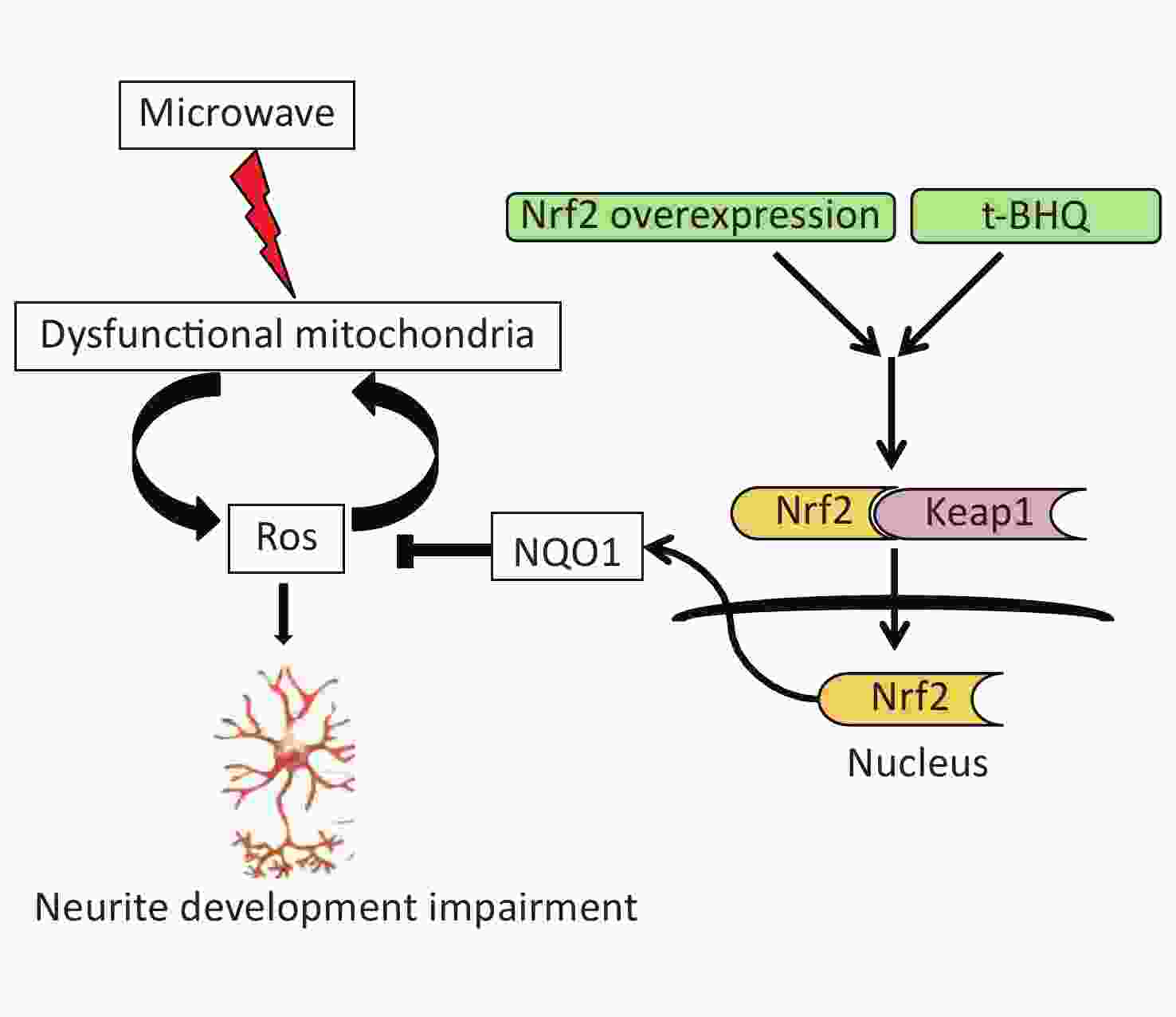
Figure S3. Schematic representation of the role of Nrf2/NQO1 pathway in the protection of neuron injury induced by microwave.
In general, the central nervous system is vulnerable to oxidative stress, as it normally produces relatively large amounts of ROS for its high energy needs. The primary causes of neuron degeneration were thought to be mitochondrial dysfunction and increased oxidative stress [9]. In degenerated cells, the increasing ROS production could not be declined by the failure of antioxidant defenses, and excessive ROS target the mitochondria and play an important role in the mitochondrial injury. The feedback process increased the damage, leading to cell dysfunction or cell death [10]. Microwave exposure impaired the learning and memory ability and the morphological and functional changes in the mitochondria. In this study, we revealed that 30 mW/cm2 microwave induced the abundant generation of ROS in neuron-like cells and neurons. There was a significant decrease in the density and average lengths of dendrites. Therefore, we showed that exposure to 30 mW/cm2 microwave for 6 min during early developmental stages might affect dendritic development, but its specific mechanism was still unclear. However, when the Nrf2/NQO1 pathway was activated by the administration of t-BHQ, the microwave-induced neurite outgrowth disorder was reversed.
In this study, we focused on the disordered effect of microwaves on neuronal cells and the regulation of the Nrf2/NQO1 pathway under microwave exposure. Nrf2 was translocated into the nucleus immediately after microwave exposure, but its activation could not be maintained for a longer duration. We also detected the upregulation of intracellular ROS levels and the disorder in neurite development. It was also confirmed that the continuous activation of the Nrf2/NQO1 signaling pathway was required for its neuroprotective effects against microwave-induced oxidative stress. In summary, the Nrf2/NQO1 pathway was involved in the neuroprotective effects against microwave, and t-BHQ was believed to be a promising neuroprotective agent against it.
Acknowledgment We would like to thank Professor Masayuki Yamamoto (Tohoku University, Sendai, Japan) for kindly providing us with the pCMV-HA-mKeap1 expression construct. We especially thank Ms. Xin Xu for her technical assistance in laser scanning confocal microscope (LSCM) image processing and analysis.
Competing Interests The authors declare that they have no competing interests.
HTML
 21009Supplementary Materials.pdf
21009Supplementary Materials.pdf
|

|







 Quick Links
Quick Links
 DownLoad:
DownLoad: